13.3
Impact Factor
Theranostics 2019; 9(6):1580-1598. doi:10.7150/thno.30302 This issue Cite
Research Paper
Nano-Structural Effects on Gene Transfection: Large, Botryoid-Shaped Nanoparticles Enhance DNA Delivery via Macropinocytosis and Effective Dissociation
1. State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
2. University of Chinese Academy of Sciences, Beijing, 100049, China
3. Institute of Tropical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, China
4. Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250011, China
5. East China Normal University School of Life Sciences, Shanghai 200241, China
6. College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
Abstract
Effective delivery is the primary barrier against the clinical translation of gene therapy. Yet there remains too much unknown in the gene delivery mechanisms, even for the most investigated polymeric carrier (i.e., PEI). As a consequence, the conflicting results have been often seen in the literature due to the large variability in the experimental conditions and operations. Therefore, some key parameters should be identified and thus strictly controlled in the formulation process.
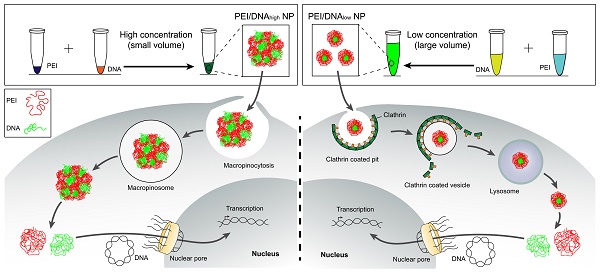
Methods: The effect of the formulation processing parameters (e.g., concentration or mixture volume) and the resulting nanostructure properties on gene transfection have been rarely investigated. Two types of the PEI/DNA nanoparticles (NPs) were prepared in the same manner with the same dose but at different concentrations. The microstructure of the NPs and the transfection mechanisms were investigated through various microscopic methods. The therapeutic efficacy of the NPs was demonstrated in the cervical subcutaneous xenograft and peritoneal metastasis mouse models.
Results: The high-concentration process (i.e., small reaction-volume) for mixture resulted in the large-sized PEI/DNA NPs that had a higher efficiency of gene transfection, compared to the small counterpart that was prepared at a low concentration. The microstructural experiments showed that the prepared small NPs were firmly condensed, whereas the large NPs were bulky and botryoid-shaped. The large NPs entered the tumor cells via the macropinocytosis pathway, and then efficiently dissociated in the cytoplasm and released DNA, thus promoting the intranuclear delivery. The enhanced in vivo therapeutic efficacy of the large NPs was demonstrated, indicating the promise for local-regional administration.
Conclusion: This work provides better understanding of the effect of formulation process on nano-structural properties and gene transfection, laying a theoretical basis for rational design of the experimental process.
Keywords: gene delivery, PEI, micropinocytosis, TRAIL, local-regional gene therapy, cervical cancer
 Global reach, higher impact
Global reach, higher impact