13.3
Impact Factor
Theranostics 2019; 9(22):6706-6718. doi:10.7150/thno.35461 This issue Cite
Research Paper
Pretargeted radioimmunotherapy and SPECT imaging of peritoneal carcinomatosis using bioorthogonal click chemistry: probe selection and first proof-of-concept
1. Université Clermont Auvergne, Imagerie Moléculaire et Stratégies Théranostiques, BP 184, F-63005 Clermont-Ferrand, France. Inserm, U 1240, F-63000 Clermont-Ferrand, France. Centre Jean Perrin, F-63011 Clermont-Ferrand, France.
2. Institut de Recherche en Cancérologie (IRCM), U1194 - Université Montpellier - ICM, Radiobiology and Targeted Radiotherapy, 34298 Montpellier cedex 5.
3. Laboratoire de Physique de Clermont, UMR 6533 CNRS/IN2P3, Université Clermont Auvergne, 63178 - Aubière Cedex
4. Department of Chemistry, Hunter College, City University of New York, New York, NY, USA 10028
5. Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA 10028
* Co-authors
Abstract
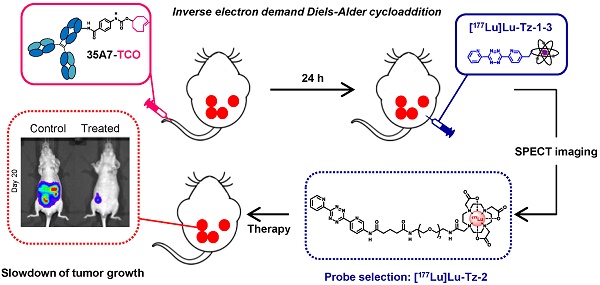
Rationale: Pretargeted radioimmunotherapy (PRIT) based upon bioorthogonal click chemistry has been investigated for the first time in the context of peritoneal carcinomatosis using a CEA-targeting 35A7 mAb bearing trans-cyclooctene (TCO) moieties and several 177Lu-labeled tetrazine (Tz) radioligands. Starting from three Tz probes containing PEG linkers of varying lengths between the DOTA and Tz groups (i.e. PEGn = 3, 7, or 11, respectively, for Tz-1, Tz-2, and Tz-3), we selected [177Lu]Lu-Tz-2 as the most appropriate for pretargeted SPECT imaging and demonstrated its efficacy in tumor growth control. Methods: An orthotopic model of peritoneal carcinomatosis (PC) was obtained following the intraperitoneal (i.p.) injection of A431-CEA-Luc cells in nude mice. Tumor growth was assessed using bioluminescence imaging. Anti-CEA 35A7 mAb was grafted with 2-3 TCO per immunoglobulin. Pretargeted SPECT imaging and biodistribution experiments were performed to quantify the activity concentrations of [177Lu]Lu-Tz-1-3 in tumors and non-target organs to determine the optimal Tz probe for the PRIT of PC. Results: The pharmacokinetic profiles of [177Lu]Lu-Tz-1-3 alone were determined using both SPECT imaging and biodistribution experiments. These data revealed that [177Lu]Lu-Tz-1 was cleared via both the renal and hepatic systems, while [177Lu]Lu-Tz-2 and [177Lu]Lu-Tz-3 were predominantly excreted via the renal system. In addition, these results illuminated that the longer the PEG linker, the more rapidly the Tz radioligand was cleared from the peritoneal cavity. The absorbed radiation dose corresponding to pretargeting with 35A7-TCO followed 24 h later by [177Lu]Lu-Tz-1-4 was higher for tumors following the administration of [177Lu]Lu-Tz-2 (i.e. 0.59 Gy/MBq) compared to either [177Lu]Lu-Tz-1 (i.e. 0.25 Gy/MBq) and [177Lu]Lu-Tz-3 (i.e. 0.18 Gy/MBq). In a longitudinal PRIT study, we showed that the i.p. injection of 40 MBq of [177Lu]Lu-Tz-2 24 hours after the systemic administration of 35A7-TCO significantly slowed tumor growth compared to control mice receiving only saline or 40 MBq of [177Lu]Lu-Tz-2 alone. Ex vivo measurement of the peritoneal carcinomatosis index (PCI) confirmed that PRIT significantly reduced tumor growth (PCI = 15.5 ± 2.3 after PRIT vs 30.0 ± 2.3 and 30.8 ± 1.4 for the NaCl and [177Lu]Lu-Tz-2 alone groups, respectively). Conclusion: Our results clearly demonstrate the impact of the length of PEG linkers upon the biodistribution profiles of 177Lu-labeled Tz radioligands. Furthermore, we demonstrated for the first time the possibility of using bioorthogonal chemistry for both the pretargeted SPECT and PRIT of peritoneal carcinomatosis.
Keywords: Pretargeting, bioorthogonal chemistry, peritoneal carcinomatosis, therapy, SPECT-CT imaging
 Global reach, higher impact
Global reach, higher impact