13.3
Impact Factor
Theranostics 2019; 9(15):4308-4323. doi:10.7150/thno.32710 This issue Cite
Research Paper
Methylation of RCAN1.4 mediated by DNMT1 and DNMT3b enhances hepatic stellate cell activation and liver fibrogenesis through Calcineurin/NFAT3 signaling
1. The Key Laboratory of Major Autoimmune Diseases, Anhui Province, Anhui Institute of Innovative Drugs, School of Pharmacy, Anhui Medical University
2. The key laboratory of Anti-inflammatory of Immune medicines, Ministry of Education
3. Institute for Liver Diseases of Anhui Medical University
Abstract
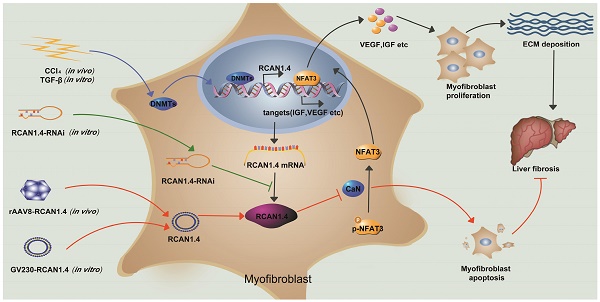
Background: Liver fibrosis is characterized by extensive deposition of extracellular matrix (ECM) components in the liver. RCAN1 (regulator of calcineurin 1), an endogenous inhibitor of calcineurin (CaN), is required for ECM synthesis during hypertrophy of various organs. However, the functional role of RCAN1 in liver fibrogenesis has not yet been addressed.
Methods: We induced experimental liver fibrosis in mice by intraperitoneal injection of 10 % CCl4 twice a week. To investigate the functional role of RCAN1.4 in the progression of liver fibrosis, we specifically over-expressed RCAN1.4 in mice liver using rAAV8-packaged RCAN1.4 over-expression plasmid. Following the establishment of the fibrotic mouse model, primary hepatic stellate cells were isolated. Subsequently, we evaluated the effect of RCAN1.4 on hepatic fibrogenesis, hepatic stellate cell activation, and cell survival. The biological role and signaling events for RCAN1 were analyzed by protein-protein interaction (PPI) network. Bisulfite sequencing PCR (BSP) was used to predict the methylated CpG islands in the RCAN1.4 gene promoter. We used the chromatin immunoprecipitation (ChIP assay) to investigate DNA methyltransferases which induced decreased expression of RCAN1.4 in liver fibrosis.
Results: Two isoforms of RCAN1 protein were expressed in CCl4-induced liver fibrosis mouse model and HSC-T6 cells cultured with transforming growth factor-beta 1 (TGF-β1). RCAN1 isoform 4 (RCAN1.4) was selectively down-regulated in vivo and in vitro. The BSP analysis indicated the presence of two methylated sites in RCAN1.4 promoter and the downregulated RCAN1.4 expression levels could be restored by 5-aza-2'-deoxycytidine (5-azadC) and DNMTs-RNAi transfection in vitro. ChIP assay was used to demonstrate that the decreased RCAN1.4 expression was associated with DNMT1 and DNMT3b. Furthermore, we established a CCl4-induced liver fibrosis mouse model by injecting the recombinant adeno-associated virus-packaged RCAN1.4 (rAAV8-RCAN1.4) over-expression plasmid through the tail vein. Liver- specific-over-expression of RAN1.4 led to liver function recovery and alleviated ECM deposition. The key protein (a member of the NFAT family of proteins) identified on PPI network data was analyzed in vivo and in vitro. Our results demonstrated that RCAN1.4 over-expression alleviates, whereas its knockdown exacerbates, TGF-β1-induced liver fibrosis in vitro in a CaN/NFAT3 signaling-dependent manner.
Conclusions: RCAN1.4 could alleviate liver fibrosis through inhibition of CaN/NFAT3 signaling, and the anti-fibrosis function of RCAN1.4 could be blocked by DNA methylation mediated by DNMT1 and DNMT3b. Thus, RCAN1.4 may serve as a potential therapeutic target in the treatment of liver fibrosis.
Keywords: DNA methylation, RCAN1.4, Proliferation, Apoptosis, Calcineurin/NFAT signaling
 Global reach, higher impact
Global reach, higher impact