13.3
Impact Factor
Theranostics 2017; 7(13):3192-3206. doi:10.7150/thno.19698 This issue Cite
Research Paper
Platelet-Targeted Delivery of Peripheral Blood Mononuclear Cells to the Ischemic Heart Restores Cardiac Function after Ischemia-Reperfusion Injury
1. Atherothrombosis and Vascular Biology Laboratory, Baker Heart and Diabetes Institute, Melbourne, VIC 3004 Australia;
2. Department of Medicine, Monash University, Melbourne, VIC 3800 Australia;
3. Vascular Biotechnology Laboratory, Baker Heart and Diabetes Institute, Melbourne, VIC 3004 Australia;
4. Experimental Cardiology Laboratory, Baker Heart and Diabetes Institute, Melbourne, VIC 3004 Australia;
5. Mammalian Functional Genetics Laboratory, Division of Blood Cancers, Australian Centre for Blood Diseases, Monash University, Melbourne, VIC 3004 Australia;
6. Vascular Biology and Atherosclerosis Laboratory, Baker Heart and Diabetes Institute, Melbourne, VIC 3004 Australia.
#Present address: NanoBiotechnology Laboratory, Australian Centre for Blood Diseases, Monash University, Melbourne, VIC 3004 Australia
*Present address: Department of Cardiology and Medical Intensive Care, Augustinerinnen Hospital, Academic Teaching Hospital University of Cologne, 50678 Cologne, Germany
Abstract
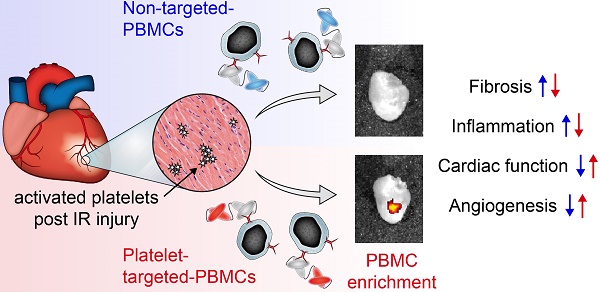
One of the major hurdles in intravenous regenerative cell therapy is the low homing efficiency to the area where these cells are needed. To increase cell homing toward areas of myocardial damage, we developed a bispecific tandem single-chain antibody (Tand-scFvSca-1+GPIIb/IIIa) that binds with high affinity to activated platelets via the activated glycoprotein (GP)IIb/IIIa receptor, and to a subset of peripheral blood mononuclear cells (PBMC) which express the stem cell antigen-1 (Sca-1) receptor.
Methods: The Tand-scFvSca-1+GPIIb/IIIa was engineered, characterized and tested in a mouse model of ischemia-reperfusion (IR) injury applying left coronary artery occlusion for 60 min. Fluorescence cell tracking, cell infiltration studies, echocardiographic and histological analyses were performed.
Results: Treatment of mice undergoing myocardial infarction with targeted-PBMCs led to successful cell delivery to the ischemic-reperfused myocardium, followed by a significant decrease in infiltration of inflammatory cells. Homing of targeted-PBMCs as shown by fluorescence cell tracking ultimately decreased fibrosis, increased capillary density, and restored cardiac function 4 weeks after ischemia-reperfusion injury.
Conclusion: Tand-scFvSca-1+GPIIb/IIIa is a promising candidate to enhance therapeutic cell delivery in order to promote myocardial regeneration and thereby preventing heart failure.
Keywords: cell delivery, single-chain antibody, targeting, myocardial infarction, PBMC, regenerative cell therapy, cell tracking.
 Global reach, higher impact
Global reach, higher impact