13.3
Impact Factor
Theranostics 2015; 5(8):847-862. doi:10.7150/thno.10854 This issue Cite
Research Paper
Photodynamic Quenched Cathepsin Activity Based Probes for Cancer Detection and Macrophage Targeted Therapy
1. The Institute of Drug Research, The School of Pharmacy, The Faculty of Medicine, Campus Ein Karem, The Hebrew University, Jerusalem, Israel.
2. Department of Plant Sciences, Weizmann Institute of Science, Rehovot, Israel,
3. Department of Biochemistry and Molecular Biology, J. Stefan Institute, Ljubljana, Slovenia,
4. Faculty of Chemistry and Chemical Technology, University of Ljubljana, Slovenia,
5. Centre of Excellence for Integrated Approaches in Chemistry and Biology of Proteins, Ljubljana, Slovenia.
Abstract
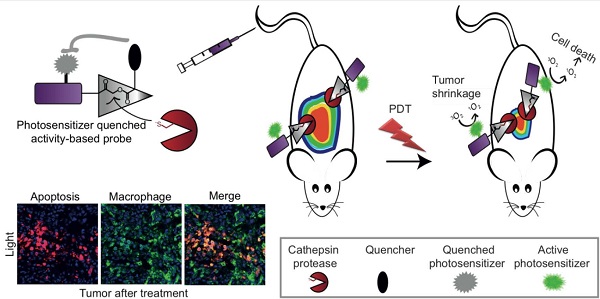
Elevated cathepsins levels and activities are found in several types of human cancer, making them valuable biomarkers for detection and targeting therapeutics. We designed small molecule quenched activity-based probes (qABPs) that fluoresce upon activity-dependent covalent modification, yielding cell killing by Photodynamic Therapy (PDT). These novel molecules are highly selective theranostic probes that enable both detection and treatment of cancer with minimal side effects. Our qABPs carry a photosensitizer (PS), which is activated by light, resulting in oxidative stress and subsequent cell ablation, and a quencher that when removed by active cathepsins allow the PS to fluoresce and demonstrate PD properties. Our most powerful and stable PS-qABP, YBN14, consists of a selective cathepsin recognition sequence, a QC-1 quencher and a new bacteriochlorin derivative as a PS. YBN14 allowed rapid and selective non-invasive in vivo imaging of subcutaneous tumors and induced specific tumor macrophage apoptosis by light treatment, resulting in a substantial tumor shrinkage in an aggressive breast cancer mouse model. These results demonstrate for the first time that the PS-qABPs technology offers a functional theranostic tool, which can be applied to numerous tumor types and other inflammation-associated diseases.
Keywords: Cathepsins, Photodynamic Therapy, Activity-Based Probes, Macrophages, Non-Invasive Imaging
 Global reach, higher impact
Global reach, higher impact