13.3
Impact Factor
Theranostics 2019; 9(6):1752-1763. doi:10.7150/thno.30977 This issue Cite
Research Paper
Convection-Enhanced Delivery of a Virus-Like Nanotherapeutic Agent with Dual-Modal Imaging for Besiegement and Eradication of Brain Tumors
1. Institute of Medical Science and Technology, National Sun Yat-sen University, 70 Lienhai Rd., Kaohsiung 80424, Taiwan
2. Institute of Biomedical Sciences, National Sun Yat-sen University, 70 Lienhai Rd., Kaohsiung 80424, Taiwan
3. Department of Neurosurgery, Chang Gung Memorial Hospital, Keelung, 222 Maijin Rd., Keelung 20401, Taiwan
4. Community Medicine Research Center and Laboratory Animal Center, Chang Gung Memorial Hospital, Keelung, 222 Maijin Rd., Keelung 20401, Taiwan
5. School of Medicine, Chang Gung University, 259 Wenhua 1st Rd., Guishan Dist., Taoyuan 33302, Taiwan
6. Department of Neurosurgery, Chang Gung Memorial Hospital, Linkou, 5 Fuxing St., Guishan Dist., Taoyuan 33305, Taiwan
7. Center for Advanced Molecular Imaging and Translation, Chang Gung Memorial Hospital, Linkou, 5 Fuxing St., Guishan Dist., Taoyuan 33305, Taiwan
*H. H. Pang and P. Y. Chen contributed equally to this work.
Abstract
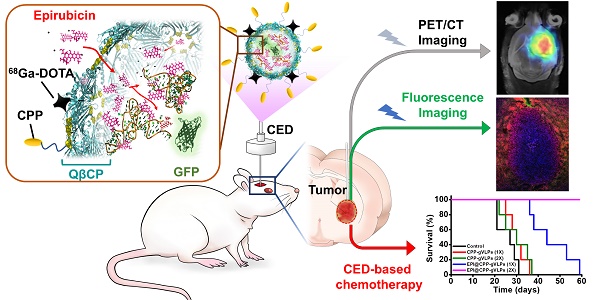
Convection-enhanced delivery (CED) is a promising technique for infusing a therapeutic agent directly into the brain, bypassing the blood-brain barrier (BBB) with a pressure gradient to increase drug concentration specifically around the brain tumor, thereby enhancing tumor inhibition and limiting the systemic toxicity of chemotherapeutic agents. Herein, we developed a dual-imaging monitored virus-like nanotherapeutic agent as an ideal CED infusate, which can be delivered to specifically besiege and eradicate brain tumors.
Methods: We report one-pot fabrication of green-fluorescence virus-like particles (gVLPs) in Escherichia coli (E. coli) for epirubicin (EPI) loading, cell-penetrating peptide (CPP) modification, and 68Ga-DOTA labeling to form a positron emission tomography (PET)-fluorescence dual-imaging monitored virus-like nanotherapeutic agent (68Ga-DOTA labeled EPI@CPP-gVLPs) combined with CED for brain tumor therapy and image tracking. The drug delivery, cytotoxicity, cell uptake, biodistribution, PET-fluorescence imaging and anti-tumor efficacy of the 68Ga-DOTA labeled EPI@CPP-gVLPs were investigated in vitro and in vivo by using U87-MG glioma cell line and U87-MG tumor model.
Results: The 68Ga-DOTA-labeled EPI@CPP-gVLPs showed excellent serum stability as an ideal CED infusate (30-40 nm in size), and can be disassembled through proteolytic degradation of the coat protein shell to enable drug release and clearance to minimize long-term accumulation. The present results indicated that 68Ga-DOTA-labeled EPI@CPP-gVLPs can provide a sufficiently high drug payload (39.2 wt% for EPI) and excellent detectability through fluorescence and PET imaging to accurately represent drug distribution during CED infusion. In vivo delivery of the 68Ga-DOTA-labeled EPI@CPP-gVLPs through CED demonstrated that the median survival was prolonged to over 50 days when the mice received two administrations (once per week) compared with the control group (median survival: 26 days).
Conclusion: The results clearly indicated that a combination of 68Ga-DOTA-labeled EPI@CPP-gVLPs and CED can serve as a flexible and powerful synergistic treatment in brain tumors without evidence of systemic toxicity.
Keywords: virus-like particles (VLPs), nanomedicine, dual-modal imaging, convection-enhanced delivery (CED), brain tumor
 Global reach, higher impact
Global reach, higher impact