13.3
Impact Factor
Theranostics 2018; 8(17):4856-4869. doi:10.7150/thno.24252 This issue Cite
Research Paper
Evaluation of antibody fragment properties for near-infrared fluorescence imaging of HER3-positive cancer xenografts
1. Department of Pathology, University of Saskatchewan, Saskatoon, Canada
2. Department of Medical Imaging, University of Saskatchewan, Saskatoon, Canada
3. Department of Medical Imaging, Royal University Hospital Saskatoon, Canada
Abstract
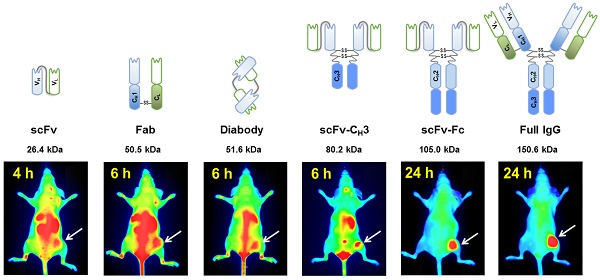
In vivo imaging is influenced by the half-life, tissue penetration, biodistribution, and affinity of the imaging probe. Immunoglobulin G (IgG) is composed of discrete domains with known functions, providing a template for engineering antibody fragments with desired imaging properties. Here, we engineered antibody-based imaging probes, consisting of different combinations of antibody domains, labeled them with the near-infrared fluorescent dye IRDye800CW, and evaluated their in vivo imaging properties. Antibody-based imaging probes were based on an anti-HER3 antigen binding fragment (Fab) isolated using phage display.
Methods: We constructed six anti-HER3 antibody-based imaging probes: a single chain variable fragment (scFv), Fab, diabody, scFv-CH3, scFv-Fc, and IgG. IRDye800CW-labeled, antibody-based probes were injected into nude mice bearing FaDu xenografts and their distribution to the xenograft, liver, and kidneys was evaluated.
Results: These imaging probes bound to recombinant HER3 and to the HER3-positive cell line, FaDu. Small antibody fragments with molecular weight <60 kDa (scFv, diabody, and Fab) accumulated rapidly in the xenograft (maximum accumulation between 2-4 h post injection (hpi)) and cleared primarily through the kidneys. scFv-CH3 (80 kDa) had fast clearance and peaked in the xenograft between 2-3 hpi and cleared from xenograft in a rate comparable to Fab and diabody. IgG and scFv-Fc persisted in the xenografts for up to 72 hpi and distributed mainly to the xenograft and liver. The highest xenograft fluorescence signals were observed with IgG and scFv-Fc imaging probes and persisted for 2-3 days.
Conclusion: These results highlight the utility of using antibody fragments to optimize clearance, tumor labeling, and biodistribution properties for developing anti-HER3 probes for image-guided surgery or PET imaging.
Keywords: antibody fragments, near-infrared fluorescence imaging, HER3, ErbB3, IRDye800CW
 Global reach, higher impact
Global reach, higher impact