13.3
Impact Factor
Theranostics 2016; 6(3):291-301. doi:10.7150/thno.13728 This issue Cite
Research Paper
Quantitative Magnetic Particle Imaging Monitors the Transplantation, Biodistribution, and Clearance of Stem Cells In Vivo
1. Department of Bioengineering, University of California at Berkeley, Berkeley, CA 94720, USA
2. Institute of Medical Technology, Hamburg University of Technology, Hamburg, Germany
3. Department of Chemical and Biomolecular Engineering, University of California at Berkeley, Berkeley, CA 94720, USA
4. Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, CA 94720, USA
5. Magnetic Insight, Inc., Newark, CA 94560, USA
6. Department of Electrical Engineering and Computer Science, University of California at Berkeley, Berkeley, CA 94720, USA
Abstract
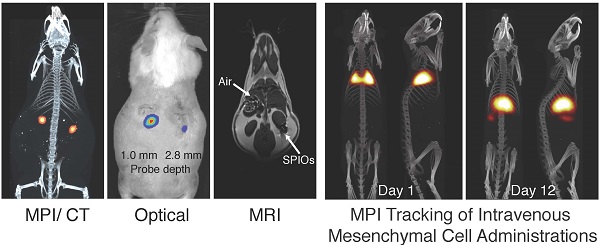
Stem cell therapies have enormous potential for treating many debilitating diseases, including heart failure, stroke and traumatic brain injury. For maximal efficacy, these therapies require targeted cell delivery to specific tissues followed by successful cell engraftment. However, targeted delivery remains an open challenge. As one example, it is common for intravenous deliveries of mesenchymal stem cells (MSCs) to become entrapped in lung microvasculature instead of the target tissue. Hence, a robust, quantitative imaging method would be essential for developing efficacious cell therapies. Here we show that Magnetic Particle Imaging (MPI), a novel technique that directly images iron-oxide nanoparticle-tagged cells, can longitudinally monitor and quantify MSC administration in vivo. MPI offers near-ideal image contrast, depth penetration, and robustness; these properties make MPI both ultra-sensitive and linearly quantitative. Here, we imaged, for the first time, the dynamic trafficking of intravenous MSC administrations using MPI. Our results indicate that labeled MSC injections are immediately entrapped in lung tissue and then clear to the liver within one day, whereas standard iron oxide particle (Resovist) injections are immediately taken up by liver and spleen. Longitudinal MPI-CT imaging also indicated a clearance half-life of MSC iron oxide labels in the liver at 4.6 days. Finally, our ex vivo MPI biodistribution measurements of iron in liver, spleen, heart, and lungs after injection showed excellent agreement (R2 = 0.943) with measurements from induction coupled plasma spectrometry. These results demonstrate that MPI offers strong utility for noninvasively imaging and quantifying the systemic distribution of cell therapies and other therapeutic agents.
Keywords: Magnetic particle imaging, mesenchymal stem cells, cell therapy tracking, quantitative imaging
 Global reach, higher impact
Global reach, higher impact