13.3
Impact Factor
Theranostics 2021; 11(7):3301-3316. doi:10.7150/thno.51988 This issue Cite
Research Paper
RGD-expressed bacterial membrane-derived nanovesicles enhance cancer therapy via multiple tumorous targeting
Department of Pharmaceutical Sciences, College of Pharmaceutical Sciences and Pharmacy, Washington State University, Spokane, WA 99202, USA
* These authors contributed equally to this work
Abstract
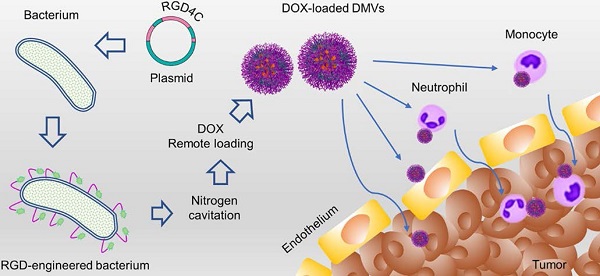
Background: A tumor microenvironment is a complicated multicellular system comprised of tumor cells, immune cells and blood vessels. Blood vessels are the barriers for drug tissue penetration. Effectively treating a cancer requires drug delivery systems to overcome biological barriers present in tumor microenvironments (TMEs).
Methods: We designed a drug delivery system made of bacterial (Escherichia coli) double layer membrane-derived nanovesicles (DMVs) with the expression of RGD peptides and endogenous targeting ligands of bacteria. The physical and biological characteristics of DMVs were assessed by cryogenic transmission electron microscopy, western blotting, flow cytometry and confocal microscopy. Doxorubicin (DOX) was loaded in DMVs via a pH gradient driven drug loading method. Therapeutical effects of DOX-loaded DMVs were studied in a melanoma xenograft mouse model.
Results: In vitro and in vivo experiments showed that DMVs can target neutrophils and monocytes that mediated the transport of DMVs across blood vessel barriers and they can also directly target tumor vasculature and tumor cells, resulting in enhanced delivery of therapeutics to TMEs. Furthermore, we developed a remote drug loading approach to efficiently encapsulate DOX inside DMVs, and the drug loading was 12% (w/w). In the B16-F10 melanoma mouse model, we showed that DOX-RGD-DMVs significantly inhibited the tumor growth compared to several controls.
Conclusion: Our studies reveal that DMVs are a powerful tool to simultaneously target multiple cells in TMEs, thus increasing drug delivery for improved cancer therapies.
Keywords: Bacterial membrane-derived nanovesicles, Doxorubicin, Remote loading, Tumor microenvironments, Inflammation response.
 Global reach, higher impact
Global reach, higher impact