13.3
Impact Factor
Theranostics 2011; 1:3-17. doi:10.7150/thno/v01p0003 This volume Cite
Research Paper
Note: A reader recently brought concerns to the attention of the Editor of Theranostics related to two of the figures in this article. This matter is currently under investigation and this expression of concern will continue to be associated with the article until the investigation is concluded.
Tunable Plasmonic Nanoprobes for Theranostics of Prostate Cancer
1. Joint American-Belarusian Laboratory for Fundamental and Biomedical Nanophotonics, Rice University, 6100 Main Street, Houston, TX, 77005, USA;
2. Belarusian State University for Informatics and Radioelectronics, 6 Browka Street, Minsk, 220013, Belarus;
3. Department of Biochemistry & Cell Biology, Rice University, 6100 Main Street, Houston, TX, 77251-1892, USA;
4. Department of Physics & Astronomy, Rice University, 6100 Main Street, Houston, TX 77005, USA
Published 2011-1-10
Abstract
Theranostic applications require coupling of diagnosis and therapy, a high degree of specificity and adaptability to delivery methods compatible with clinical practice. The tunable physical and biological effects of selective targeting and activation of plasmonic nanobubbles (PNB) were studied in a heterogeneous biological microenvironment of prostate cancer and stromal cells. All cells were targeted with conjugates of gold nanoparticles (NPs) through an antibody-receptor-endocytosis-nanocluster mechanism that produced NP clusters. The simultaneous pulsed optical activation of intracellular NP clusters at several wavelengths resulted in higher optical contrast and therapeutic selectivity of PNBs compared with those of gold NPs alone. The developed mechanism was termed “rainbow plasmonic nanobubbles.” The cellular effect of rainbow PNBs was tuned in situ in target cells, thus supporting a theranostic algorithm of prostate cancer cell detection and follow-up guided destruction without damage to collateral cells. The specificity and tunability of PNBs is promising for theranostic applications and we discuss a fiber optic platform that will capitalize on these features to bring theranostic tools to the clinic.
Keywords: Plasmonic nanobubble, gold nanoparticle, laser, photothermal, prostate cancer, theranostics.
Introduction
The biomedical applications of plasmonic nanoparticles (NPs) use their optical scattering properties for imaging and diagnostics [1-4], and their photothermal properties for various types of therapies through the generation of heat [1,5,6], bubbles [6-12] and acoustic waves [13,14]. The specificity and sensitivity of imaging and diagnostics, as well as the selectivity and efficacy of the therapeutic methods, depend upon the selectivity of NP delivery and activation within their targets, typically diseased cells or tissues. The initial application of various NPs was based on passive targeting [15-17], which did not allow the NPs to recognize their specific molecular targets and resulted in the low selectivity of NP-based methods and also in potentially toxic loads of NPs. The situation was improved by using active molecular targeting with cell-specific molecules (peptides, antibodies, aptamers) attached to NPs and coupling to cognate receptors at the membranes of specific target cells [18-21]. This approach is currently used for the in vitro and in vivo application of NP-based imaging, diagnostics and therapies including drug delivery with gold NPs [22-26] and, ultimately, for theranostics, the method that unites diagnosis and therapy in one procedure [27-33].
However, NP conjugates still do not provide “absolute” targeting: certain amounts of NPs miss their targets and are taken up nonspecifically by neighboring cells and tissues. Consequently, the specificity and sensitivity of imaging and diagnostics, and the selectivity and efficacy of the therapeutic methods, are reduced. On the other hand, the major promise of NP-based medicine is associated with the unique properties of NPs and the nanoscale processes they support. To realize this promise, one needs targeting and activating mechanisms that provide selective delivery and activation of the NPs only in target cells without influencing other normal cells. Otherwise NP-based medicine (often referred to as nanomedicine) often defaults to its “macro” version by employing only the bulk "macro" effects of NPs, and thus losing their main advantage over macro- materials and methods.
To address these problems, we recently introduced the antibody-endocytosis based mechanism of gold NP targeting and activation [34-36]. This mechanism works through the selective formation of NP clusters (tightly aggregated groups of 5-50 NPs) in specifically targeted cells and the cluster size-dependent mechanism of optical activation of such NP clusters through the generation of plasmonic nanobubbles (PNBs) [35-38]. Endocytosis provides the selective formation of big NP clusters only in diagnosis-specific cells, while fewer NPs incidentally accumulated by non-specific cells are insufficient to form an NP cluster. PNBs are generated as transient vapor bubbles around intracellular NP clusters under their exposure to short laser pulses. Unlike other thermal, acoustical and optical phenomena related to plasmonic NPs, the generation of PNBs is a threshold process: a PNB does not emerge if the laser pulse energy is below a specific threshold. The PNB threshold energy significantly depends upon the NP cluster size [37-39], thus allowing the selective generation of PNBs only around big enough clusters (the lowest PNB threshold) that cannot be formed in non-specific cells. The dynamically tunable optical (i.e. diagnostic) and mechanical (i.e. therapeutic) properties of PNBs make them ideal candidates for cell-level imaging, diagnostics, therapy and, ultimately, theranostics [34,38,40].
However, a general heterogeneity of biological properties (including the expression of specific receptors and their ability to support endocytosis) often reduces the selectivity of NP targeting and the precision of the following diagnosis and therapy [21,41-43]. As a result, NP-based biomedical technologies often cannot provide cell-level diagnosis and therapy. The other significant limitation of various nanoprobes is the limited (or nonexistent) tunability of their function in a cell. We hypothesized that a further improvement in the selectivity and tunability of NP-based imaging, diagnosis, and therapy, and the unification of these steps into one theranostic procedure could be achieved with the simultaneous activation of the several different and co-locolized plasmon resonances in one NP cluster, and through the generation of tunable localized PNBs. Our current work is focused on the experimental evaluation of this hypothesis for gold NPs and associated PNBs. Below we report the experimental results for a new selective mechanism of PNB generation around clusters of gold NPs, and the evaluation of the developed mechanism at cell level for the theranostics of prostate cancer cells growing amidst normal stroma.
Methods
Generation of rainbow plasmonic nanobubbles
The generation of a vapor bubble around a plasmonic NP (Figure 1A) requires a specific threshold of the fluence of the laser pulse. When the threshold is exceeded, the optical energy is converted in the NP by the mechanism of plasmon resonance into a sufficient amount of thermal energy that is rapidly transferred to the NP nano-environment, evaporates this nano-environment, and provides the expansion of the vapor into the nanobubble. The clustering of plasmonic NPs was shown to reduce the PNB generation threshold [37,38].
The described mechanism employs one plasmon resonance as the source of heat. We expanded this approach into a principally new mechanism that employs the simultaneous effect of several different plasmon resonances in one NP cluster. This was realized through using two different types of plasmonic (gold) NPs, rods and spheres, and their clustering through collective endocytosis (Figure 1B). If this multi-NP cluster is irradiated with one laser pulse at sub-threshold fluence (below the PNB threshold for clusters of a similar size though built of only one type of NP) there will be no bubble (Figure 1C). However, if we irradiate this multi-NP cluster with two simultaneous pulses at two different wavelengths (matching the plasmon resonances of the NPs), their cumulative thermal effect will exceed the PNB threshold and will result in a PNB (Figure 1C).
Principle of optical generation and detection of (A) standard PNB generated with single laser pulse around mono-NP cluster and (B) rainbow PNB generated with several laser pulses (shown with green and purple arrows) and multi-NP cluster (yellow and purple NPs with different plasmon resonances), (C) thermal outputs of single NPs, mono-NP clusters and multi-NP clusters under identical optical excitation with two laser pulses (as in case (B)): only the synergistic effect of two simultaneous plasmon resonances in one NP cluster deliver the thermal energy sufficient for PNB generation. Red arrows in (A) and (B) show PNB detection through optical scattering of an additional probe laser beam.
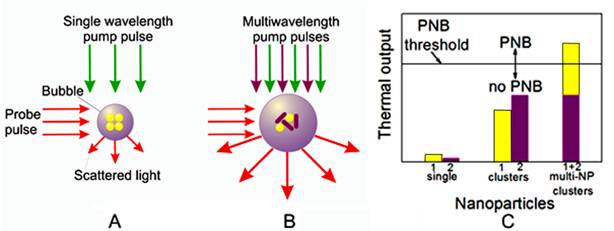
This new, “rainbow” mechanism is principally different from the standard one in the following ways:
- A single source of optical energy (a laser pulse at a single wavelength) is replaced by several sources (several pulses of different wavelengths), while the fluence of each pulse is reduced below the PNB generation threshold for each corresponding NP type. In such cases, the simultaneous exposure of the mono-NP cluster or the single pulse exposure of the mono- or multi-NP cluster will not generate a PNB, because the thermal output in both cases will be insufficient, and only the simultaneous excitation of the multi-NP cluster will cause the bubble.
- A reduced fluence of laser pulses results in a reduced initial temperature of individual corresponding NPs compared with the case of a single pulse excitation. The simultaneous heating of closely packed different NPs results in a rapid fusion (superposition) of their thermal fields into one joint thermal field surrounding the NP cluster. This thermal field acts as the energy source for the vapor bubble formation and expansion, and, compared with the single plasmon resonance case, the thermal load on the NPs is reduced, thus better protecting them from melting and enabling multiple activations.
- The independent control of the fluence of each laser pulse allows us to optimize the excitation of the multi-NP cluster for maximal selectivity of PNB generation, whereas excitation with a single pulse does not offer this opportunity.
From the biomedical point of view the above features can be considered as a reduction of the radiation load on a tissue and an improvement in PNB selectivity in a heterogeneous cell environment when different NPs are targeted with the different vectors (antibodies, peptides etc). Optical generation and detection of the PNBs was performed with a previously developed photothermal laser microscope [44] equipped with a dual pulsed laser (STH-01, Standa Ltd, Vilnius, Lithuania): each pulse 0.5 ns, the wavelength of 532 nm (matching plasmon resonances of gold spheres of 60 nm) and tunable near-infrared (matching plasmon resonances of gold nanorods and nanoshells that were employed). Three types of gold NPs were used to form the multi-NP clusters: gold spheres (50 and 60 nm), rods (25x75 nm) and shells (52 nm).
The optical detection of PNBs was realized in two parallel modes: time-resolved scattering imaging (Figure 2A, C, E) and time response (Figure 2B, D, F). Time-resolved scattering imaging was realized by using side illumination of the sample with a pulsed probe laser beam at a wavelength of 690 nm, different from the pump laser wavelengths (of 532 nm, 787 nm and 675 nm). Optical scattering was also used for the imaging of single NPs and their clusters. For objects whose dimensions are smaller than a wavelength, the size of their image does not represent their actual size. However, if the image is formed by the light scattered by the object, the brightness of the scattered light (measured as the pixel image amplitude) correlates to the size of the object. We used this well-known rule for the quantitative control of the relative size of the gold nanoparticle clusters. This provided a comparison of the clusters of similar size. The probe laser radiation scattered by NP or by PNB was imaged with a CCD camera (Luka, Andor Technologies, Ireland). While allowing us to “see” the PNB or NP, a time-resolved scattering imaging cannot provide a kinetic measurement. The latter was realized with a simultaneous time response mode [44]. An additional continuous probing beam (633 nm) was directed at the sample and focused on it collinearly with pump laser beams, and its axial intensity was monitored by a high-speed photodetector (PDA110AC, Thorlabs Inc.) and a 400-MHz Lecroy digital oscilloscope. The PNB-induced scattering of a part of the probe beam decreased its axial amplitude, resulting in a dip-shaped output signal [44]. This mode provided the monitoring of PNB growth and collapse, and delivered the PNB lifetime that characterizes its maximal diameter [44]. The PNBs were quantified by measuring their lifetime, bubble generation threshold fluence and the pixel amplitude of their scattering images. The PNB generation threshold fluence of the pump laser pulse describes both the efficacy of the photothermal conversion by NPs and the hydrodynamic conditions of PNB generation. The laser fluence that provides PNBs generation probability 0.5 was defined as pump laser pulse fluence.
Results and Discussion
Mechanism of rainbow plasmonic nanobubbles
Initially we studied the generation of PNBs around mono-NP and multi-NP clusters in water. Optical detection of the PNBs was realized in two parallel modes: time-resolved scattering imaging showed individual transient PNBs at the moment of their generation and delivered the information about the brightness of PNBs (Figure 2A, C, E), and time response mode employed a continuous probe laser (633 nm) to monitor the PNB growth and collapse and thus to obtain the PNB lifetime that characterizes the maximal diameter of the PNB [44] (Figure 2B, D, F). We studied several combinations of NPs and their optical excitation in order to compare the single wavelengths excitation of one type of NPs with the multi-wavelength excitation of the mixed types of NPs (Table 1). For each condition, we measured the lifetime of individual PNBs that were generated around individual NP clusters under a single pulse excitation.
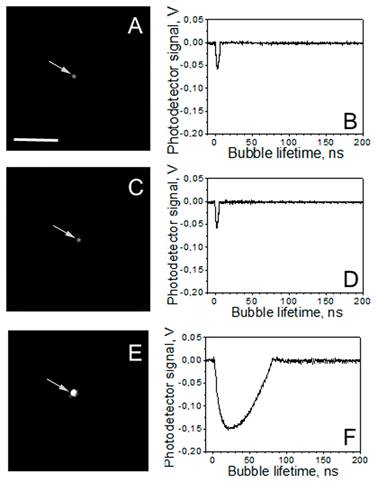
Under identical excitation conditions (532 nm: 6 mJ/cm2, 787 nm 14 mJ/cm2) the clusters of spheres (Table 1, Figure 2A, B) and rods (Table 1, Figure 2C, D) returned no or small PNBs, while the multi-NP clusters of the same size returned relatively large PNBs (Table 1, Figure 2E, F) that were associated with apparently over-threshold conditions. Both fluence levels were chosen to be significantly below the PNB generation thresholds for single NPs (46 mJ/cm2@532 nm for NSP and 32 mJ/cm2@787 nm for NRs) and slightly below the PNB generation thresholds for the mono-NP clusters of NSPs (16 mJ/cm2@532 nm) and NRs (20 mJ/cm2@787 nm), in order to compare the standard and rainbow mechanisms of PNB generation.
A,C,E: time-resolved scattering optical images show individual transient PNBs generated around single NP clusters during exposure to the two simultaneous laser pulses (0.5 ns each, 532 nm and 787 nm): A - the mono-NP cluster of gold spheres, C - the mono-NP cluster of gold rods, E - the multi-NP cluster of the same diameter as in A and C but consisting of a mixture of gold spheres and rods; the pixel image amplitude is shown in the gray scale. B, D, F: corresponding time responses obtained simultaneously with the images A,C,E and characterize maximal diameter of the PNB through the duration of the PNB-specific signal. Scale bar is 10μm.
Similar results were obtained for the clusters of two different hollow gold nanoshells with the absorbance peaks at 575 nm and 690 nm (Table 1). We applied 532 nm@10 mJ/cm2 pulses and 675 nm@10 mJ/cm2 pulses separately and simultaneously. Single pulse excitation of the mono-NS clusters returned no or small PNBs. Maximal lifetime and probability of PNBs were obtained under dual excitation of the mixed NS clusters that included both types of NS (Table 1). This lifetime, 133 ns, was 2-3 times longer (indicating a two to three fold bigger diameter of the PNB) compared with the dual excitation of the NS clusters of a similar size but consisting of one type of NS.
PNB lifetime (ns) (measure of its maximal diameter) obtained for individual gold NP clusters of similar size for the different combinations of NP types and the wavelengths of optical excitation (@optical fluence, mJ/cm2).
| NP cluster composition | Spheres (532 nm) | Rods (750 nm) | Spheres +rods | Shells (560 nm) | Shells (690 nm) | Shells + shells |
|---|---|---|---|---|---|---|
| Optical absorbance peak, nm | 532 | 800 | 560 | 690 | ||
| Laser pulse wavelength, nm | ||||||
| 532 @10 mJ/cm2 | 0 | 0 | 0 | 0 | 0 | 46±7 |
| 675@10 mJ/cm2 | 0 | - | - | 14±3 | 26±4 | 40±5 |
| 787@14mJ/cm2) | 0 | 0 | 15±2 | - | - | - |
| 532(6 mJ/cm2)+787(14 mJ/cm2) | 10±3 | 14±3 | 63±4 | - | - | - |
| 532+675@10 mJ/cm2 for both pulses | 58±5 | - | - | 47±3 | 72±5 | 133±13 |
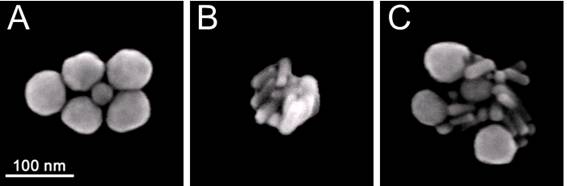
The size of NP clusters was quantified and compared through the amplitudes of their optical scattering images (obtained for individual NP clusters). We studied the clusters that yielded similar scattering image amplitudes for all three cluster types. In addition, the composition and dimensions of the clusters were verified with scanning electron microscopy (SEM, Figure 3). We used 60 nm spheres (NSP), 25x75 nm nanorods (NR) and multi-NP (NSP-NR) clusters consisting of spheres and rods (Figure 3). According to their SEM images, the size of these clusters was 130-180 nm. Figure 3C clearly shows the multi-NP nature of the clusters that delivered the images and signals shown in Figure 2E, F.
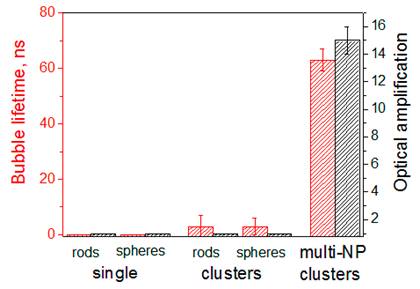
The therapeutic effect of PNBs is associated with their mechanical impact [34,37,40,45] that is characterized by the maximal diameter of the expanding PNB and is measured through the lifetime of the PNB (Figure 4). The diagnostic effect of a PNB is associated with its brightness that is characterized through the pixel image amplitudes (Figure 2A,C,E), normalized by the pixel image amplitudes of the corresponding NP clusters as measured prior to their optical excitation and calculated as an amplification of optical scattering by the PNB relative to that by the NP cluster (Figure 4). The results obtained (Table 1, Figures 2, 4) show the definite priority of the novel “rainbow” mechanism over the standard excitation of a single plasmon resonance: the lifetime and brightness of rainbow PNBs increased by almost one order of magnitude compared with the identical excitation of the mono-NP clusters.
SEM images of the clusters prepared from: (A) gold solid spheres (NSP) 60 nm, (B) gold rods (NR) 25x75 nm, (C) gold NRs and NSPs (multi-NP cluster).
Lifetime and brightness (relative to that of the corresponding NPs) of individual PNBs generated in water with two simultaneous laser pulses (0.5 ns, 532 nm@6 mJ/cm2 and 787 nm@14 mJ/cm2): around single NPs, spheres (plasmon peak close to 532 nm) and nanorods (plasmon peak close to 787 nm); around mono-NP clusters consisting of spheres (NSP) or rods (NR) and around multi-NP clusters that included both NSP and NR (rainbow mechanism).
To compare the efficacy of the rainbow and standard mechanisms, we determined the fluence of the single laser pulse that provided the same lifetime of the PNB around a mono-NP cluster as was achieved for the rainbow PNB. The PNB lifetimes close to those of the rainbow PNBs required the following fluences of the single 532 nm laser pulse:
- for NSP cluster - 3-4 fold (48 mJ/cm2 vs. 14 mJ/cm2 in rainbow mode);
- for NS cluster - 2-3 fold (27 mJ/cm2 vs. 10 mJ/cm2 in rainbow mode).
Therefore, the simultaneous dual pulse excitation of the mixed NP cluster pulses resulted in a significant increase in the PNB generation probability and lifetime for all combinations of gold NPs tested. Thus the rainbow PNB mechanism provides an opportunity for improving the selectivity and efficacy of the generation of rainbow PNBs in biomedical applications. Another interesting opportunity is related to the tunability of PNB size (which is the measure of its therapeutic and diagnostic effect). According to Table 1 and Figure 4 the single pulse excitation delivered PNBs with a lifetime below 100 ns, the threshold that was earlier found by us to separate non-invasive PNBs from ablative ones [34,40]. However, the dual pulse excitation mode returned PNBs with a lifetime above 100 ns, that can be considered as ablative for cells. Therefore, varying the number of the pump laser pulses we can support and switch between the non-invasive diagnostic and ablative therapeutic modes. This option was studied in detail below.
Rainbow plasmonic nanobubbles in living cells
The internalization and clustering of NPs in living cells (unlike the water model studied above), involves several biological processes that not only form such clusters, but also create significant heterogeneity in their size. The latter factor determines the specificity of NP- and PNB-based diagnosis and the efficacy and selectivity of the NP- and PNB-based therapy. We studied NP clustering and PNB generation in a co-culture of living bone metastatic prostate cancer (C4-2B) and human bone marrow stromal (HS-5) cells in vitro under the following conditions:
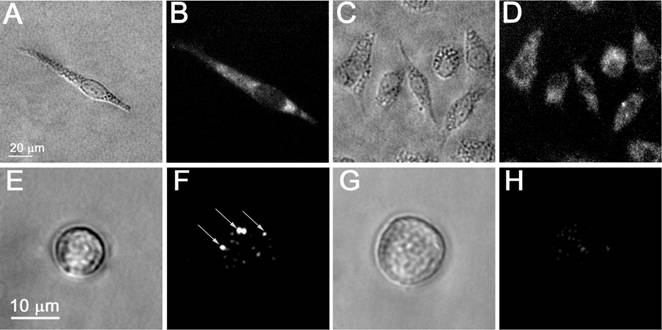
- The initial targeting of the cells with gold NPs was supported by using a combination of the two different antibodies that were conjugated to specific different gold NPs. We applied prostate cancer-specific gold conjugates of NSP with PSMA (prostate specific membrane antigen) and gold NS conjugated with C225 (Erbitux, the antibody raised against human EGF receptor) that is tumor-associated, although it is less specific to prostate cancer cells than PSMA. The rods were replaced by other NPs with NIR plasmon resonances, by nanoshells (NS), because the latter turned out to be less toxic and easier to conjugate. The specificity and efficacy of PSMA-based targeting was independently verified with fluorescent confocal microscopy (LSM-710, Zeiss) of C4-2B and HS-5 cells that were identically treated with the conjugate of fluorescent dye with PSMA, AlexaFluor488-PSMA (Figure 5A,D). This step did not provide absolute specificity of the targeting and, therefore, we applied the next targeting step.
- The clustering of the membrane-accumulated NPs was provided by their endocytosis and subsequent aggregation in endosomal compartments in the cytoplasm. This process was monitored in C4-2B and HS-5 cells that were incubated with gold NPs by using the reflecting mode of the confocal microscope with a 633 nm laser (Figure 5B,E). The size of NP clusters was analyzed through the image pixel amplitude of the scattering images of the NP clusters in cells. According to this metrics, the C4-2B cells formed much bigger clusters of NPs than those in HS-5 cells (Figure 5B,C,E,F).
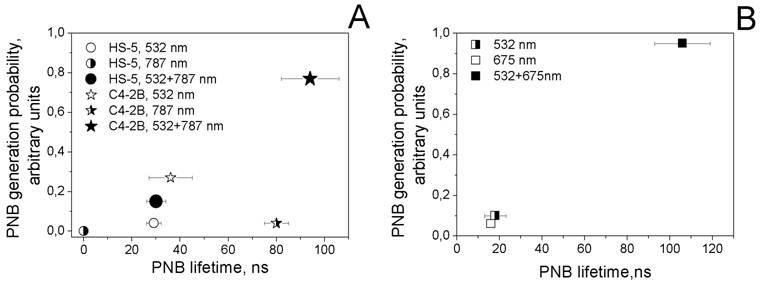
Next we studied the process of the generation of PNBs in individual cells. Living cells were individually exposed to single laser pulses of a specific wavelength and fluence and to pairs of laser pulses. PNBs were detected and quantified through their responses and time-resolved scattering images. Thus we obtained the probability of PNB generation, lifetime and scattering image amplitude. Firstly, each cell was exposed to laser pulses at 532 nm@16 mJ/cm2, 787 nm@19 mJ/cm2 and to the simultaneous pair of these pulses. The PNB lifetimes and the probabilities of their generation were plotted as shown in the diagram (Figure 6A).
The rainbow mechanism (which mixed targeting the cells with NS-C225 and NSP-PSMA and exposing them to the two simultaneous laser pulses, 532 nm and 787 nm) resulted in an increase in PNB size (lifetime) of almost one order of magnitude compared with the response of the same cells to mono-pulse treatments (Table 2). This result confirmed our previous findings (obtained for the water model), that the efficacy of rainbow PNB generation was much higher than for standard PNBs. In addition to the improved efficacy (increased lifetime of the rainbow PNB), we observed the better selectivity (C4-2B versus HS-5 cells) of the rainbow PNBs compared with the mono-pulse generated PNBs in the same cells (Figure 6A). Next, we increased the fluence of 532 nm to the level that provided the lifetime of a PNB close to that achieved in the rainbow mode (95 ns). This required increasing the fluence of the 532 nm laser pulse to 86 mJ/cm2. This fluence level exceeded the summarized fluence of the two laser pulses (16+19=35 mJ/cm2) by 2.5 times. Such a difference in the fluences indicates that the simultaneous activation of several plasmon resonances in multi-NP clusters resulted in the synergistic effect of the co-localized excitation of several plasmon resonances in spheres (532 nm) and in shells (787 nm).
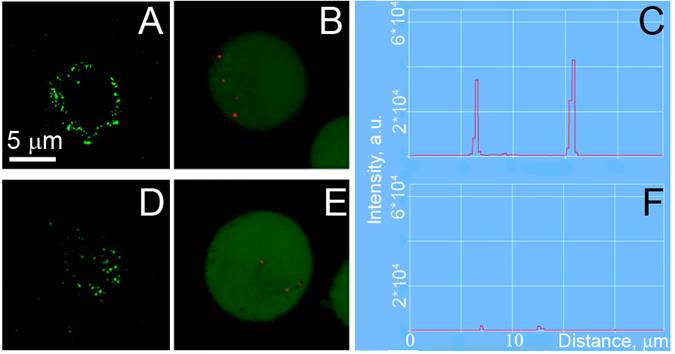
Confocal fluorescent, confocal scattering images of prostate cancer C4-2B (A,B) and stromal HS-5 (D,E) cells: (A,D) - confocal fluorescent images of AlexaFluor488 conjugated to PSMA antibody, (B,E) - confocal scattering images of gold NPs clusters (shown in red on the green fluorescent background that shows cell tracker dye) and (C,F) the corresponding profiles of scattering signal amplitudes by gold NPs clusters in cells.
PNB lifetime-probability of generation diagrams obtained for the cell samples. A - prostate cancer (C4-2B) and stromal (HS-5) cells that were treated with the mixture of gold NP conjugates (NSP60-PSMA and NS110-C225) and were exposed to single and paired laser pulses (532 nm@16 mJ/cm2 and 787 nm@19 mJ/cm2): for C4-2B cells: hollow star - single pulse 532 nm, half right star - single pulse 787 nm, solid star - pair of laser pulses at 532 nm and 787 nm (rainbow mechanism); for HS-5: hollow circle - single pulse 532 nm, half right circle - single pulse 787 nm, solid circle - pair of laser pulses at 532 nm and 787 nm (rainbow mechanism). B - prostate cancer cells (C4-2B) that were treated with hollow gold shells 52 nm and were exposed to single and paired laser pulses (532 nm@10 mJ/cm2 and 675 nm@8 mJ/cm2): half right square - single pulse 532 nm, hollow square - single 675 nm pulse, solid square - pair of laser pulses at 532 nm and 675 nm.
C4-2B cell population averaged PNB lifetimes (ns) under various NP targeting and laser excitation conditions (wavelength, nm /fluence, mJ/cm2).
| NPs (antibody/diameter/plasmon resonance peak) | NS (C225/52nm/690 nm) | NSP+NS (C225/110nm/800) |
|---|---|---|
| 532 nm, 10 mJ/cm2 | 1.8±2 | - |
| 675 nm, 8 mJ/cm2 | 1.0±1 | - |
| 532 nm (10 mJ/cm2)+675 nm (8 mJ/cm2) | 105.0±13 | - |
| 532 nm, 16 mJ/cm2 | - | 12.0±3 |
| 787 nm, 19 mJ/cm2 | - | 3.2±1 |
| 532 nm (16 mJ/cm2)+787 nm (19 mJ/cm2) | - | 72.0±4 |
The advantage of gold NSs over gold NSPs of the same diameter for the generation of PNBs was discovered and discussed earlier in terms of the physical mechanism of PNB generation [38]. Based on the above, we additionally studied different excitation modes for PNB generation in C4-2B cells treated with the NS52-C225 conjugates (plasmon peak around 690 nm) and applied a single pulse (532 nm) and dual pulse (532 nm and 675 nm) optical excitation under identical fluences. In this case we intentionally applied the fluences of each pulse at the level below the PNB generation threshold for a single-pulse mode, 532 nm, 10 mJ/cm2 and 675 nm, 8 mJ/cm2. Almost no PNBs were generated in cells in single-pulse mode (Figure 6B, Table 2). However, the dual-pulse mode returned PNBs with a lifetime above 100 ns. This was achieved without increasing the fluence of laser pulses (that was, in this case, within the FDA-approved safe limit, 20 mJ/cm2 [46]) and through the rainbow mechanism of PNB generation. To achieve a similar PNB lifetime in single pulse mode in the same cells, we had to increase the fluence of the 532 nm pulse to 27 mJ/cm2, a level higher than the summarized fluence of 532 nm and 675 nm pulses (18 mJ/cm2) and also higher than the FDA-approved safety limit. The obtained lifetimes of PNBs (Table 2, Figure 6B) indicated a significant difference between the single pulse and dual pulse (rainbow) excitation modes. Again, like in the previous experiment, the lifetime of the rainbow PNBs was one to two orders of magnitude higher than the lifetimes of PNBs under a single pulse excitation. The latter were mainly not generated at all under the reduced level of laser fluences.
These experiments clearly demonstrate that the rainbow mechanism of PNB generation (i.e. dual-pulse optical excitation of PNBs) improves the selectivity of PNB generation and also reduces the laser fluence required to generate PNBs to the minimal level. Our next step was to study in detail the potential of rainbow PNBs for diagnostic and therapeutic applications for the same cell model of prostate cancer cells growing amongst normal stromal cells.
Diagnostic properties of plasmonic nanobubbles
The diagnostic specificity and sensitivity of PNBs were evaluated by comparing the brightness and optical contrast of rainbow PNBs with those of gold NPs (scattering) and AlexaFluor488 label (PSMA-specific fluorescence) (Figure 7). We applied the reduced fluence (532 nm@10 mJ/cm2 and 787 nm@12 mJ/cm2) in order to generate relatively small, non-invasive PNBs with a lifetime below 100 ns (that had previously been found to be the threshold for transition from non-invasive to ablative bubbles [34,40]). The viability of each individual cell was monitored by measuring the level of fluorescence of specific vital dyes that leak out from the damaged cell (PNB-induced cell damage was shown to have a disruptive mechanical nature [40,45,47]).
We fluorescently labeled stromal (HS-5) and prostate cancer cells (C4-2B) with calcein vital dyes of red (cancer) and green (stromal) colors and mixed them in the proportion HS-5:C4-2B of 5:1 (Figure 8A). The brightness of the PNBs in cancer C4-2B cells (measured as the pixel image amplitude of the PNB, Figure 7F) was found to be 71 times higher than that for stromal cells (Figure 7H). Such an optical contrast exceeded the optical contrast of the fluorescent labels (that were targeted to C4-2B and HS-5 cells using the same prostate cancer-specific PSMA antibody, see Figure 7B, D) by 31 times. The optical contrast (measured as a ratio of the fluorescent image amplitudes for C4-2B to HS-5) of fluorescent imaging for cancer versus stromal cells was 2.3 (Figure 7B, D). Such a high contrast of PNB imaging was achieved due to their threshold mechanism: no PNBs were generated in HS-5 cells (Figure 7H), and their images were formed by the optical scattering by gold NPs that did not produce PNBs.
The above results were achieved with a single exposure of each cell to the pair of pump laser pulses. As can be seen from Figure 8B, the PNBs did not cause detectible changes in the level of Calcein AM fluorescence. Therefore, this diagnostic stage of PNB treatment can be considered as non-invasive for all cells being treated - target prostate cancer C4-2B cells and non-specific stromal HS-5 cells.
Microscopy images of living cells: bright field (A,C,E,G), time-resolved optical scattering (F,H) and fluorescent (Alexa Fluor 488 conjugated to PSMA antibody, B,D) images of prostate cancer C4-2B (A,B,E,F) and stromal HS-5 (C,D,G,H) cells; (F) and (H) - time-resolved scattering images of the cells presented in (E) and (G), respectively, that were exposed to the pair of laser pulses at 532 nm and 787 nm: image (F) shows the rainbow PNBs in a C4-2B cell while the image (H) shows no PNB and only the scattering by residual gold NPs in HS-5 cell.
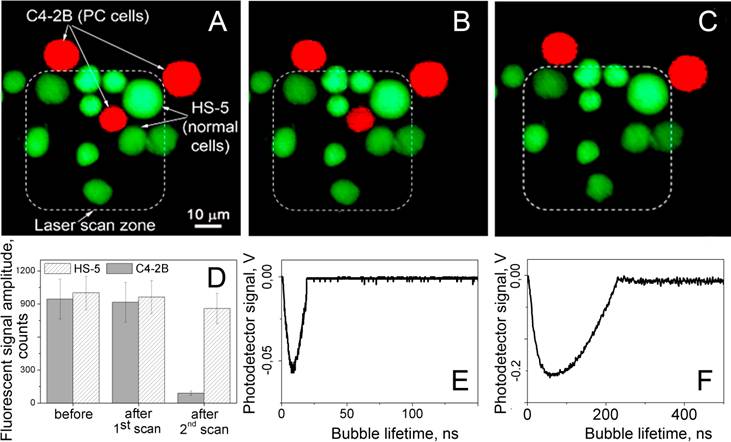
Fluorescent images of the mixture of prostate cancer C4-2B (red) and stromal HS-5 (green) cells: (A) before, (B) after the first scan and (C) 60 s after the second scan, exposure to the pair of laser pulses (532 nm and 787 nm) that selectively generated cell damaging PNBs causing fading of red fluorescence due to leaking of red calcein out through the disrupted membrane. Dashed line shows the area that was exposed to the laser pulses. D - cell population averaged fluorescent signal amplitudes for C4-2B and HS-5 cells before and after treatment with laser pulses. E and F - the time responses of PNBs in C4-2B cell obtained during the first and the second scans.
Therapeutic properties of plasmonic nanobubbles
The next, therapeutic, stage of PNB treatment was realized by increasing the energy of the pump laser pulses to the level that provided the PNB lifetime well above cell damage level (i.e. longer than 100 ns). We evaluated the rainbow PNBs for the selective ablation of cancer cells surrounded by non-cancer stromal cells (a typical cancer microenvironment described above for the diagnostic stage). Next, we treated the mixture of cells with the two gold conjugates and with two laser pulses, 532 nm@16 mJ/cm2 and 787 nm@19 mJ/cm2. The sample was scanned through the laser beam and each cell was identified according to its fluorescence (Figure 8B), and was then exposed to a single pair of laser pulses (Figure 8C). Based upon the PNB generation probabilities and lifetimes (Figure 6), the stable PNBs were generated under the rainbow mechanism and in cancer cells only. PNBs were also observed on occasion in stromal cells, but their probability was below 20%, and their size was small compared to the size of cancer-cell generated PNBs. This latter result inspired an additional test: because cell damage by a PNB depends upon its size (lifetime [34,40,44]), we monitored and compared the vital fluorescence of PNB treated cancerous and stromal cells.
Within the area irradiated with laser pulses (shown within a dashed line in Figure 8B,C) we discovered that only the cancer cell was damaged. The vital dye stays in the cell with an intact membrane and quickly leaks out of the cell with a compromised membrane [48]. The damage (ablation) of the cancer cell in the center (observed as the loss of the red fluorescence) was in line with the increased size (lifetime) of the PNBs generated specifically in C4-2B cells, and with the mechanical nature of cell damage that is associated with the disruption of the cellular structures including the plasma membrane [34,40,44,45,49,50]. This final test clearly demonstrates the selectivity of the rainbow PNBs that were generated locally in a single specific (cancer) cell and were not generated in the surrounding stromal cells (or were generated in such cells with a probability and size that prevented their damage). While the vital fluorescent images in Figure 8B,C demonstrate the potential of rainbow PNBs for selective therapy, the optical scattering of these PNBs has a high diagnostic potential.
The destruction of the cells was a result of a "single pulse" procedure (the pair of simultaneous pulses) and was associated with the mechanical, non-thermal disruption of cellular membrane and other components. A discussion of the cell damage mechanism due to PNBs can be found in our previous work [34,40]. Here we would like to point out the difference in the optical parameters of ablative (destructive) and non-invasive (diagnostic) PNBs: their lifetimes and optical brightness (measured as the image pixel amplitude) differ significantly (Figure 4,7). Long lifetimes of PNBs correlate with the damage to prostate cancer cells. Therefore, this optical parameter of PNBs can be used as a real-time guidance of the PNB effect on a specific cell, in particular, to confirm the cell damage that has occurred.
Rainbow plasmonic nanobubbles as theranostic probes
The described in vitro experiments with the co-culture of prostate cancer and stromal cells demonstrate three functions of PNBs: diagnosis, ablative therapy and real-time guidance. Providing that each stage is supported by a single exposure to a short pulse laser radiation (in a sub-nanosecond range) and the typical time of the PNB signals is in a sub-microsecond range, there is a direct possibility to unite these three vital steps of diagnosis, therapy and therapy confirmation (guidance) into one connected theranostic procedure. Next, the developed rainbow mechanism has demonstrated a single cell specificity and selectivity, which was not previously achieved with other NP-based methods. Plasmonic NPs alone were widely employed for imaging through optical scattering [1-4], which demonstrated good in vitro results. The problems of NP scattering imaging begin when the background scattering of non-specific cells and tissues dominates the NP signals, a common in vivo case. An increase in the sensitivity of scattering imaging can be achieved through generating PNBs around NPs, as we demonstrate above, because the scattering of such vapor nanobubbles is brighter than that of gold NPs [37,38,47,51]. In addition to higher brightness, the threshold mechanism of PNB generation (unlike the non-threshold mechanism of optical scattering and fluorescence) further improves the optical contrast of gold NPs. The rainbow PNB method further develops the unique properties of PNBs though the multi-resonance mechanism. There are several mechanisms for the optical activation of plasmonic NPs. Excitation, the most common, employs continuous radiation [52-54], which is a relatively simple and low cost solution. However, the continuous delivery of optical energy to an NP compromises the efficacy and spatial selectivity of the photothermal output of such an NP due to thermal diffusion [55]. This problem was solved by using short laser pulses [11,12,56-58]. Very short (fs) single pulses improve the photothermal efficacy by minimizing heat loss by NPs, though at the same time they limit the efficacy of PT conversion, since maximal energy is limited by the optical breakdown threshold. For this reason, many fs-pulsed lasers are used in continuous mode [26,59]. Other than as thermal sources, plasmonic NPs are employed as sources for acoustic, shock waves and vapor bubbles. Among these mechanisms, the gold nanoparticle-generated vapor bubbles (PNBs) were shown to provide the best selectivity and accuracy for cell-level biomedical applications.
Using the data for NSP clusters, we estimated fluences of a single 532 nm pulse required to generate the "standard" PNBs with a lifetime close to those of the rainbow PNBs as in the experiments reported above. Such a “projected fluence” of a single 532 nm pulse was found to be higher that the sum of the fluences of the two pulses (532 nm and 787 nm) employed for the excitation of the rainbow PNB. This difference increased with the fluence and PNB lifetime from 1.5 to 4. This means that the mechanism of the excitation of rainbow PNBs has a synergetic nature. Compared to a “standard” mechanism of nanobubble generation around a gold NP in a cell (antibody-receptor targeting, activation of mono-NP cluster with a single optical wavelength), the rainbow PNB mechanism introduces several specific features:
- Nanobubble source: clusters of different NPs, rather than clusters of NPs of one type. This feature decreases the bubble generation threshold fluence below the threshold level for mono-NP clusters.
- Excitation optical wavelength and fluence: several simultaneous pulses at the wavelengths that match the plasmon resonances of the corresponding NPs in the cluster. This feature also reduces the fluence of each laser pulse below the threshold for a nanobubble generated through a “single pulse - mono-NP” mechanism.
- Independent control and tuning of the fluence: each excitation laser pulse provides maximal flexibility in manipulating the lifetime (i.e. size) of the rainbow PNB in a wide range of cluster size and composition, thus circumventing the problem of the heterogeneous formation and content of NP clusters in cells.
All three above features are unique to rainbow PNBs and can be especially useful during in vivo applications where the heterogeneity of NP clustering and optical propagation and scattering create challenges both for nano- and for optical technologies. It should be noted that a rainbow PNB depends much less upon the variations of its NP content (this could be caused by the heterogeneous interaction and internalization of gold NPs by the cell). This result, together with the tunable and multifunctional properties of PNBs in vitro and in vivo [34,40,50] that support diagnosis, therapy and theranostics (diagnosis and treatment united in one procedure) at the cellular level and with single cell selectivity, justify the development of PNB theranostic technologies despite the general limitations mentioned above.
PNB theranostics in clinic
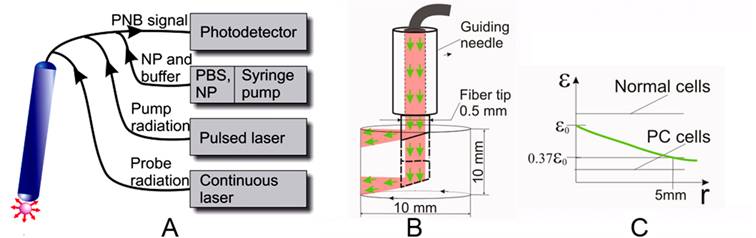
Rainbow nanobubbles offer the ability to target specific pathology at cell level based on their theranostic functions. This includes their ability to generate bright optical probes at low fluence indicating they will provide an excellent imaging and guiding tool in addition to their therapeutic applications. The application of PNB theranostics for focal prostate cancer treatment requires that the technology that can support PNB generation and detection in vitro be extended to in vivo conditions. The goal is to achieve high cell level selectivity of tumor cell detection and ablation, with minimal harm to nearby vital tissues such as urethra and nerve. Fiber optical technology can provide local delivery and guidance of PNB based treatment while minimizing the negative effects of optical scattering of the tissue. We envision a clinical solution for PNB theranostics of cancers and other pathologies through a fiber optical system (Figure 9). This system (Figure 9A) will support the essential features of PNBs and rainbow bubbles specifically including simultaneous delivery of several pump pulses of specific dynamically variable fluence and wavelength, continuous probe laser radiation and delivery of the suspension with NPs and, if necessary, of optically transparent buffer fluid. The radius of propagation of near-infrared laser radiation in soft and tumor tissue (determined as a length that attenuates the laser fluence by 63% relative to the fluence level near the fiber tip) was reported to be in the range of 5-8 mm [60-67]. This will be realized with a system based on a rotating and sliding fiber probe that is guided though the needle and can be shifted in and out for approximately 10 mm. By rotating and sliding the fiber tip (Figure 9B) with side illumination (similar solutions were already realized for laser surgery [68-71]) we will generate and detect the PNBs in a cylindrical volume with a diameter of 10 mm and a height of 10 mm (Figure 9B). The diameter of the tip in clinical design can be minimized to 0.5-1.0 mm in order to allow its minimally invasive penetration (up to 10 mm) into the tissue. This fiber tip will be used for several functions:
- PNB generation: several pulses of different wavelengths (preferably in NIR region, shown in green in Figure 9) will be delivered through the side of the rotating fiber. The effective radius of laser pulse propagation also will be determined by the PNB generation thresholds for normal and prostate cancer cells (Figure 9B). By increasing the difference between these two thresholds, we will increase the effective radius of PNB treatment by allowing a high (though still safe) level of laser pulse fluence at the fiber tip exit (Figure 9B). According to ANSI standard, the safety threshold for NIR radiation in tissues is 25-40 mJ/cm2 [46]. The PNB generation threshold was found to be lower (less than 10 mJ/cm2 for the clusters of gold nanoshells in cells). The speed of the PNB treatment can be estimated through pulse energy and frequency: the maximal surface to be scanned with the tip shown in Figure 9B can be estimated by 3 cm2. A single pulse may irradiate the area of 10-3 cm2 (required energy will be about 100 μJ per pulse). Commercial lasers deliver a pulse frequency up to 104 Hz, which results in a treatment time of less than 1 second per 1 cm3 (or per 3 cm2). This time includes the PNB generation and guidance and characterizes the complete PNB theranostic procedure for a single fiber probe. A bigger volume can be treated with several fiber probes simultaneously applied to the different locations (similar to the strategy used in brachytherapy [72]). The PNB treatment times are much shorter than those reported for brachytherapy and prostatectomy (tens minutes).
- PNB guidance: a continuous low power laser beam will be directed through the fiber coaxially with the pump pulses (Figure 9). The radiation scattered by PNB will be collected by peripheral optical fibers and detected by a high-sensitive photodetector. Relatively high scattering by the tissue will provide a relatively permanent background signal, while the PNB will deliver a transient signal of a specific shape that can be recognized in its time and frequency domains that are very different from those of the background. The duration of the detected signal will be used to monitor the size of the PNB, and, hence, will allow the guided destruction of the cells. This procedure will provide a real-time detection of PNBs, an estimation of their size and a dynamic adjustment of the fluences of the pump laser pulses while scanning the sample in order to adjust the diameter of PNBs to specific tissues or tumors.
- NP delivery: the same needle-fiber system can be used for advanced injection of gold NP conjugates.
The described system will use the following algorithms: (1) single pulse guided treatment: the pulse fluence will be automatically maintained to provide the PNB lifetime (size) above the cell ablation threshold. Each detected PNB-specific signal of such duration will be counted as a confirmation of PC cell destruction; (2) theranostic dual-pulse treatment: the first pulse will be close to the PNB generation threshold to generate small non-invasive PNBs; upon detecting such signals the system will increase the energy to the ablative level and will generate the second pulse in the same direction to expose the same area or volume and to detect ablative PNBs. In the case of the absence of PNB-specific signals, the system will continue the scan at the minimal fluence level until it detects the next target.
Fiber optic PNB system for treatment of mulitfocal cancers such as prostate. A: general scheme that shows fiber optical probe connected through the fiber bundle to photodetector, through the multimode fiber to the pump and probe lasers, and through the capillaries to the syringe pump with the NPs and optical buffer fluid. B: scheme of the operation of fiber optic probe in tissue, volume scan is provided by sliding and rotating the probe in the guiding needle. C: the green line shows the attenuation of optical fluence in scattering tissue relative to the PNB thresholds for normal and prostate cancer (PC) cells, the difference between these thresholds determines an effective radius of PNB-based selective ablation of prostate cancer cells.
Conclusions
We conclude that the suggested method of cell theranostics with “rainbow plasmonic nanobubbles” demonstrates the following advantages over other methods of photothermal excitation of plasmonic NPs and PNBs:
- The synergistic excitation of several plasmon resonances in the clusters of gold (plasmonic) NPs significantly improves the efficacy of the generation of plasmonic nanobubbles and is referred to as “rainbow plasmonic nanobubbles”.
- Rainbow plasmonic nanobubbles can be generated under reduced fluences of the excitation laser pulses that are below the PNB generation threshold for single NPs and for clusters consisting of only one type of NPs.
- The mechanism of rainbow plasmonic nanobubbles significantly improves the selectivity of bubble generation in specific target cancer cells compared to non-specific stromal (non-cancer) cells and can therefore provide the cell level selectivity needed for diagnosis and therapy.
- Rainbow plasmonic nanobubbles are less sensitive to the NP cluster content than the plasmonic nanobubbles generated around mono-NP clusters whose content can vary in cells due to the heterogeneous nature of NP uptake by living cells.
- PNBs demonstrate the tunability of their function in individual cells, thus performing the diagnosis and guided treatment that can be united into a single connected theranostic procedure.
As a result rainbow plasmonic nanobubbles can be considered as a novel platform for developing cell level diagnostic, therapeutic and theranostic solutions for treating various pathologies such as prostate cancer.
Acknowledgements
This work was supported by the National Institute of Health, grants 1R21CA133641 and R01GM094816 and Hamill Innovation Award to D.O.L., P01CA098912 to M.C.F.C. Authors thank Professor S. Link (Rice University) for providing gold nanorods, Professor R.A. Drezek for providing silica-gold shells and the members of the Carson laboratory, especially Drs. Brian Danysh and Pam Constantinou, for many helpful discussions. Ms. S. Parminter has kindly copy-edited the manuscript. Confocal microscopy was performed on equipment obtained through a Shared Instrumentation Grant from the National Institutes of Health (S10RR026399‑01).
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Hirsch LR, Gobin AM, Lowery AR. et al. Metal nanoshells. Ann Biomed Eng. 2006;34:15-22
2. Murphy CJ, Gole AM, Stone JW. et al. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res. 2008;41:1721-30
3. Javier D, Nitin N, Roblyer D, Richards-Kortum R. Metal-based nanorods as molecule-specific contrast agents for reflectance imaging in 3D tissues. J Nanophotonics. 2008;2:023506
4. Hu M, Chen J, Li Z. et al. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev. 2006;35:1084-94
5. Cherukuri P, Glazer ES, Curley SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev. 2010;62:339-45
6. Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23:217-28
7. Farny H, Wu T, Holt R. et al. Nucleating cavitation from laser-illuminated nano-particles. Acoust Res Lett Online. 2005;6:138-43
8. Kotaidis V, Dahmen C, von Plessen G. et al. Excitation of nanoscale vapor bubbles at the surface of gold nanoparticles in water. J Chem Phys. 2006;124:184702
9. Prasad V, Mikhailovsky A, Zasadzinski JA. Inside-out disruption of silica/gold core-shell nanoparticles by pulsed laser irradiation. Langmuir. 2005;21:7528-32
10. Liu Z, Hung WH, Aykol M. et al. Optical manipulation of plasmonic nanoparticles, bubble formation and patterning of SERS aggregates. Nanotechnology. 2010;21:105304
11. Wang S, Chen K-J, Wu T-H. et al. Photothermal effects of supramolecularly assembled gold nanoparticles for the targeted treatment of cancer cells. Angew Chem Int Ed. 2010;49:3777-81
12. Wu T-H, Kalim S, Callahan C. et al. Image patterned molecular delivery into live cells using gold particle coated substrates. Opt Express. 2010;18:938-46
13. McLaughlan JR, Roy RA, Ju H, Murray TW. Ultrasonic enhancement of photoacoustic emissions by nanoparticle-targeted cavitation. Opt Lett. 2010;35:2127-9
14. Wang Y, Xie X, Wang X. et al. Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Letters. 2004;4:1689-92
15. Taylor U, Klein S, Petersen S. et al. Nonendosomal cellular uptake of ligand-free, positively charged gold nanoparticles. Cytometry A. 2010;77:439-46
16. Oberdörster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for concern? J Nanosci Nanotechnol. 2009;9:4996-5007
17. Lasagna-Reeves C, Gonzalez-Romero D, Barria MA. et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393:649-55
18. Gu Y, Cheng J, Lin C. et al. Nuclear penetration of surface functionalized gold nanoparticles. Toxicol Appl Pharmacol. 2009;237:196-204
19. Kogan MJ, Olmedo I, Hosta L. et al. Peptides and metallic nanoparticles for biomedical applications. Nanomedicine. 2007;2:287-306
20. Li J, Wang L, Liu X. et al. In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 2009;274:319-26
21. Arnida Malugin A, Ghandehari H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: a comparative study of rods and spheres. J Appl Toxicol. 2009;30:212-7
22. Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv Drug Deliv Rev. 2010;62:346-61
23. Tong L, Wei Q, Wei A, Cheng JX. Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. Photochem Photobiol. 2009;85:21-32
24. Huang X, El-Sayed IH, El-Sayed MA. Applications of gold nanorods for cancer imaging and photothermal therapy. Methods Mol Biol. 2010;624:343-57
25. Chen J, Glaus C, Laforest R. et al. Gold nanocages as photothermal transducers for cancer treatment. Small. 2010;6:811-7
26. Tong L, Zhao Y, Huff T. et al. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv Mater. 2007;19:3136-41
27. Hooper JW. The genetic map to theranostics. Med Lab Obs. 2006;38:22-3
28. Picard FJ, Bergeron MG. Rapid molecular theranostics in infectious diseases. Drug Discov Today. 2002;7:1092-101
29. Chanda N, Shukla R, Katti KV, Kannan R. Gastrin releasing protein receptor specific gold nanorods: breast and prostate tumor avid nanovectors for molecular imaging. Nano Lett. 2009;9:1798-805
30. Daou TJ, Li L, Reiss P. et al. Effect of poly(ethylene glycol) length on the in vivo behavior of coated quantum dots. Langmuir. 2009;25:3040-4
31. Rosi NL, Giljohann DA, Shad Thaxton C. et al. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027-30
32. Bhojani M, Van Dort M, Rehemtulla A, Ross BD. Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol Pharmaceutics. 2010;7:1921-9
33. Chen W, Bardhan R, Bartels M. et al. A molecularly targeted theranostic probe for ovarian cancer. Mol Cancer Ther. 2010;9:1028-38
34. Lukianova-Hleb E, Hanna E, Hafner J, Lapotko D. Tunable plasmonic nanobubbles for cell theranostics. Nanotechnology. 2010;21:085102
35. Lapotko D, Lukianova-Hleb E, Oraevsky A. Clusterization of nanoparticles during their interaction with living cells. Nanomedicine. 2007;2:241-53
36. Hleb EY, Hafner JH, Myers JN. et al. LANTCET: elimination of solid tumor cells with photothermal bubbles generated around clusters of gold nanoparticles. Nanomedicine. 2008;3:647-67
37. Lapotko D. Optical excitation and detection of vapor bubbles around plasmonic nanoparticles. Opt Express. 2009;17:2538-56
38. Lukianova-Hleb EY, Hu Y, Latterini L. et al. Plasmonic nanobubbles as transient vapor nanobubbles generated around plasmonic nanoparticles. ACS Nano. 2010;4:2109-23
39. Lapotko D, Lukianova E, Оraevsky A. Selective laser nano-thermolysis of human leukemia cells with microbubbles generated around clusters of gold nanoparticles. Lasers Surg Med. 2006;38:631-42
40. Wagner DS, Delk NA, Lukianova-Hleb EY. et al. The in vivo performance of plasmonic nanobubbles as cell theranostic agents in zebrafish hosting prostate cancer xenografts. Biomaterials. 2010;31:7567-74
41. Brandenberger C, Mühlfeld C, Ali Z. et al. Quantitative evaluation of cellular uptake and trafficking of plain and polyethylene glycol-coated gold nanoparticles. Small. 2010;6:1669-78
42. Mailänder V, Landfester K. Interaction of nanoparticles with cells. Biomacromolecules. 2009;10:2379-400
43. Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145-50
44. Lapotko D, Lukianova K, Shnip A. Photothermal responses of individual cells. J Biomed Optics. 2005;10:014006
45. Lapotko D. Plasmonic nanoparticle-generated photothermal bubbles and their biomedical applications. Nanomedicine. 2009;7:813-45
46. Laser Institute of America. American national standard for safe use of lasers (ANSI Z136.1-2000). US: Laser Institute of America. 2000
47. Hleb E, Hu Y, Drezek R. et al. Photothermal bubbles as optical scattering probes for imaging living cells. Nanomedicine. 2008;3:797-812
48. Mueller H, Kassack MU, Wiese M. Comparison of the usefulness of the MTT, ATP, and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines. J Biomol Screen. 2004;9:506-15
49. Lapotko D, Romanovskaya T, Gordiyko E. Photothermal monitoring of redox state of respiratory chain in single live cells. Photochem Photobiol. 2002;75:519-26
50. Lukianova-Hleb E, Santiago C, Wagner D. et al. Generation and detection of plasmonic nanobubbles in zebrafish. Nanotechnology. 2010;21:225102
51. Hleb E, Lapotko D. Influence of transient environmental photothermal effects on optical scattering by gold nanoparticles. Nano Letters. 2009;9:2160-66
52. Stern JM, Stanfield J, Kabbani W. et al. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J Urol. 2008;179:748-53
53. Huang HC, Rege K, Heys JJ. Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano. 2010;4:2892-900
54. Govorov O, Richardson H. Generating heat with metal nanoparticles. Nano Today. 2007;1:30-8
55. Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-7
56. Pitsillides M, Joe EK, Wei X. et al. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys J. 2003;84:4023-32
57. Green DE, Longtin JP, Sitharaman B. The effect of nanoparticle-enhanced photoacoustic stimulation on multipotent marrow stromal cells. ACS Nano. 2009;3:2065-72
58. Volkov AN, Sevilla C, Zhigilei LV. Numerical modeling of short pulse laser interaction with au nanoparticle surrounded by water. Appl Surf Sci. 2007;253:6394-9
59. Huang X, Qian W, El-Sayed IH, El-Sayed MA. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Lasers Surg Med. 2007;39:747-53
60. van Gemert MJC, Welch AJ. Clinical use of laser-tissue interactions. IEEE Eng Med Biol Mag. 1989;8:10-3
61. Svaasand LO. Dosimetry for laser induced hyperthermia. Lasers Med Science. 1989;4:309-15
62. O'Neal DP, Hirsch LR, Halas NJ. et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Letters. 2004;209:171-6
63. Day ES, Thompson PA, Zhang L. et al. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J Neurooncol. 2010 [Epub ahead of print]
64. Terentyuk GS, Maslyakova GN, Suleymanova LV. et al. Laser-induced tissue hyperthermia mediated by gold nanoparticles: toward cancer phototherapy. J Biomed Optics. 2009;14:021016
65. Olafsson R, Bauer DR, Montilla LG, Witte RS. Real-time, contrast enhanced photoacoustic imaging of cancer in a mouse window chamber. Optics Express. 2010;18:18625-32
66. Shah J, Park S, Aglyamov S. et al. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J Biomed Optics. 2008;13:034024
67. Kim C, Favazza C, Wang LV. In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths. Chem Rev. 2010;110:2756-82
68. Hmelar M, Manokian N. Side firing fiber optic laser probe. Patent number 5772657. 1998
69. van Swol CFP, Verdaasdonk RM, van Vliet RJ. et al. Side-firing devices for laser prostatectomy. World J Urol. 1995;13:88-93
70. Sountoulides P, Tsakiris P. The evolution of KTP laser vaporization of the prostate. Yonsei Med J. 2008;49:189-99
71. Wosnitzer MS, Rutman MP. KTP/LBO laser vaporization of the prostate. Urologic Clinics of North America. 2009;36:471-83
72. Podder TK, Buzurovic I, Yu Yan. Multichannel robot for image-guided brachytherapy. BIBE. 2010:209-13
Author contact
![]() Corresponding author: Dmitri O. Lapotko, PhD, Rice University, Physics and Astronomy - MS 61, 6100 Main Street, Houston, TX 77005. Tel: 713-348-3708; Fax: 713-348-4150; E-mail: dl5edu
Corresponding author: Dmitri O. Lapotko, PhD, Rice University, Physics and Astronomy - MS 61, 6100 Main Street, Houston, TX 77005. Tel: 713-348-3708; Fax: 713-348-4150; E-mail: dl5edu
 Global reach, higher impact
Global reach, higher impact