13.3
Impact Factor
Theranostics 2025; 15(9):4147-4174. doi:10.7150/thno.108705 This issue Cite
Review
The role of manganese-based MRI contrast agents for cancer theranostics: Where do we stand in 2025?
1. Lab of Molecular Imaging and Medical Intelligence, Department of Radiology, Longgang Central Hospital of Shenzhen, Shenzhen, 518116, China.
2. Shenzhen Clinical Medical College, Guangzhou University of Chinese Medicine, Shenzhen, Shenzhen, 518116, China.
3. Longgang Clinical Institute of Shantou University Medical College Shenzhen, 518116, China.
4. School of Science, Shenzhen Key Laboratory of Advanced Functional Carbon Materials Research and Comprehensive Application, Harbin Institute of Technology, Shenzhen, 518055, China.
Received 2024-12-12; Accepted 2025-1-30; Published 2025-3-15
Abstract
Magnetic resonance imaging (MRI) guidance in the realm of anticancer therapy is crucial to visualize the spread of tumors in deep tissues, accumulate the therapeutics, and trigger them for precise therapy. Recent studies bridge this gap by integrating MRI contrast agents (CAs) with different therapeutic regimes for a better outcome. In this context, manganese-based materials hold great potential owing to their T1/T2 dual-modal MR-relaxation, less toxicity, and other therapeutic capabilities such as chemodynamic therapy, immunotherapy, etc., which have gained increasing interest among researchers and medical professionals. This work offers a timely update on the last three years for Mn-based MRI-guided theranostic applications, including chemodynamic therapy, chemotherapy, photo therapies, sonodynamic therapy, and radiotherapy against cancer. Further, several combinatory therapies and surgical intersections have also been summarized in the light of MRI guidance. The design rationale of these Mn-based agents has been discussed to understand the existing challenges and plausible outcomes shortly. Herein, we deep dive into the stimulus-based probes including pH, temperature, etc. to show the unexplored potential of Mn-complexes in this domain. This state-of-the-art review will guide innovations in Mn-based CAs to expedite safer anticancer theranostic modules for clinical translation.
Keywords: Manganese, Cancer, Magnetic resonance imaging, Nanotheranostics, Combinatory therapy
1. Introduction
Cancer has caused severe harm to human health by spreading exponentially worldwide irrespective of the age of the patient. Recent studies highlight several therapeutic modalities that can cure cancer to a certain level and extend the life expectancy of a patient. However, detecting cancer is crucial to inhibit its spread and start the therapy in time [1]. It is observed that probing the cancer in time could expedite the therapeutic capability and restrict metastasis [1]. Thus, a combination of precise therapy with diagnosis is needed for an advanced therapeutic regimen. Currently, theranostic nanoplatforms (especially imaging-guided delivery systems) combining therapeutic and diagnostic modalities have shown promise for cancer treatment [2-3]. The real-time visualization of the therapeutics, accumulation of them in the diseased tissues, and the outcomes of the therapy make these systems advanced compared to conventional chemotherapy and immunotherapy alone [4]. Recently, fluorescence imaging (FI), magnetic resonance imaging (MRI), computed tomography (CT), X-ray imaging, etc. have become important modalities owing to their high signal-to-noise ratio (SNR), higher tissue penetration, and low autofluorescence [5]. Although FI in the NIR-II domain can penetrate up to several millimeters and image soft tissues and veins quite clearly, they have limited accuracy when subjected to bone imaging. On the other hand, X-rays and CT can precisely detect bone lesions, but the use of ionizing radiations and toxic contrast agents (CAs) limits their biocompatibility [6]. On the other hand, MRI can detect infections both in soft tissues and bone with high accuracy and utilizes the magnetic response of the CAs without damaging any cells or tissues. More importantly, it can penetrate through skin and tissues to the highest depth [7]. Thus, MRI has become a favorable choice to image cancer margins even in deep tissues [8-9].
Paramagnetic materials as contrast agents are popular owing to their stronger magnetic properties. The number of unpaired electrons in the Gd3+ ion is 7, whereas, for Mn2+, the number of unpaired electrons is 5 [11]. As the number of unpaired electrons determines the magnetic moment, Gd3+ possesses a higher magnetic moment with better MR imaging capability. MRI contrast agents have higher relaxation rates (T1 and T2) of nearby water protons. Mn ions (Mn2+) are highly efficient at T1-weighted contrast enhancement, leading to bright images. Free Gd3+ ions are highly toxic because they interfere with calcium-dependent biological processes. To mitigate this, Gd3+ is used in chelated forms, such as Gd-DTPA [10]. The gadolinium deposition in tissues, especially in the brain, can cause massive toxicity. Gd is a heavy metal, and in its free ionic form, it is highly toxic to human tissues. When the kidneys process Gd-based contrast agents (GBCAs), they are exposed to potential toxicity, especially in patients with pre-existing kidney impairment [11]. Excessive or unregulated doses of gadolinium (Gd) can lead to severe kidney damage, including conditions like nephrogenic systemic fibrosis (NSF) in some cases. This highlights the critical need for a safer and more biocompatible alternative, such as contrast agents (CA), to minimize potential risks while ensuring effective diagnostic imaging [12]. Mn, on the other hand, functions as both a T1 and a T2 contrast agent in magnetic resonance imaging (MRI), demonstrating dual-mode functionality [13]. The magnetic characteristics of contrast agents, such as manganese (Mn), affect the relaxation times (T1) (longitudinal) and (T2) (transverse) of the surrounding water protons. A powerful local magnetic field produced by Mn's unpaired electrons interacts with the nuclear spins of neighboring water protons. Because Mn effectively promotes energy transfer from excited protons to their surroundings, T1 relaxation, which is linked to increased signal intensity in T1-weighted MRI is shortened. Conversely, field inhomogeneities of Mn also impact T2 relaxation, which is associated with the loss of phase coherence among proton spins and results in signal decay in T2-weighted MRI [14]. These effects, which allow for customized imaging contrast for certain diagnostic requirements, are dependent on Mn content, particle size, and surface chemistry. To improve image quality in magnetic resonance imaging (MRI), gadolinium-based contrast agents (GBCAs) minimize the relaxation durations T1 of surrounding water protons. The seven unpaired electrons in gadolinium (Gd³⁺) produce a powerful magnetic moment that interacts with neighboring protons. In T1-weighted images, this interaction increases the signal intensity and speeds up T1 relaxation, highlighting pathological and anatomical details. Usually chelated with ligands to lower toxicity, Gd³⁺ ensures safe excretion while retaining its effectiveness. When it comes to finding anomalies in the central nervous system, tumors, and blood arteries, GBCAs are especially useful [15]. Yet, worries about long-term tissue preservation have spurred research into less harmful substitutes, like manganese-based contrast agents, which have comparable advantages but less toxicity.
Mn can induce Fenton-like reactions and initiate the cGAS-STING pathway to promote chemodynamic therapy (CDT) and immunotherapy [16]. Moreover, Mn can be coordinated with organic dyes like porphyrins to yield MRI-functionalized dyes for various applications such as MRI-guided PTT, and sonodynamic therapy. Additionally, it can be doped/mixed in nanoplatforms via covalent and/or non-covalent linkages yielding multiple functional theranostic nanomedicines with MRI guidance. These features enable Mn to serve both imaging and therapeutic purposes in cancer. Table 1 compares the (r1) relaxivity of typical gadolinium-based agents with manganese-based contrast agents, highlighting that Mn-based agents exhibit a higher relaxivity than Gd agents.
Under specific circumstances, Mn-based treatments can also produce reactive oxygen species (ROS), which can help destroy cancer cells. [30]. Furthermore, Mn can enhance immune responses against tumors by activating the cGAS-STING immunological system, which increases immunological responses by inducing the production of pro-inflammatory cytokines and type I interferons [31]. Transforming hydrogen peroxide into oxygen, Mn-based treatments can improve oxygen availability and boost therapeutic efficacy while reducing tumor hypoxia [32]. Manganese (Mn) improves cancer treatment through a variety of interactions with the tumor microenvironment (TME) [33]. By causing oxidative stress and producing reactive oxygen species (ROS), which harm cancer cells and encourage cell death, Mn2+ ions can alter the TME. Mn interacts with the TME to facilitate medication transport and treatment responses, in part because of its capacity to modify pH, control the tumor extracellular matrix, and influence redox processes [34]. Being a common nutrient, Mn is a safer substitute compared to traditional agents due to its biodegradability and low toxicity. By reducing off-target effects and increasing therapeutic accuracy, Mn-based compounds can be designed for tailored drug delivery. Besides, Mn promotes drug distribution and penetration by modifying the extracellular matrix and controlling pH levels. The potential of Mn-based contrast agents to enhance imaging and therapeutic precision in cancer treatment is highlighted by these interactions. Furthermore, Mn-based medicines can be used with immunotherapy, photothermal therapy, chemotherapy, and other treatments to provide a multimodal strategy for more successful cancer treatment [35].
The comparison of the r1 relaxivity of typical gadolinium agents compared to manganese-based contrast agents where it shows that the Mn-based agents achieved a higher r1 relaxivity rate than Gd agents
| Sample name | r1 relaxivity (mM-1 s-1) | References |
|---|---|---|
| MnO-PEG-RGD | 12.1 | [17] |
| Mn0.5Mg2.6Al1-LDH | 7.60 | [18] |
| Mn3Fe1-LDH | 7.83 | [19] |
| MnOx-SiO2 hollow@PEG | 8.81 | [20] |
| MnO@Fe3O4-OH-PEG-PH | 22.8 | [21] |
| Gd2O3 | 9.14 | [22] |
| GdDTPA- conjugated Fe3O4@SiO2@mSiO2 | 6.13 | [23] |
| MnO-PEG-AS1411 Aptamer | 12.9 | [24] |
| Gd-DOTA | 4.2 | [25] |
| Mn-M48SN | 8.4 | [26] |
| Polyacrylic acid-coated MnO nanoparticles | 9.3 | [27] |
| USMnO@ZDS | 15.6 | [28] |
| Gd-DOTA | 5.77 | [29] |
Apart from the therapeutic or diagnostic efficacy of Mn, in its ionic form Mn can detect cancer in several ways. Notably, cancer cells can uptake Mn ions due to their strong affinity towards Mn. Manganese chloride (Mn chloride), specifically the Mn²⁺, enters cells via calcium channels. However, its application in cancer research has been relatively limited. In breast cancer, studies using MCF7 and MDA-MB-231 tumor models demonstrated that areas of significant Mn enhancement on MRI correlated with strong CaSR expression. However, these studies did not compare enhancement levels between the two cell lines, nor did they quantify Mn uptake [36]. Mn interacts with the tumor microenvironment (TME) significantly to improve cancer treatment [37]. MnO2 in the nanoparticle form can be catalyzed through the tumor microenvironment by the local H2O2 and internalized by the cancer cells in many works [38]. These works greatly support the importance of Mn-based systems in cancer theranostics.
Very recently the emergence of Mn-based small molecules, polymer dots, quantum dots, and biomimetic NPs made these systems very efficient and provided maximum theranostic efficacy (Figure 1). Mn can be tumor-microenvironment (TME) responsive and stimulate different pathways to achieve such functionalities. Various Mn-based systems and their applications have been reviewed to modulate the TME. These methods include producing reactive oxygen species, increasing pH, oxygen generation, glutathione/hydrogen peroxide depletion, glucose exhaustion, and activating innate or adaptive immunity [39]. Increased imaging signals always accompany all of these mechanisms for response monitoring. Due to these significant benefits, Mn-based systems have a wide range of uses in TME-responsive cancer therapies, such as chemotherapy, gene therapy, gas therapy, immunotherapy, photodynamic therapy (PDT), sonodynamic therapy (SDT), radiotherapy, starvation therapy, chemodynamic therapy (CDT), ferroptosis-mediated therapy, and immunotherapy [40]. There are several reviews highlighting Mn-based STING immunotherapy, Mn as MRI agents, designing rationale of Mn-based contrast agents, etc till the end of the last decade [41]. These reviews also described cancer treatments that include heat production in TME, such as photothermal therapy (PTT), microwave dynamic therapy (MDT), magnetic hyperthermia, and microwave thermal therapy (MTT), paving a path to understanding innovative therapeutic approaches [32]. Likewise, Huang et al. showed the physicochemical properties and synthesis techniques of biomaterials derived from Mn, which are thoroughly examined in his review article, with a focus on their application in tumor diagnostics, encompassing magnetic resonance imaging, photothermal and photoacoustic imaging, ultrasound imaging, multimodal imaging, and detection. The benefits of using Mn-based materials for tumor treatment applications are also covered. These applications include tumor immunotherapy, drug delivery, tumor microenvironment control, synergistic photothermal, photodynamic, and chemodynamic therapies, and imaging-guided therapy [42]. However, scientists focus on increasing the efficiency of such contrast agents by making them 'all-in-one' theranostic systems for more effective and personalized cancer treatment. Notably, very recent research on Mn-based STING immunotherapy, Fenton reaction-based chemodynamic therapy, lipid peroxidation mediated anticancer therapy, and multimodal imaging-guided anticancer therapies have not been included in such review, creating a scope of further summarizing these new domains and analyzing their future prospects.
Herein, we offer a timely update on the recent progress, especially from 2022 to the present time, on how to design Mn-based theranostic platforms for optimal efficacy. This work also highlights the novelty of manganese-based agents in MRI-guided theranostics by emphasizing their T1/T2 dual-modal MR imaging capabilities, superior biocompatibility, and multifunctional therapeutic potential. Unlike conventional MRI contrast agents, Mn-based systems provide enhanced tumor visualization and precise therapeutic triggering, bridging imaging with therapies like chemodynamic therapy, chemotherapy, and phototherapies. The integration of stimulus-responsive probes sensitive to pH, temperature, and other microenvironmental changes further distinguishes this approach, offering dynamic control over therapeutic delivery. Importantly, the combinatory therapeutic platforms with Mn-based MRI imaging guidance are also pointed out. Afterward, some key nanotoxicity challenges, potential obstacles, and prospects are also discussed. In the end, Mn-based nanomaterial-derived cancer nanomedicine is expected to progress in terms of development and prospects. By summarizing the latest advancements and addressing challenges in Mn-based theranostics, this review sets a foundation for innovative designs, ensuring safer and more effective cancer treatments for clinical translation.
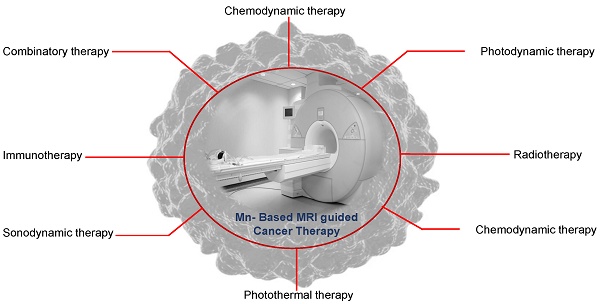
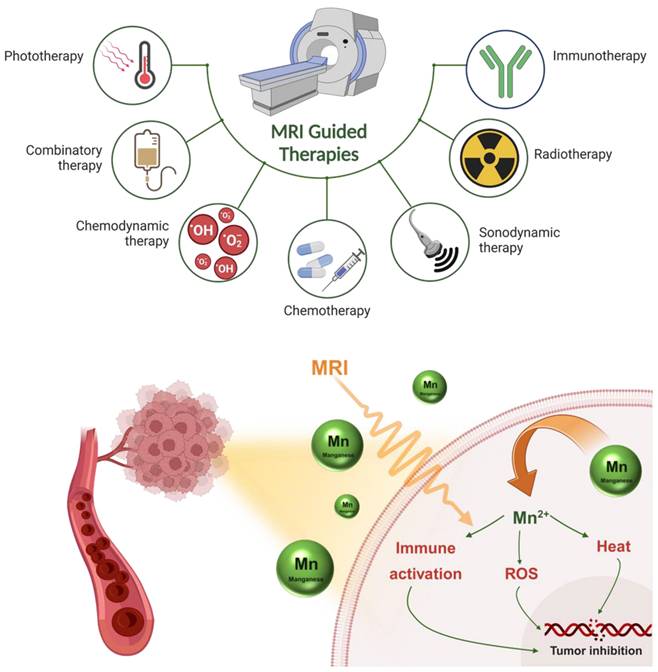
Manganese-based MRI-guided therapies like immunotherapy, radiotherapy, sonodynamic therapy, chemotherapy, phototherapy, etc. for cancer theranostics. This figure also illustrates different pathways involving Mn to promote ROS, heat, and immunity-based activation processes in a biological system for anticancer therapy.
2. Theranostic agents and their implications on biomedical applications
The concept known as "theranostic," which represents the combination of imaging and therapy, is referred to by several groups [43-46]. It is described as the endeavor to combine investigative and therapeutic capabilities into a single agent to create personalized, targeted therapies for a range of diseases [47]. John Funkhouser initially introduced this term, and it has more recently been found to be applicable in the context of image-guided therapy and therapeutic agents that also possess imaging capabilities. In the past, the conventional approach involved the diagnosis and subsequent therapy to address the specific disease. Consequently, medical research primarily concentrated on characterizing the ailment and creating standardized treatments or medications. However, some diseases like cancer, which exhibit diverse expressions, demand tailored and personalized treatment approaches [48]. Theranostic strategies herein play a crucial role in developing advanced medical efficacy.
The rapidly advancing field of nanotheranostics is distinguished by its focus on drug delivery, drug release, and the assessment of therapeutic effectiveness, all of which are simultaneously monitored through a single nanoscale carrier [49]. Nanoparticles within the range of 1 to 100 nm are believed to display discriminating interactions with biomolecules, including receptors, antibodies, and enzymes located either inside or outside of cells [50].
Nanotechnology not only elevates the performance of the theranostic systems but also introduces imaging guidance along with high therapeutic efficiencies. MRI-imaging guided therapy is one of such recent advancements [51]. One of the significant contenders in creating theranostic nanoplatforms has been the transformation of MRI contrast agents (CAs). The precise delivery of contrast agents, like iron oxide NPs, Mn2+, and Gd3+ complexes, to the specific tumor site through custom-designed nanoplatforms such as block copolymers, liposomes, graphene oxide, and hydrogels, plays a vital role in conferring highly sensitive MRI contrast capabilities to the theranostic nanosystem [52].
Among these MRI theranostics systems, Mn-based MRI probes have become a prevalent choice owing to their inertness, higher biocompatibility, and lower cytotoxicity. In the following chapter such Mn-based MRI theranostic systems have been discussed.
3. Mn-based agents for MRI-guided theranostic applications
3.1 Mn-based contrast agents for MRI-guided chemotherapy
Chemotherapy is a medical therapy that employs medications to destroy or slow down the proliferation of cancer cells or other fast-growing cells in the body [53]. Chemotherapy is a crucial therapeutic modality for cancer treatment [54]. The side effects and overcoming drug resistance are critical factors. One of the primary factors contributing to this resistance is the overexpression of ATP-binding cassette (ABC) transporters on the surface of tumor cells, which could be minimized if other therapeutic modalities could be integrated into the existing chemotherapy [55]. Herein, using Mn could be beneficial as it has multiple therapeutic and imaging abilities. Chemotherapeutic drugs such as doxorubicin, gemcitabine, and paclitaxel are commonly used in cancer treatment to reduce or eradicate tumors, frequently by focussing on rapidly proliferating cancer cells [56]. Nevertheless, these medications are poorly soluble and have brief in vivo half-lives. Nanocarriers are employed to improve controlled release and focused distribution. Using manganese ions (Mn²⁺) to cause toxicity in cancer cells through redox processes, manganese-based chemotherapy is a novel strategy that aims to target tumors while minimizing harm to healthy organs efficiently [57].
The potential of manganese-based (Mn-based) contrast agents in MRI-guided chemotherapy is drawing more attention [58]. By clearly visualising tumors, these medicines improve MRI imaging and allow for more accurate treatment planning and monitoring. Additionally, Mn-based compounds help improve drug administration and uptake, overcome obstacles including tumor hypoxia, and modify the tumor microenvironment [59]. Mn-based drugs are promising options for maximizing chemotherapeutic efficacy and minimizing negative effects because of their dual role. MRI-guided chemotherapy can provide spatial information and show improved cancer therapeutic efficacy. The MRI-based chemotherapy follows the ''sense and release" approach, usually getting triggered under the tumor microenvironment [60]. It has been established that Mn-MOF complexes possess a high affinity for tumor cells and can be considered a suitable choice for delivering chemotherapeutic drugs [61]. For example, Song et al. loaded DOX into Mn nanoparticles to diagnose breast cancer cells in 4T1 mice and enhanced treatment via chemotherapy [62]. In another study, Pan and colleagues created a multifunctional nanoplatform consisting of BSA-MnO2 (~10 nm) for tumor and renal imaging and chemotherapy. Indocyanine green and paclitaxel, which act as photothermal and chemotherapeutic agents, are loaded in the nanostructure [63]. The T1 relaxivity was found to be 7.9 mM-1 s-1. Such work opens up new avenues for the development of new and integrated biomaterials for cancer therapy. Another biocompatible and biodegradable multifunctional integrated nano platform was reported by Zhou et al. [64]. Researchers created Mn-doped calcium phosphate nanoparticles for targeted DOX delivery in MRI-guided cancer therapy. These nanoparticles demonstrated improved therapeutic effects against BxPC-3 cells and robust T1-MR relaxation, providing a viable method for imaging-guided cancer treatment [65].
Recently, by focussing on the tumor microenvironment (TME), researchers are improving MR relaxation rates and chemotherapeutic capabilities. For instance, carbon dot nanozymes based on manganese and toluidine blue (TB) demonstrated a 225-250% enhancement in dual T1/T2 MR relaxation. Due to their increased permeability and retention effect, these enzymes also demonstrated peroxidase-like activity because of the Mn and were responsive to the TME, which encouraged aggregation at tumor locations (Figure 2) [66].
Nevertheless, by improving tumor imaging, facilitating targeted drug distribution, and increasing therapeutic efficacy, Mn-based contrast agents improve MRI-guided chemotherapy and present a viable strategy for integrated cancer treatment with few adverse effects.
3.2 Mn-based contrast agents for MRI-guided chemodynamic therapy
Chemodynamic therapy (CDT) is an emerging therapeutic approach in the field of cancer treatment that harnesses chemical reactions to selectively generate toxic species, such as reactive oxygen species (ROS), within tumor cells [67]. Unlike traditional chemotherapy, which relies on delivering cytotoxic drugs, CDT exploits the unique biochemical and microenvironmental characteristics of cancer cells to induce cell death. The use of manganese-based (Mn-based) contrast agents for MRI-guided chemodynamic treatment (CDT) is becoming more popular [68]. Their paramagnetic characteristics improve tumor localization and treatment planning by enabling accurate tumor imaging via MRI. Reactive oxygen species (ROS) are produced in the tumor microenvironment by Mn-based therapies, which promote oxidative stress and tumor cell death [69]. Mn-based contrast agents are a promising technique for enhancing the efficacy of chemodynamic cancer therapies because of their dual functionality, which combines therapeutic activity with diagnostic imaging.
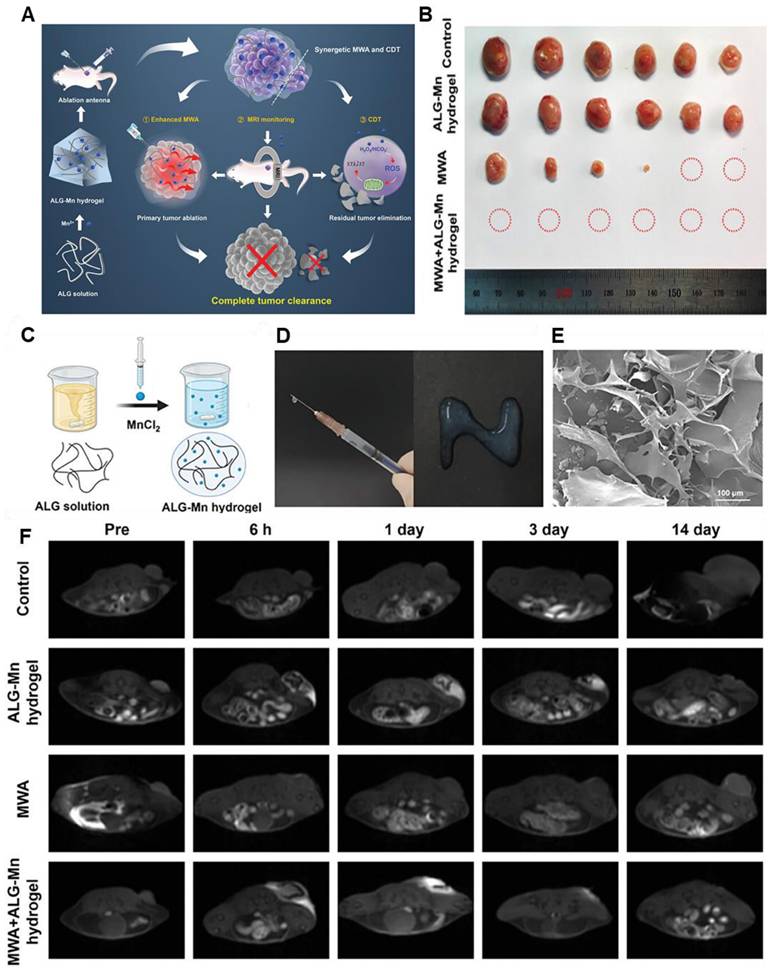
The outstanding efficacy of chemodynamic therapy has further been elevated to a new level when scientists attached the MRI imaging guidance. According to a recent study, Gao et al. synthesized a Pt-MnO2 complex that can perform synergistic chemo/chemo dynamic therapies for tumor cells. The tumor cells could specifically internalize L/D-MnO2@Pt NPs, and the efficient depletion of glutathione (GSH) through redox reaction was achieved, resulting in the release of Mn2+ and Pt. A strong chemodynamic effect was exhibited by the released Mn2+ through a Fenton-like reaction. The chemodynamic therapy (CDT) efficiency was further improved by the depletion of GSH. Such results in recent times provoked other groups to take up imaging-guided chemodynamic treatment of diseased organs and cells. For instance, Lin et al. designed a MnO2-based self-reinforcing CDT nano-agent (MS@MnO2) that can react with the GSH in the tumor site and produce GSSH, along with a reduction of MnO2 to Mn2+ [70]. This Mn2+ catalyzes the •OH from H2O2 with high oxygen activity. This reactive •OH is toxic to the cancer cells as it hampers the DNA, proteins, and lipids of tumor cells, ultimately killing the cells. The Mn2+ ions also help in CDT tracking via MRI. He and his team developed metastable γ-MnS@BSA for MRI-guided CDT and simultaneous gas therapy [71]. The Mn nanoparticle was synthesized via the wet chemical method. In the tumor site, Mn2+ and S2- act as the donor of reactive species and H2S gas, respectively. At acidic pH, H2S gas increased to 12 μM, which is effective enough to kill the cancer cells. Although there are several limitations of chemodynamic therapy such as excessive ROS/RNS generation, uncontrolled reaction rate, etc. However, imaging guidance seems to have promising qualities to probe such inconsistencies making this modality a futuristic candidate in MRI-imaging-guided theranostic modules. Besides, several minimalistic approaches have been adopted by some groups to undergo tumor treatment (Figure 3) [72]. Injectable hydrogels containing Mn have been used for this purpose. It is seen that the presence of Mn2+ not only promoted the entanglement of the hydrogel but also facilitated the Fenton reaction for a precise CDT. Moreover, it produced an enhanced T1 MR relaxation for in vivo imaging of tumors. Thus, the use of Mn could aid not only in the Fenton reaction and MRI guidance but also in preparing the hydrogel matrix and integrating other therapies in a minimalistic way.
(A) Schematic illustration of Manganese-doped TB CDs for MRI-guided light-enhanced CDT and PDT. (B) Catalytic activity of Mn-CDs using TMB as the chromogenic substrate. (C) ESR spectra of Mn-CDs in aqueous solution under 660, 808, 660 + 808 nm laser irradiation. (D-G) T1 and T2 relaxation performance of the sample. (H) Cross-sectional and coronal T1- and T2-weighted pictures, as well as pseudocolor images, of the mice model before and after Mn-CD injection at various time points for 4T1 tumors. (I) Pictures of the 4T1 in vivo tumor-bearing mice in each group after the therapy. Adapted with permission from [66], copyright 2023 Wiley-VCH.
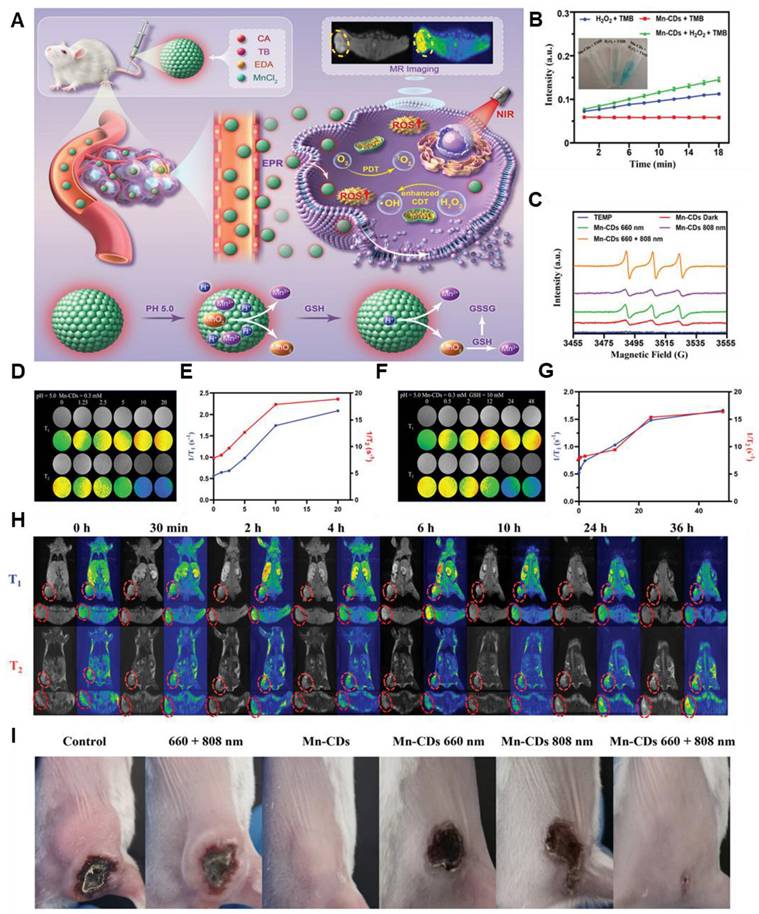
Therefore, through its ability to catalyze the Fenton-like reaction, which produces hydroxyl radicals, oxidative stress, and cancer cell damage, manganese (Mn) improves MRI-guided chemodynamic treatment (CDT). Mn2+ enhances CDT effects by decreasing tumor hypoxia, and its paramagnetic properties enhance T1-weighted MRI imaging for accurate tumor visualization and therapeutic tracking. Mn integrates therapeutic and diagnostic activities to effectively treat cancer by facilitating regulated drug release in the acidic tumor microenvironment.
(A) The synthesis and mechanism of ALG-Mn hydrogel for synergistic MWA and CDT oncotherapy towards total tumor clearance are shown schematically. (B) Pictures of the tumor tissues taken from the mice on the 15th day following different treatments (C) Diagrammatic representation of the preparation of ALG-Mn hydrogel. (D) Assessment of the syringeability of ALG-Mn hydrogel, and (E) SEM images of the prepared hydrogel. (F) Different groups of in vivo MR images demonstrating the potential of the samples as an MRI agent. Adapted with permission from [72], copyright 2024 Wiley-VCH.
3.3 Mn-based contrast agents for MRI-guided photothermal therapy
MRI-guided photothermal treatment (PTT) shows promise with manganese-based (Mn-based) contrast agents. For accurate tumor monitoring and localization, their paramagnetic qualities improve MRI imaging. When light is activated, Mn-based compounds can also produce heat, which effectively kills tumor cells and promotes photothermal effects. Furthermore, Mn ions improve therapy results by altering the tumor microenvironment. When imaging and therapy are combined, Mn-based compounds are a great option to improve the accuracy and effectiveness of photothermal cancer treatments. Phototheranostics or phototherapy typically involves the synergistic use of photosensitizers and light in cancer treatment. Photothermal therapy (PTT) is a form of hyperthermia treatment that entails subjecting bodily tissues to elevated temperatures above the normal range as part of cancer therapy [73]. In most cases, NIR irradiation is used with photothermal agents that can potentially convert the light into heat. Photosensitizers should possess the property of moving to an excited state at a particular frequency and then releasing the energy as heat to kill the localized/targeted cells [74].
It is observed that photobleaching is a vital phenomenon that affects the overall optical properties of a system. For an efficient photostable system, photobleaching must be optimized. In this context, MacDonald et al. reported that Mn3+ ions incorporated building blocks of porphysome nanoparticles can efficiently resist photobleaching and thereby enhance the MRI signal. According to them, it can rival Gd-DTPA for MRI contrast generation. Apart from that, this sample can show stable photothermal efficacy under 680 nm laser (0.75 W) and effectively maintain over 40 °C for longer durations [75]. These types of MRI-guided PTT agents could be effective in clinical research as they have efficient photostability along with bioavailability.
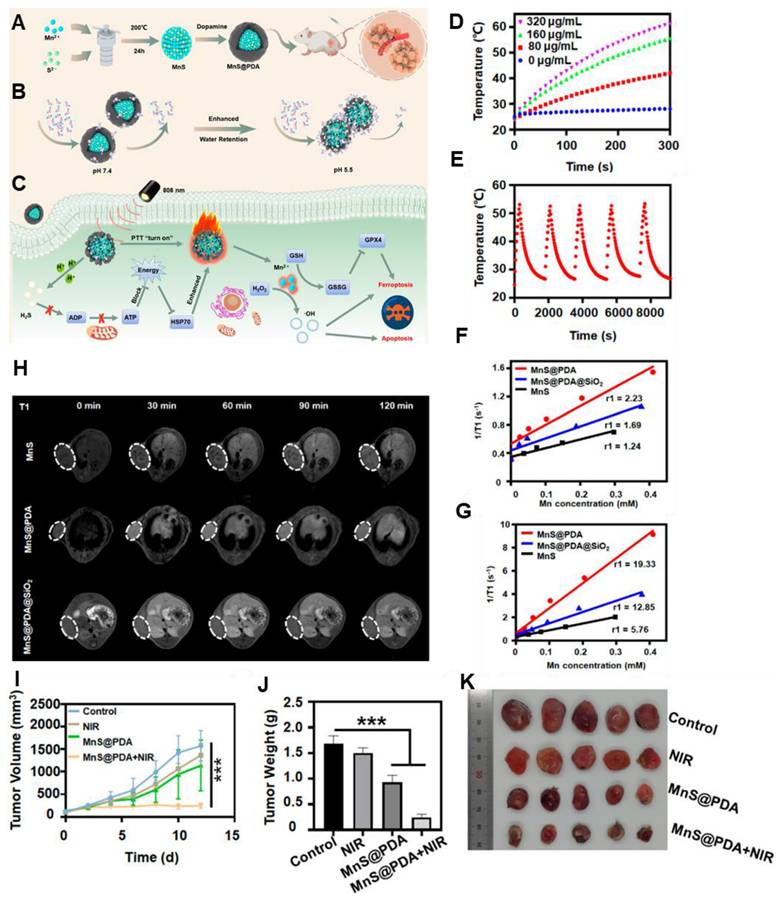
Currently used photothermal agents like Au nanoparticles or graphene and graphene oxide sheets are not biodegradable and may have a poor pharmacokinetic profile. In this regard, Cheng et al. reported MRI-guided PTT using manganese carbonate nanoparticles coated with polydopamine (MnCO3@PDA NPs) [76]. Other than BSA or PDA coating, Mn(III) porphyrins can also be used. For example, Jing et al. conjugated Au nanoshells with Mn(III) porphyrin [77]. To increase the colloidal stability and for longer blood circulation time, PEG was covalently linked to its surface. The complex entrapped DOX to develop DOX@PLA@AuPEG-MnP nanoparticle (diameter of 123.6 nm). Under NIR light irradiation, efficient photo-hyperthermic effect and triggered release of DOX were observed. The Mn(III) porphyrin provided a relaxivity at 0.5 T of 22.18 mM-1 s-1. In vivo MRI relaxation was evaluated in HT-29 tumor-bearing mice and a positive MRI contrast at the tumor site was observed. Polydopamine (PDA) has also been used in this regard by several groups. It is observed that PDA cloaking can increase the pH response of the MnS nanoclusters and is effectively attributed to PTT. Furthermore, it released Mn2+ in a sustained manner and achieved 19.33 mM-1s-1 T1 relaxation (Figure 4) [78]. Besides, BSA-stabilized MnO2 with cross-linked indocyanine green has also been developed for MRI-guided PTT. Under NIR irradiation of 808 nm (laser power of 0.5 W cm-2), tumors recovered completely after 13 days of intratumoral administration [63]. Moreover, a higher T1 rate of relaxation is found (70.6 mM-1 s-1 at 0.5 T). Since 2D MnO2 nanosheets possess an inherent photothermal conversion capability of η: 21.4 % and can exhibit T1-weighted MR imaging capabilities as pH and redox response, it can be useful for inhibiting tumor growth [79].
Heat shock protein (HSP) production may be induced by heterogeneous heat distribution during photothermal treatment (PTT), which could result in heat resistance and decreased effectiveness. To improve therapeutic effects and enable on-demand drug release, Jin et al. created a Co-P@mSiO2@Dox-MnO2 theragnostic agent for pH-activated T1/T2 MRI-guided simultaneous photothermal and chemotherapy. Furthermore, 'cold PTT' is being investigated to lessen harm to healthy tissues, and PTT in combination with other therapies can increase the effectiveness of treatment [80].
So, Mn-based contrast agents for MRI-guided photothermal therapy have the potential to improve tumor visualization and enable targeted hyperthermic therapy at the same time. They are an important tool in the development of non-invasive cancer treatments because of their capacity to produce reactive oxygen species and increase imaging accuracy, both of which boost therapeutic efficacy.
3.4 Mn-based contrast agents for MRI-guided sonodynamic therapy
The integration of sonodynamic therapy (SDT) with nanotechnology offers a novel approach to cancer treatment by enhancing reactive oxygen species (ROS) production in tumor cells. Nanomaterials, such as sonosensitizers, are engineered to improve ultrasonic responsiveness and target tumor tissues specifically, minimizing harm to healthy cells. This strategy increases the therapeutic efficacy of SDT while reducing side effects, leading to tumor cell death and improved cancer treatment outcomes. For instance, Huang et al. reviewed the anti-tumor mechanism of SDT, highlighted advancements in nanotechnology-based SDT treatments, and explored the role of nanomaterials in SDT combination therapies, paving the way for designing innovative sonosensitizers[81].
(A) Schematic Illustration of (A) the Detailed Synthesis mechanism of MnS@PDA, (B) With varying pH conditions, the mechanism of PDA Shell Enhancing Water Molecules Interaction (C) Mechanism of MnS@PDA for PTT and Ferroptosis Therapy of Tumors (D) With different concentration, photothermal temperature changes of MnS@PDA (E) Temperature changes of MnS@PDA with 5 times NIR irradiation cycle. (F) Longitudinal relaxation detection under pH 7.4 and (G) pH 5.5. (H) Longitudinal relaxation detection of mice at 30, 60, 90, and 120 min postinjection of various samples with an Mn dose of 3.8 mg·kg-1 (I) Tumor growth curves, (J) final tumor weight, and (K) Tumor photographs after various treatments (***P < 0.001). Adapted with permission from [78], copyright 2024 American Chemical Society.
The perovskite-type sonosensitizer manganese vanadate (MnVO₃) improves sonodynamic treatment (SDT) by generating reactive oxygen species (ROS) during ultrasound, which destroys cancer cells. While its biodegradability guarantees its safe elimination from the body, its combination with chemodynamic treatment (CDT) enhances its effectiveness by raising ROS production and decreasing glutathione in acidic tumor settings. MnVO₃ provides the possibility for safe, biodegradable cancer treatments, low systemic toxicity, and strong antitumor efficacy [82].
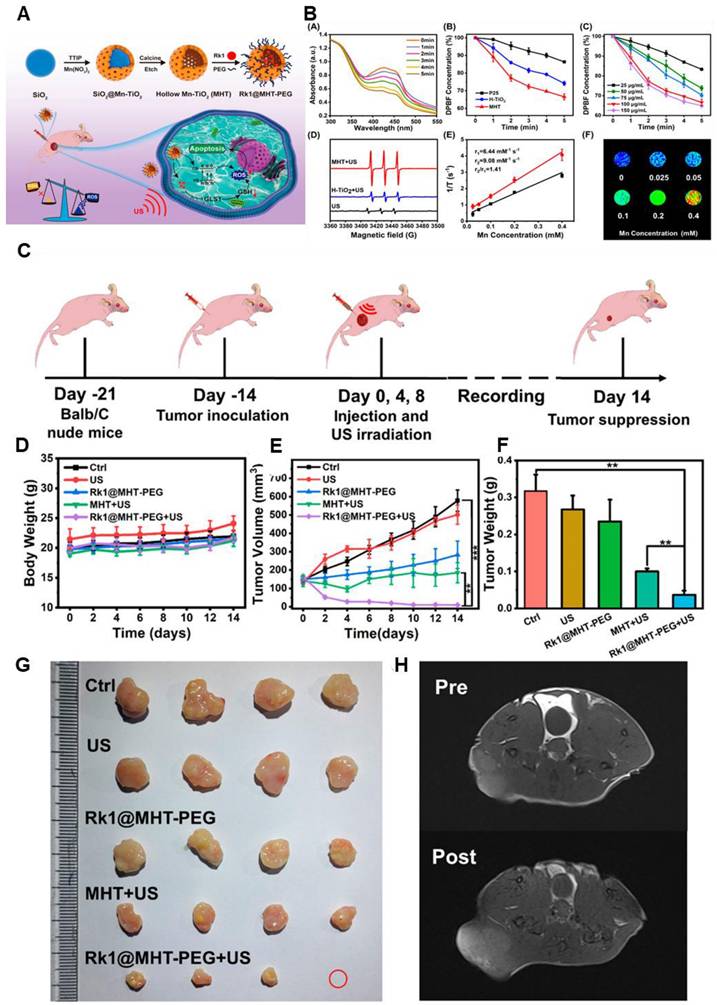
Nonetheless, Ginsenoside Rk1-loaded manganese-doped hollow titania nanoparticles (Rk1@MHT) improve drug transport and boost ROS generation, which improves tumor sonodynamic treatment (SDT). ROS formation is catalyzed by manganese doping, and drug loading efficiency is maximized by the hollow titania structure. By increasing oxidative stress, ginsenoside Rk1 prevents the growth of cancer cells. By raising ROS levels and decreasing the bandgap of titania, the Rk1@MHT sonosensitizer increased the efficacy of SDT and eliminated tumors in mice. High-performance, noninvasive SDT-based cancer treatment appears to be possible with this strategy (Figure 5) [83].
An efficient template for creating metallic oxide nanoparticles, including MnO₂ (MnNPs@Keratin) and GdO₃ (GdNPs@Keratin), was shown by Yan Li et al. These nanoparticles show good colloidal stability, biocompatibility, and outstanding in vitro and in vivo MR imaging capabilities. The therapeutic potential of keratin is increased by its cysteine-rich composition, which imparts redox-responsive drug-release behavior [84]. MRI-guided SDT can be utilized to precisely treat deep-seated resistant-pathogen infections in addition to diagnosing cancer and treating it with SDT [85]. A bacterial-targeting peptide, a porphyrin sonosensitizer (MnTCPP), and a PEG shell were combined to create a polymer-peptide-porphyrin conjugate (PPPC), which allows for accurate real-time monitoring of infected areas. Nano-aggregates and increased sonosensitizer accumulation result from the PEG layer dissociating at the infection site following intravenous injection because of over-expressed gelatinase. This makes it possible for MRI-based monitoring and effective ultrasonic irradiation-based bacterial removal.
Despite its benefits—such as deep tissue penetration, non-invasiveness, and no harm to nearby organs—sonodynamic therapy (SDT) has drawbacks, including lengthier treatment times and limited penetration depth because of ultrasound attenuation. MRI guidance can help with these problems and increase the efficacy of treatment. The chosen sonosensitizer and the medical problem being treated determine the safety and effectiveness of SDT. Future developments in SDT as a treatment alternative could improve and broaden its application.
3.5 Mn-based contrast agents for MRI-guided surgery
The potential of manganese-based (Mn-based) contrast agents to improve tumor visibility has led to an increase in their use in MRI-guided surgery. They have several advantages such as their lower toxicity, higher bio-compatibility when used in a particular amount. Not only that, it has a natural affinity for cellular transport mechanisms, such as calcium channels, which enables targeted imaging, further improving surgical accuracy. Their paramagnetic characteristics enhance T1-weighted imaging, facilitating accurate surgical planning and tumor localisation. Additionally, Mn-based medicines lower the risk of surgery by helping to define the boundaries of the tumor and the surrounding tissues. By guaranteeing more precise, targeted tumor removal during treatments, these medicines present a promising strategy for enhancing surgical outcomes and have the possibility for real-time monitoring. MRI-guided surgery refers to surgical procedures in which magnetic resonance imaging (MRI) technology provides real-time, high-resolution images that guide the surgeon during the operation. This approach offers several advantages in terms of precision, visualization, and the ability to navigate through complex anatomical structures [86]. MRI-guided surgery is commonly employed in various medical fields, including neurosurgery, orthopedic surgery, and certain cancer treatments [87]. It enables surgeons to make informed decisions and enhance the accuracy of their procedures, ultimately improving patient outcomes. It has recently received enormous attention owing to its specificity and safety. Especially, MRI imaging has been proven to have multiple advantages in clinics. It is observed that Mn2+-based MRI CAs are capable of being used in such applications.
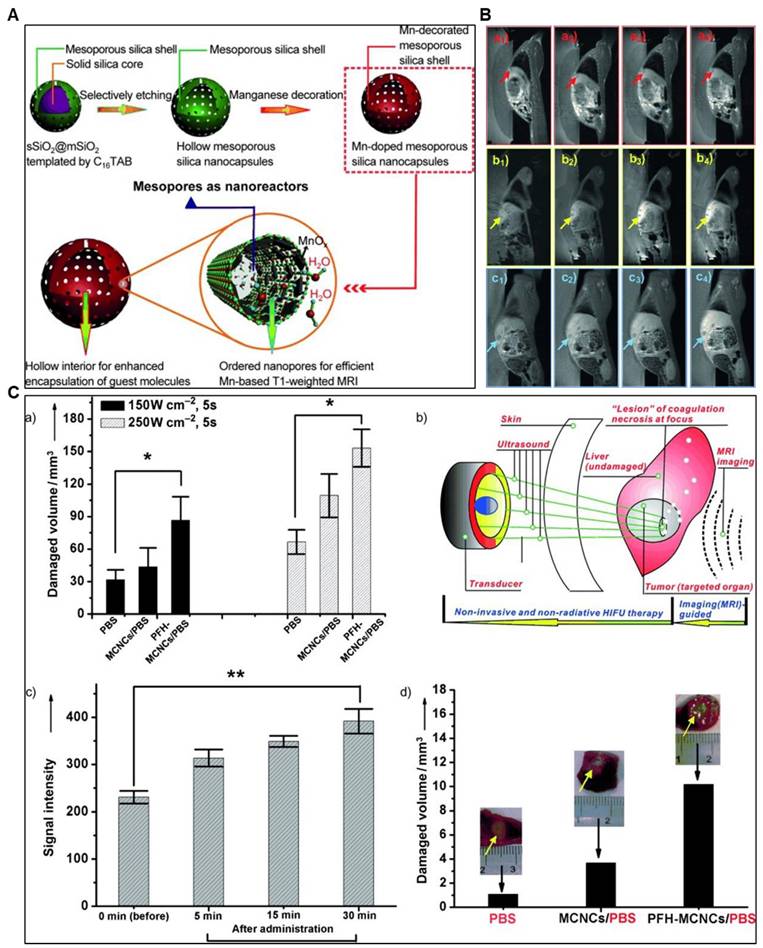
Chen et al. synthesized manganese-based multifunctional mesoporous composite nanocapsules (MCNCs) as contrast agents (CAs) and synergistic agents (SAs) for MRI-guided High-Intensity Focused Ultrasound (HIFU) cancer surgery. The MCNCs' unique structure offers key advantages: a paramagnetic mesoporous shell with confined manganese oxide for efficient T1-weighted MRI and hollow interiors for PFH delivery, enhancing HIFU synergy. In rabbit liver tumors, MCNCs enabled precise ultrasound targeting and improved therapeutic outcomes. These nanostructures show potential for delivering molecules like chemotherapeutics, paving the way for MRI-based diagnosis, thermal chemotherapy, and imaging-guided HIFU cancer surgery (Figure 6) [88].
(A) Schematic Illustration of Rk1@MHT-PEG Preparation and Antitumor Mechanism. (B) A) DPBF UV-vis absorption curve in MHT aqueous solution under various ultrasonic irradiation times. B) The relative DPBF concentrations of MHT, H-TiO2, and P25 were exposed to ultrasound radiation, respectively. C) Relative absorption of DPBF following coexistence with varying MHT concentrations via ultrasonic irradiation. D) ESR tests of various groups with the 1O2 probe TEMP. E) The MHT relaxation curve. F) MHT T1-weighted MR imaging at varying Mn concentrations. In the relevant groups, US irradiation (1.0 MHz, 2 W/cm2, 50% duty cycle) was administered. *p < 0.05, **p < 0.01, and ***p < 0.001 are the means ± SD of three separate experiments. (C) Diagrammatic representation of the Rk1@MHT-PEG nanoprobe animal experiment procedure. (D-F) Body weight, tumor weight on day 14, and tumor volume changes in mice treated for 14 days. (G) Photos of the dissected tumor were taken on day 14 by several groups. (H) T1-weighted MR imaging before and following the insertion of a nanoprobe. In the relevant groups, ultrasonic irradiation (1.0 MHz, 1.5 W/cm2, 50% duty cycle) was administered for 3 minutes. The means ± SD of at least three separate experiments are used to display all data. **p<0.01, ***p<0.001, *p<0.05. Adapted with permission from [83], copyright 2023 American Chemical Society.
(A) Schematic illustration of the synthetic protocol for MCNCs and their corresponding microstructures. (B) In vivo T1-weighted MRI of rabbits with VX2 liver tumors before (a1, b1, c1) and after agent administration at 5 min (a2, b2, c2), 15 min (a3, b3, c3), and 30 min (a4, b4, c4) via the ear vein. Agents: PBS (a1-a4), MCNCs/PBS (b1-b4), PFH-MCNCs/PBS (c1-c4). Arrows mark the tumor. PBS = phosphate-buffered saline. (C) a) Coagulated-tissue volume of degassed bovine liver was analyzed after injecting PBS, MCNCs/PBS, or PFH-MCNCs/PBS (200 μL each) under 150 W/cm² or 250 W/cm² irradiation for 5 seconds (*P < 0.05). b) MRI-guided HIFU enables precise surgery for hepatic neoplasms in rabbits by targeting tumors under real-time imaging. c) T1-weighted MRI signal intensities of tumors significantly increased after intravenous PFH-MCNCs/PBS administration (**P < 0.005). d) Coagulated necrotic tumor volume was measured after MRI-guided HIFU exposure (150 W/cm², 5 s) in rabbit liver tumors treated with different agents (inset: tumor photos post-HIFU). Copyright (2023) with permission from the American Heart Association Adapted with permission from[88], copyright 2011, Wiley-VCH.
Notably, MRI has already been successfully established as an imaging modality in clinics. Therefore, using this technique in imaging-guided surgery would incur nominal modifications and costs. However, extensive research may be needed to enhance rationality and reduce the secondary toxicity of imaging contrast agents. Herein, manganese complexes can play a crucial role shortly.
Nonetheless, by improving imaging contrast, Mn-based contrast agents for MRI-guided surgery provide several benefits, including enhanced tumor visualization. They are a promising tool for accurate, minimally invasive cancer therapies, improving surgical outcomes and therapeutic efficacy because of their capacity to offer real-time monitoring, lower surgical risks, and enhance tumor targeting.
3.6 Mn-based contrast agents for MRI-guided radiotherapy
Manganese-based (Mn-based) contrast agents are becoming cutting-edge instruments for MRI-guided radiation therapy, fusing therapeutic enhancement with accurate imaging. By improving T1-weighted MRI, their paramagnetic qualities allow for precise tumor localization and treatment planning. Additionally, by upsetting hypoxia and encouraging the production of reactive oxygen species (ROS), Mn ions alter the tumor microenvironment and improve radiosensitivity. Because of their dual purpose, Mn-based medicines are bright prospects for increasing the accuracy and effectiveness of radiation therapy and enhancing the results of cancer treatment.
Radiotherapy is necessary for about half of cancer patients. It uses high-energy X-rays or γ-rays to create ROS, which damages DNA, triggers apoptosis, and destroys tumors. However, the effectiveness of radiation is limited by hypoxic tumor microenvironments (TME) in solid tumors. This is addressed by manganese-based nanomaterials, especially manganese oxides, which increase the body's natural generation of oxygen. Dose optimization and better radiation results are made possible by MRI-guided imaging, which guarantees ideal pharmacokinetics, pharmacodynamics, and accurate accumulation of contrast agents [89].
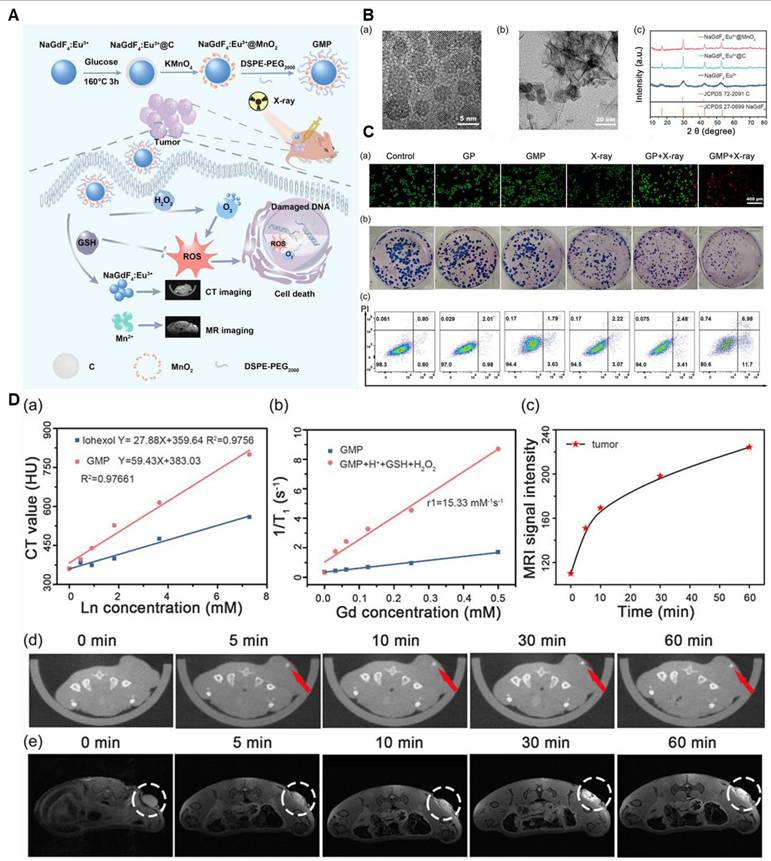
For instance, a review by Cai et al. emphasizes manganese oxide (MON) nanoparticles (NPs) as potentially useful tools for MRI-based theranostics. The study discusses their use as MRI contrast agents for tumor diagnosis and detection as well as how they may improve the effectiveness of chemotherapy and radiation therapy by tackling issues with the tumor microenvironment. The uses of MONs in photothermal and photodynamic therapy are also examined, demonstrating their adaptability in transforming the treatment of cancer. According to the results, MONs are a versatile tool for enhancing cancer treatment and detection [90]. Moreover, researchers have played a significant role in advancing intelligent drug delivery systems based on MnO2. Among these breakthroughs, Song and colleagues detailed the application of MnO2 nanoparticles loaded with Doxorubicin (Dox), modified with hyaluronic acid (HA), and conjugated with mannan (referred to as Man-HA-MnO2 NPs). This innovative approach aimed at targeting 4T1 mouse breast cancer cells, enhancing chemotherapy, and enabling imaging. Moreover, Li and his team successfully reverse radiotherapy-resistant factors for radiotherapy sensitization and MRI (Figure 7). They integrated Gd inside the Mn-complex to attain CT imaging for 3D visualization. It also enhanced the radiotherapeutic efficacy [91].
Tumor hypoxia is addressed by tumor-associated macrophages (TAMs) in hypoxic tumor locations, in conjunction with MnO2 nanoparticles (NPs) that react with hydrogen peroxide (H2O2) to produce oxygen (O2) and control pH. In addition to targeting tumor cells, hyaluronic acid (HA) also transforms anti-inflammatory M2 TAMs into pro-inflammatory M1 macrophages, increasing the effectiveness of nanoparticles and overcoming chemoresistance. Mn2+ ions are released when Man-HA-MnO2 NPs react with H2O2, which enhances tumor imaging and T1/T2-weighted MRI detection capabilities. [92] MRI agents based on hyaluronic acid (HA) present several advantages, including their biocompatibility, ability to target specific tissues or cells, biodegradability, and enhanced retention [93]. However, they have limitations, such as potential issues with relaxivity, stability, synthesis complexity, size constraints, potential immunogenicity, and variability. The choice to employ HA-based MRI agents depends on the precise imaging goals and the meticulous assessment of these pros and cons, tailored to the unique requirements of the given application [94].
(A) Schematic diagram of the MRI/CT guided radiotherapy. (B) TEM (a,b) and XRD of NaGdF4:Eu3+ and NaGdF4:Eu3+@MnO2 nanoparticles. (C) (a) Fluorescence images of HeLa cells with different treatments after the live/death staining by Calcein and PI. PI stained the necrotic cells with red fluorescence and Calcein stained the live cells with green fluorescence. (b) Colony formation assay of HeLa cells under different treatments. (c) Flow cytometric analysis of HeLa cells apoptosis with different treatments. Apoptotic ratio: a sum of the early apoptotic ratio in the Q3 region and the late apoptotic ratio in the Q2 region. The dose of X-ray was 6 Gy. (D) a) In vitro CT values of GMP NP aqueous solutions with different concentrations of Ln (Gd + Eu). b) The longitudinal relativities (r1) of GMP NPs in different solutions. c) The corresponding signal intensity of MR in tumor after intratumor injection of GMP NPs solution at different times. d) In vivo CT images and e) MR images of HeLa-tumor-bearing nude mice at 0, 5, 10, 30, and 60 min after intratumor injecting GMP NPs solution (4 mg mL-1, 20 μL). The circled part was the tumor. Adapted with permission from[91], copyright 2024 Wiley-VCH.
Similarly, Liu and colleagues created a dye-based MnO2 nanoparticle (NP) system for MRI-guided radiation. MnO2 nanoparticles were effectively loaded with the radiosensitizer acridine orange (AO), which was then released under regulated conditions. By increasing DNA damage, the device produced oxygen (O2) inside cells, improving the results of radiation. Furthermore, MnO2 nanoparticles break down in acidic settings, releasing Mn2+ for MRI monitoring. According to the study, mMnO2 presents a suitable platform for the development of composite radiosensitizers for use in radiation treatment [95].
MRI-guided radiotherapy makes Adaptive treatment planning possible, which permits real-time modifications for the best possible radiation delivery. Manganese complexes, especially nanoparticles (NPs), are now essential for MRI-guided radiation therapy because they can efficiently target a variety of malignancies. To investigate their potential for boosting theranostic properties and boosting therapeutic efficacy, more study is required on other Mn-complexes. Hence, for MRI-guided radiotherapy, Mn-based contrast agents provide improved tumor imaging and treatment accuracy. Mn compounds successfully combine diagnostic and therapeutic roles by enhancing MRI contrast, enabling regulated drug delivery, and intensifying oxidative stress through catalytic processes, increasing radiation outcomes with fewer side effects and greater efficacy.
3.7 Mn-based contrast agents for MRI-guided photodynamic therapy
In MRI-guided photodynamic treatment (PDT), manganese-based (Mn-based) contrast agents are becoming more and more popular as dual-purpose materials. Because of the paramagnetic nature of Mn ions, these medicines offer improved T1-weighted MRI, allowing for accurate tumor monitoring and localization. By producing reactive oxygen species (ROS) in response to light activation, they serve as catalytic agents in PDT and efficiently target cancer cells [96]. The stability, biocompatibility, and therapeutic effectiveness of Mn-based medicines are improved when they are combined with nanocarriers or photosensitizers (PS). Furthermore, Mn ions help modulate the tumor microenvironment by interfering with hypoxia, which enhances PDT results. Mn-based contrast agents are useful in advanced cancer theranostics because of their multifunctionality, which seamlessly bridges imaging and treatment by combining diagnostic and therapeutic properties.
Photodynamic therapy (PDT) is a biomedical procedure that employs a photosensitizing substance and particular light wavelengths to specifically eliminate or harm designated cells, including cancerous cells or irregular tissue. PDT entails the introduction of a photosensitizer into the desired region, where it accumulates and is subsequently subjected to light of the suitable wavelength [97]. Through the combination of photosensitizers (PS), oxygen, and light, photodynamic treatment (PDT) produces reactive oxygen species (ROS), which leads to necrosis or apoptosis and necrobiosis. It is extensively used in ophthalmology, dermatology, and cancer treatment. Theranostic properties of PS formulations are improved by advancements, which allow for precision imaging-guided PDT by triggering diagnostic signals in response to stimuli unique to tumors. To provide optimal treatment planning and better results, PDT relies on precise dosimetry and monitoring, taking into account tumor features, PS concentration, location, light distribution, and oxygen levels. Additionally, it involves post-treatment assessment to gauge the response, potential recurrence, and alleviation of symptoms (Figure 8) [99]. This setup effectively suppressed singlet oxygen (1O2) production, resulting in the "off" state for both PDT and MR imaging due to the stable MnO2 shield. Nevertheless, in the acidic tumor microenvironment, the MnO2 case underwent a reaction with H2O2, causing its degradation and activating both MR imaging and photodynamic therapy (PDT) concurrently. The MR imaging demonstrated a longitudinal relaxation rate of 25.31 mM-1 s-1, ensuring a 74% yield of singlet oxygen (1O2) owing to the sufficient generation of oxygen (O2) [99-100].
Though, photodynamic therapy (PDT) offers localized, non-ionizing treatment with reduced radiation risks but faces challenges like limited penetration depth and variable outcomes based on photosensitizers and patient factors. MRI-guided PDT enhances efficacy by refining treatment precision. Combining PDT with other modalities may further improve effectiveness while maintaining minimal invasiveness.
Apart from metal nanoparticles and nanoclusters, scientists nowadays are working on biodegradable theranostic probes for this purpose. Such initiatives may be crucial for designing new-age theranostic probes having multiple activation-induced imaging capabilities.
Therefore, Mn-based contrast agents improve MRI-guided photodynamic therapy by producing reactive oxygen species (ROS), enhancing tumor imaging, and enabling targeted therapy. Their simultaneous role in treatment and imaging, along with the catalytic qualities of Mn, boosts therapeutic efficacy and presents a viable strategy for accurate, non-invasive cancer treatment.
3.8 Mn-based contrast agents for MRI-guided immunotherapy
Manganese-based (Mn-based) contrast agents combine therapeutic advantages with diagnostic imaging to improve MRI-guided immunotherapy. Their paramagnetic characteristics enhance T1-weighted imaging, allowing for accurate tumor monitoring and localization. Mn ions also alter the tumor microenvironment by promoting immune cell infiltration and upsetting hypoxia. Because of their dual purpose, Mn-based medicines can effectively combine immunotherapy with MRI diagnostics, providing a prospective means of enhancing the effectiveness of treatment for immune-related disorders and cancer.
(A) AuNCs@mSiO2@MnO2 nanozyme synthesis process for H2O2-responsive "Off/On" regulation and upgrade of MR imaging and photodynamic therapy. (B) TEM, STEM, high-amplification STEM, and component planning pictures of the synthesized samples. (a-d) AuNCs@mSiO2; (e-h) AuNCs@mSiO2@MnO2; and (I-l) AuNCs@mSiO2 @MnO2 (H2O2). (C) H2O2 -responsive MR, O2, and 1O2 age execution. (a) "Off/on" tweak and upgrade of MR imaging, PDT; (c) T1 - weighted MR imaging; (d) longitudinal MR unwinding bends of samples. (D) In vivo MR imaging and PDT. (a) T1 - T1-weighted MR imaging of mice infused intravenously with the sample. (b) growth volume change bends of various gatherings; (c) body weight change bends of various gatherings. (d) photos of mice in various gatherings; and (e) DAPI and TUNEL staining pictures of MDA-MB-435 growths in various gatherings. Adapted with permission from [99], copyright 2021, the American Chemical Society.
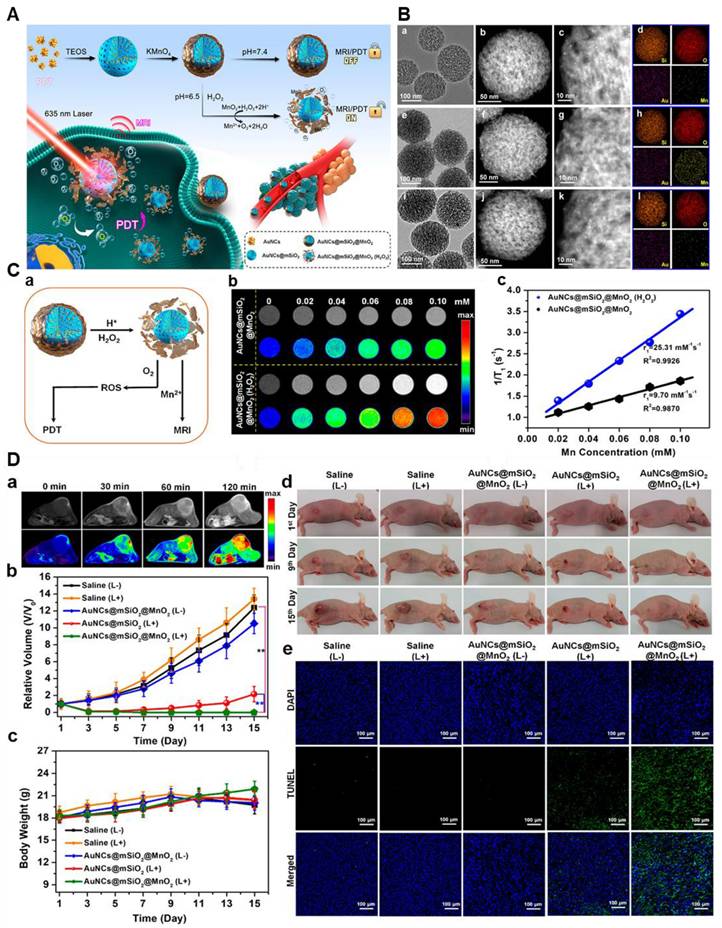
The immunomodulatory qualities of manganese (Mn) are used in manganese-based (Mn-based) tumor immunotherapy to strengthen the body's anti-cancer defenses. Mn ions improve tumor identification and removal by activating immune cells. By delivering immune-stimulating chemicals, Mn-based nanoparticles encourage the generation of cytokines and the entry of immune cells into tumors. Improvements in Mn-based nanoplatforms for imaging-guided methods, immunotherapy, and multimodal synergistic medicines are highlighted by Zhang et al. The study highlights Mn's potential to improve cancer immunotherapy outcomes by addressing its characteristics, therapeutic potential, and obstacles [101].
Cyclic dinucleotides (CDNs) are a class of molecules made up of two nucleotides combined in a cyclic structure. They participate in cellular signaling, specifically in the activation of the STING (stimulator of interferon genes) pathway, which is essential for triggering immune responses, particularly in the context of cancer and viral infections. Cyclic dinucleotides, like cyclic GMP-AMP (cGAMP), are used as agonists to stimulate the STING pathway, which improves immune system activation against tumors and pathogens. This STING pathway is activated by cyclic dinucleotide-manganese particles (CDN-Mn), which also increase immunological defense by producing type I interferon and cytokines. Cyclic dinucleotides are delivered to immunological and tumor cells by CDN-Mn particles, which enhance immune cell infiltration and initiate systemic reactions. Using Mn²⁺ and CDN STING agonists, Sun et al. created a metalloimmunotherapy prototype that produced the self-assembling nanoparticle CDN-Mn²⁺ (CMP). Strong anti-tumor immunity and great therapeutic efficacy with modest STING agonist dosages were shown by CMP in animal models, enhancing delivery and efficacy [102]. In the tumor microenvironment, the cGAS-STING pathway is activated by the ATP-responsive manganese-based bacterial material (E. coli@PDMC-PEG). Elevated ATP levels cause the material to break down, producing Mn2+ ions that increase the sensitivity of the cGAS enzyme to bacterial extracellular DNA, thereby activating the pathway in concert. According to in vivo research, E. coli@PDMC-PEG and VNP20009@PDMC-PEG successfully prevented the formation of liver cancer in rabbits and melanoma in mice, indicating their potential as therapeutic agents [103]. Subsequently, A manganese-based nanoplatform (MPCZ NPs) that triggers the cGAS-STING pathway was created by He and his associates. When subjected to a NIR laser and elevated endogenous GSH levels, MPCZ NPs, which coat ZPP on hMnO2 NPs and load PDA with NH4HCO3, release Mn²⁺ in tumor cells. This increases innate immunity by inducing the creation of ROS and strengthening the STING pathway. MPCZ NPs successfully suppressed tumor growth in the 4T1 tumor-bearing mouse model, demonstrating their potential to improve anticancer immunotherapy [104]. Consequently, Li et al. conjugated biocompatible cationic biopolymers with cGAMP to generate nanocomplexes that were affixed to APC-targeting microbubbles (MBs), thereby developing a platform for ultrasound (US)-guided cancer immunotherapy. By delivering cGAMP into the cytosol, sonoporation primed antigen-specific T cells by triggering proinflammatory and cGAS-STING pathways [105]. Simultaneously, Cai et al. developed a strategy using a bispecific antibody (BsAb) with a manganese oxide-doped silicate nanosystem to deliver minicircle DNA. It activated the cGAS-STING pathway, redirected host lymphoid cells to target cancer, and achieved a high rate of antibody discharge over five days, illustrating manganese's indirect role in supporting immune functions for cancer therapy [106].
Moreover, manganese-based nanoparticles are regarded as biocompatible and exhibit diverse applications in the field of nanomedicine. Sun et al. used such a biodegradable Mn-based theranostic agent for MRI-guided dual immuno-chemodynamic combination therapy. In reality, they developed MnO nanoparticles (NPs) coated with hollow mesoporous silica and further modified them with the tumor-homing peptide iRGD (CRGDKGPD). The resulting NPs, termed MnO@mSiO2-iRGD NPs, were utilized in an MRI-guided tumor immuno-chemodynamic combinatory therapy. These NPs offered multiple benefits, including activation of the cGAS-STING pathway for immunotherapy, upregulation of reactive oxygen species through Fenton-like reactions, and T1-weighted MRI capabilities [106]. Additionally, Ding et al. synthesized Manganese oxide nanospikes (MnOx NSs) which are responsible for the tumor microenvironment (TME)-responsive nano adjuvants and immunogenic cell death (ICD) drugs for cancer nanovaccine immunotherapy. With high antigen-loading capacity, they combine ICD-based chemodynamic therapy, ferroptosis induction, and antigen stimulation, offering synergistic immunopotentiation, dual-mode imaging (MRI/photoacoustic), and effective inhibition of tumor growth and metastasis (Figure 9) [107].
Manganese is essential for immunological function, but too much of it can be harmful. If properly optimized, MRI-guided immunotherapy presents a viable clinical alternative to maintaining a balanced intake. Nevertheless, Mn-based contrast agents improve tumor targeting and immune response, which improves MRI-guided immunotherapy. They are potential prospects for clinical cancer treatment applications because of their capacity to strike a balance between safety and efficacy.
(A) Illustration of MnOx-OVA/tumor cell fragment (TF) nanovaccines for MR/PA dual-mode imaging-induced cancer immunotherapy. (B) In vivo MR/PA dual-mode imaging. (C) Relative tumor-growth curves of distant tumors. Adapted with permission from[107], copyright 2020, Wiley-VCH GmbH.
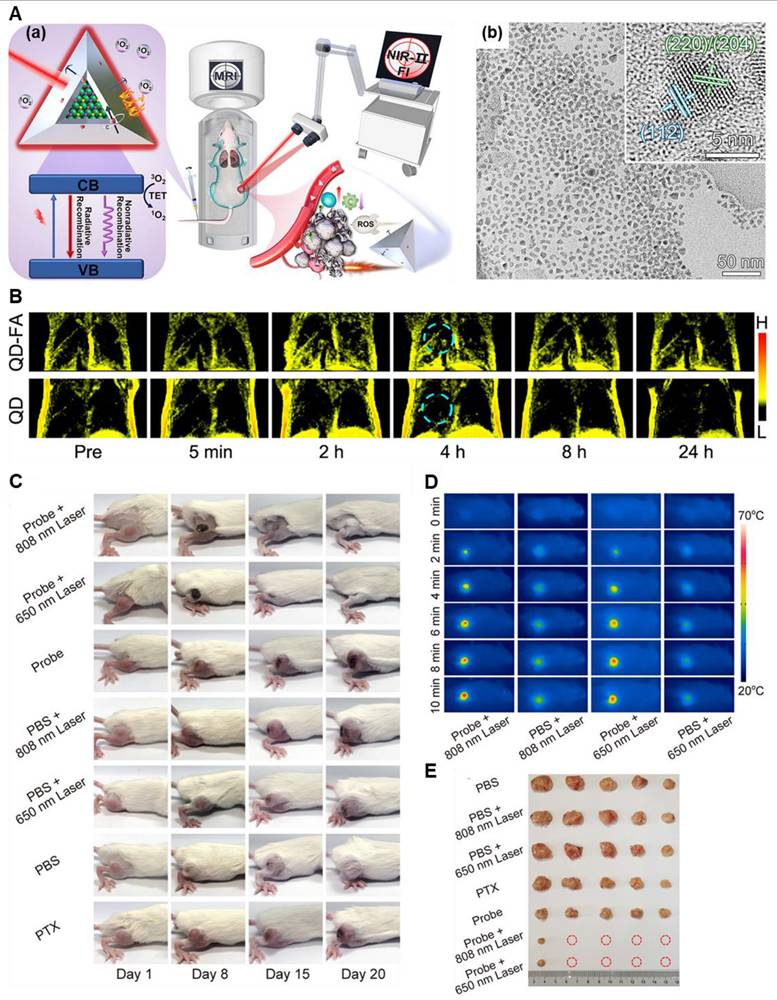
(A) (a) Scheme of a CISe@ZnS: Mn QD) manufactured having a lot of functionality. (b) structure obtained by the TEM image of the quantum dot. (B) lung metastasis tumor detection through T1-weighted MR imaging after the IV of the QD-FA probes. In vivo, NIR-II FI of hypodermal tumors was taken at variable time points after the IV injection. (C) Sample images of tumor-bearing mice undergoing the various treatments as shown. (D) Thermal images of mice having 4T1 tumors injected with PBS or the QD-FA probe were recorded at different irradiation times with 808 nm laser (1.0 W·cm-2, 10 min) and 650 nm laser (1.0 W·cm-2, 10 min). (E) Images of 4T1 tumors from each group were collected 20 days after treatment. Adapted with permission from [112], copyright 2022, the American Chemical Society.
3.9. Mn-based contrast agents for MRI-guided combinatory therapy
Multimodal/synergistic/combinatory therapy has become a dominant choice owing to its particular and targeted approach toward the therapeutic modality [108,109]. In a combinatory setup, two or more different therapeutic regimens must be introduced simultaneously for higher efficacy. Often a part of these therapeutic modalities takes quick action against the disease, whereas the other modalities support the previous one by maintaining a low and continuous therapeutic profile. For instance, photothermal therapy (PTT) is a quick and effective therapy against tumors, while other therapies like chemodynamic therapy or drug delivery act as supportive systems with PTT in a combinatory setup [73,110]. There are a lot of combinatory therapies accessible. Herein, we highlight a few examples to illustrate their diverse applications. To make Mn-based combinatory theranostic systems, scientists have followed several strategies. Initially, they started with complex nanosystems and nanocomposites containing Mn ions to achieve multimodal theranostics. For example, Zhu and colleagues have developed a nanoplatform for cancer theranostics comprising manganese-loaded Gox (Glucose oxidase), paclitaxel (PTX), and a near-infrared (NIR) fluorescent dye. This nanoplatform exhibited pH-dependent behavior, resulting in the release of both manganese ions and therapeutic payloads specifically within tumor cells. Through in vitro assessments and cellular experiments, it was observed that NanoMn-GOx-PTX catalyzed the conversion of glucose into reactive oxygen species (ROS) through a cascade Fenton-like reaction, concurrently releasing PTX. The combined effects of glucose depletion, ROS generation, and the therapeutic action of PTX synergistically induced increased cytotoxicity and apoptosis in 4T1 cancer cells [111].
Notably, when MRI has been used with other imaging modalities, the efficacy of the theranostic regimen is enhanced. In very recent work, Li et al. developed such a theranostic system consisting of CuInSe2@ZnS: Mn quantum dots (QDs). This probe can accurately detect the localization of small metastases by virtue of MRI/ NIR-II dual-modal imaging techniques and ablate the tumor by combined PDT/PTT/IT modalities (Figure 10) [112]. This multimodal approach increased the detection efficiency up to 31.2 % and prevented tumor regrowth in 80% of mice. Such works are crucial in determining the tumor proximities and they will provide safe and non-recurring ablation of the tumor growth under the imaging guidance.
A potent cancer treatment strategy is provided by immunotherapy in conjunction with MRI-guided sonodynamic therapy (SDT). With the use of targeted SDT, which uses ultrasound to activate sonosensitizers that produce ROS and cause tumor cell death, precise tumor localization, and monitoring are made possible by MRI. Concurrently, SDT increases the immunogenicity of the tumor, increasing its vulnerability to an immune response. This dual approach, when combined with immunotherapy, enhances the body's immune response against cancer, improving therapeutic outcomes by precisely targeting the main tumor as well as any metastases. For instance, Tian et al. created a phenolic nanoadjuvant through metal-phenolic coordination, which allowed the sonosensitizer polymer (PEG-b-IR), GSH inhibitor (sabutoclax), Mn2+, and TME acidity-sensitive phenolic polymer (PEG-b-Pho) to self-assemble. The activation of the cGAS-STING pathway was accelerated by the combined action of Mn2+ and SDT-mediated ICD effect, which greatly increased the maturation of DCs (dendritic cells). Moreover, this phenolic nano adjuvant significantly increases the susceptibility of the tumors to PD-L1 checkpoint blockade immunotherapy, which effectively prevents lung metastasis and delays the growth of distant tumors [113]. Consequently, the therapeutic constraint of inadequate antitumor immunity for improved cancer immunotherapy may be addressed by this approach.
By precisely locating tumors and continuously monitoring therapy, MRI-guided chemotherapy, and immunotherapy offer a highly tailored approach to cancer treatment. Cancer cells are destroyed by cytotoxic chemicals delivered by chemotherapy, and tumors are better recognized and destroyed by the immune system when immunotherapy is added. When drugs are delivered accurately with MRI guidance, the likelihood of side effects is reduced and the effectiveness of treatment is increased. This combined method offers a synergistic strategy for more precisely treating metastases as well as locally located tumors by stimulating the immune system in addition to actively attacking the tumor. For instance, small-sized telodendrimer and Mn2+-based nanodriver (PLHM) were designed that efficiently target lymph nodes via blood circulation and show tumor-preventive effects at low Mn2+ doses (3.7 mg kg-1). However, through triggering in vivo innate immune responses, the PLHM nanodriver also shows apparent anticancer effects in mice bearing GBM. Because cytoplasmic DNA and Mn2+ synergistically potentiate the efficacy of the STING pathway, PLHM and doxorubicin nanoparticles (PLHM-DOX NPs) provide superior tumor growth inhibition in mice bearing GBM. These results show that PLHM-DOX NPs can successfully induce innate immunity, boost maturation of dendritic cells, and coordinate the cascaded infiltration of CD8 cytotoxic T lymphocytes into low-immunogenicity glioblastomas. By triggering the STING pathway, these nanodivers chelated with Mn2+ exhibit encouraging potential for tumor prevention and anticancer effects on glioblastoma [114].
Luo et al. created a pH-responsive nanomodulator by co-loading MnO2, CaCO3, and curcumin (CU), a Ca2+ enhancer, into nanoparticles that were covered in a cancer cell membrane. The goal of this nanoplatform was to use ion fluctuation to reprogram the tumor microenvironment (TME) and provide an anticancer treatment. The resultant nanoplatform, known as CM NPs, could produce Ca2+ and release CU, raising Ca2+ levels and encouraging ROS formation in the mitochondria and endoplasmic reticulum, ultimately leading to immunogenic cell death. They could also neutralize protons by breaking down CaCO3 and reducing cellular acidity. To alleviate hypoxia and improve cGAS sensitivity, Mn2+ may break down endogenous H2O2 into O2, triggering the cGAS-STING signaling pathway [115]. A biomimetic mineralization technique is suggested to strengthen RT-induced systemic antitumor immune responses by easily synthesizing MnO2 nanoparticles with high anti-programmed death ligand 1 (αPDL1) encapsulation efficiency (αPDL1@MnO2). By reprogramming the immunosuppressive tumor microenvironment (TME) and overcoming hypoxia-induced radio-resistance, therapeutic nanoplatforms-mediated RT can successfully elicit ICD and considerably increase tumor cell death. Additionally, under an acidic tumor pH, the produced Mn2+ ions from αPDL1@MnO2 can activate the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway, which helps to promote the maturation of dendritic cells (DCs). When αPDL1 was released from αPDL1@MnO2 nanoparticles, it stimulates systemic antitumor responses and intratumoral infiltration of cytotoxic T lymphocytes (CTLs), which has a potent abscopal impact that effectively prevents tumor metastasis. Overall, the biomineralized MnO2-based nanoplatforms are promising for improved RT immunotherapy because they provide a straightforward method for TME regulation and immune activation [116]. The production of an immunostimulatory MOF (ISAMn-MOF) based on manganese ion (Mn2+) for cancer metalloimmunotherapy was suggested using a facile and environmentally friendly technique. In bone marrow-derived dendritic cells (BMDCs), ISAMn-MOF dramatically accelerates the activation of signaling pathways and genes linked to cyclic GMP-AMP synthase-stimulator of interferon (cGAS-STING). Compared to BMDCs treated with comparable MnCl2, ISAMn-MOF-treated cells release 4-fold more type I interferon and 2- to 16-fold more proinflammatory cytokines. Either by itself or in conjunction with immune checkpoint antibodies, ISAMn-MOF dramatically inhibits tumor growth and metastasis while extending the longevity of mice. Mechanistic investigations reveal that the administration of ISAMn-MOF promotes the infiltration of immune cells that stimulate tumor growth and lymphoid organs. This research sheds light on how to create bioactive MOFs for more effective cancer metalloimmunotherapy [117]. In a similar manner, Zhou et al. reported on manganese-enriched zinc peroxide nanoparticles (MONPs) that are responsive to the tumor microenvironment (TME) for synergistic cancer immunotherapy. STING-stimulating cancer cells undergo immunogenic death (ICD) and are activated. When MONPs come into contact with acidic tumor tissue, they specifically dissociate and produce •OH in situ, which contributes to the ICD mechanism. Furthermore, Mn2+ triggered the STING and jointly stimulated the release of inflammatory cytokines and type I interferon to trigger certain T-cell responses. Concurrently, MONPs reduced Tregs and polarised M2 macrophages to the M1 type, which released a cascade adaptive immune response, relieving the immunosuppression of TME. MONPs have shown significantly greater effectiveness in halting tumor growth and averting lung metastases when combined with the anti-PD-1 antibody [117]. This research indicates a promising approach for cancer immunotherapy by demonstrating the viability of using functional nanoparticles to enhance STING innate stimulation.
Nonetheless, potential improvements in the treatment of cancer can be achieved with Mn-based contrast agents for MRI-guided combinatory therapy. They are perfect for multimodal therapies because they can improve drug transport efficiency, catalyse therapeutic processes, and increase imaging precision, combining therapeutic and diagnostic capabilities for more effective, tailored cancer treatments.
4. Challenges of Mn-based CAs and their plausible remedies
Due to its unique magnetic properties and biocompatibility, iron (Fe) is a promising candidate for MRI contrast agents in cancer theranostics. It enhances imaging contrast, improving tumor visualization for early diagnosis and treatment monitoring. Fe-based agents can also be tailored to target specific cancer celIn MRI-guided photodynamic treatment (PDT), manganese-based (Mn-based) contrast agents are becoming more and more popular as dual-purpose materials. Because of the paramagnetic naturls, allowing for integrated diagnosis and therapy [118] . While iron (Fe) has notable advantages as an MRI contrast agent, there are also disadvantages compared to manganese (Mn)-based contrast agents in cancer theranostics [119]. One major drawback of Fe is its limited relaxivity, which can result in less effective imaging at lower concentrations compared to Mn. Additionally, Fe-based agents may lead to the formation of aggregates or precipitation, which could affect their stability and distribution within the body. Conversely, Manganese has a higher atomic number, contributing to better signal intensity and contrast enhancement. Furthermore, Mn-based agents can offer more efficient cellular uptake and targeting capabilities, potentially improving diagnostic precision [120].
But still, some drawbacks are using Mn-based contrast agents such as the potential toxicity of excessive manganese accumulation, which can have a negative impact on the immune system and general health, which is one of the difficulties faced by Mn-based contrast agents (CAs). For extended in vivo use, it is also essential to guarantee their stability and biocompatibility because prolonged exposure to high manganese concentrations may result in undesirable side effects [121]. Achieving the ideal balance between safety and therapeutic efficacy—especially when paired with other therapies like immunotherapy-remains a persistent obstacle for the clinical application of Mn-based CAs. Herein, we aim to explore such clinical challenges and thereby find a rationale to remediate them.
Wang and the team synthesized an Mn-chelate composed of O-carboxymethyl chitosan derivatives. This hybrid Mn-chelate-based MRI contrast agent shows significant enhancement in the image contrast in the case of the kidney and liver of Sprague Dawley (SD) rats. The in vitro and in vivo studies suggest that this compound is biocompatible and can be completely excreted from the SD rats within ten days of administration without any significant accumulation or toxicity [122].
Gene mutations that have significant pathogenic roles in Parkinsonism and the control of manganese transport and metabolism are present in these diseases. In manganese-related neurotoxicity, liver function is also crucial, and subclinical liver dysfunction may raise the risk of Parkinson's disease [123]. Since mitochondria are an essential organelle for the synthesis of ATP, they are vulnerable to toxicity from Mn. Reduced energy production results from abnormal mitochondrial function caused by excess Mn. Gunter and associates demonstrated that ATP synthesis in mitochondria isolated from rat liver, heart, and brain could be inhibited by elevated Mn levels [124]. Elevated intracellular Mn causes oxidative stress, mitochondrial failure, autophagy dysregulation, apoptosis, and protein dyshomeostasis, all of which impair regular cellular function and ultimately change neurotransmission. These lead to a range of neurological illnesses by disrupting regular neural function [125]. Schrantz et al. found that Mn treatment caused dose- and time-dependent cell death in human B lymphocytes, activating the apoptotic marker proteins caspase-1 and 3 [126]. Neurotransmission may change dramatically as a result of Mn exposure. Zhang and colleagues observed a drop in dopaminergic (DAergic) neurons in the substantia par compacta (SNpc) and tyrosine hydroxylase (TH) protein in the rats that got Mn injection. This was linked to a decrease in dopamine (DA) and the D1 DA receptor [127].
Besides, folate-PEG-modified dihydroartemisinin (DHA) loaded manganese-doped mesoporous silica nanoparticles have been developed by Fei and his team to combat MRI-guided ferroptotic cancer therapy. The folate receptor herein acts as the binding molecule to the GSH-overexpressed cancer cells and thus produces enhanced MRI contrast [128]. Hence, it can be said that scientists are working on microenvironment-based targeting moieties to prolong and enhance the contrast of the MRI images besides their clearance. It is observed that the designing approach may be attributed to the targeting efficiency of the contrast agents. In reality, Gallo et al. coated the MnO NPs with PEG and cRGDfK cyclopeptide and varied the chain length of the PEG to observe the targeting efficiency in tumors. It is found that the MnO NPs having a higher molecular weight (i.e., chain length) are more capable of targeting the tumor cells [129].
With the use of Mn2+ to reduce hypoxia and increase ROS production, Zhou's group created manganese-based theranostic nanoparticles for MRI-guided breast cancer treatment. Using glucose oxidase and manganese oxide, Korupalli et al. developed a multifunctional MnOx NSs@BSA-IR780-GOx nanocomposite that uses light-responsive dual-modal treatment to produce ROS in the tumor microenvironment [129]. Such studies will surely unlock new avenues in the domain of MRI-imaging-guided theranostic modules.
Manganese-based MRI contrast agents in particular have problems with renal clearance and may be toxic to the liver and spleen due to Mn2+. Chevallier et al. addressed this by wrapping MnO NPs in a PEG-phosphonate dendron, which improved renal excretion and increased imaging potential [130]. Currently, Mn-based MRI probes have been made of different biopolymers and other biomimetic systems having better renal clearance. However, Mn-based coordination complexes show enormous potential in this domain. We hope these coordination complexes and small molecules may be used in clinics in the near future.
The dual aptitudes of MnCAs in diagnostic and therapy make them highly promising for use in cancer theranostic applications. MnCAs are naturally eliminated by the body and show less toxicity than gadolinium-based drugs. By reducing T1 relaxation time and enabling distinct tumor visualization, they improve MRI imaging [131]. Targeting ligands, including peptides or antibodies, can be functionalized to MnCAs to enable selective accumulation in cancer cells. They are more effective in cancer theranostics when coated with biomimetic nanoparticles with cell membranes. Through homologous binding or immunological interactions, MnCAs can be cloaked in membranes from cancer or immune cells to improve tumor targeting, prolong circulation, and avoid immune detection [132]. On Mn-based contrast agents, cell membrane coating promotes selective accumulation in tumor tissues, lowers toxicity, and increases biocompatibility. By producing ROS, this improves MRI imaging and therapeutic potential for the diagnosis and treatment of cancer.
By promoting osteoblast activity, collagen synthesis, and calcium absorption, manganese (Mn) aids in bone development. Mn can repair damaged bone tissue and deliver therapeutic drugs by being included in scaffolds or nanoparticles used in bone cancer treatment. Materials containing Mn also improve imaging to detect cancer and track its progress throughout therapy. For instance, a multifunctional nanoplatform based on PAH-MnO2@BP nanosheets loaded on thermosensitive gel poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide) (PDLLA-PEG-PDLLA, PLEL) for in situ osteosarcoma synergy therapy (photothermal/photodynamic/chemotherapy, PTT/PDT/CHT), was developed by Fu et al. [133]. By turning excess hydrogen peroxide into oxygen, manganese dioxide on BP NSs improves photodynamic treatment (PDT) and decreases tumor hypoxia. The photothermal effect of BP NSs also controls the release of doxorubicin. The hydrogel composite optimizes tumor efficacy while reducing relapse by integrating photothermal therapy (PTT), photodynamic therapy (PDT), and chemotherapy (CHT). This strategy has the potential to treat osteosarcoma and provide individualized cancer care.
Moreover, since the late 1980s, neurotoxicity has been a great concern for mankind. It is the damage caused by toxic substances or conditions to the nervous system, potentially leading to temporary or permanent impairment of neural function. Neurotoxicity can arise from various sources such as exposure to heavy metals (e.g., manganese, lead, mercury), pesticides, solvents, industrial chemicals overdose, or chronic use of certain drugs, etc. Paramagnetic properties of the Manganese make manganese-enhanced MRI (MEMRI) a useful tool for neuroimaging since it makes it possible to observe the activity and structure of neurons. However, because of its ability to build up in the brain and disrupt regular neuronal function, manganese (Mn) neurotoxicity is still a serious problem. The severity of Mn toxicity depends on the dose, mode of administration, and species-specific sensitivity. It can cause severe neurological effects, such as motor and mental disorders. MEMRI offers valuable insights into neural function, but minimizing neurotoxic risks requires careful consideration of dosing and species-specific differences. Researchers and clinicians should balance its benefits against potential adverse effects by following safety guidelines and using the lowest effective doses [134].
Nonetheless, it takes several steps to translate MnCAs for cancer theranostic applications into clinical use. To begin with, comprehensive preclinical research has to validate safety, biocompatibility, and therapeutic and imaging efficacy. To reduce toxicity, MnCA formulations must be optimized to target certain tumors and ensure effective renal clearance [135]. Evaluation of patient outcomes, treatment efficacy, diagnostic accuracy, and cooperation between researchers, physicians, and industry should be the main goals of clinical studies for translating MnCAs from the lab to the clinic.
5. Summary
Manganese (Mn)-based MRI contrast agents have made significant contributions to cancer therapy by offering enhanced imaging capabilities, superior biocompatibility, and targeted delivery to cancerous tissues. Their strong paramagnetic properties improve T1-weighted imaging, enabling better differentiation between healthy and diseased tissues, while their natural role as an essential trace element reduces the risks of toxicity. Mn-based agents also demonstrate a unique ability to target specific cellular pathways, such as calcium transport, facilitating precise tumor localization and monitoring of therapeutic responses. However, some major concerns regarding manganese ions need to be addressed to make them efficient MRI contrast agents. First, the neurotoxicity of Mn2+ should be controlled by encapsulating them into suitable hosts. Manganese complexes and chelates can be formed with organic and inorganic complexes for this purpose. Second, the use of biomimetic and biocompatible probes must be explored. Numerous research groups are currently working in this field, making manganese-based probes a more feasible alternative. Third, pharmacokinetic/pharmacodynamic profiles should be investigated before deploying such theranostic agents. The accumulation of manganese in the brain and other organs could worsen patients' health conditions. Therefore, proper excretion profiles of these agents must be determined well in advance. Last, but not least, more work is needed to achieve better T1 relaxation in Mn-based contrast agents. So far, Mn-based agents cannot qualify as contrast agents like Gd due to the lower number of spin quantum states available in the Mn ions. However, the future research could also focus on optimizing Mn-chelated complexes for greater stability and specificity, developing multimodal Mn-based agents for combined diagnostic and therapeutic (theranostic) applications, and exploring nanoformulations to improve biodistribution and controlled release. This will not only position Mn-based contrast agents as an alternative to commercial Gd-based agents but also potentially make them superior in many aspects.
Acknowledgements
This work was supported by to the “Chunhui Plan” cooperative scientific research project of the Ministry of Education, China (HZKY20220312), Special Foundation for General Basic Research Program of Shenzhen (JCYJ20210324132816039, JCYJ20240813105122030), General project of Guangdong Natural Science Foundation (2022A1515011781), and Shenzhen Key Laboratory of Advanced Functional Carbon Materials Research and Comprehensive Application (ZDSYS20220527171407017).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dewson G, Eichhorn PJA, Komander D. Deubiquitinases in cancer. Nat Rev Cancer. 2023;23:842-62
2. Abdel-Wahab M, Giammarile F, Carrara M. et al. Radiotherapy and theranostics: a Lancet Oncology Commission. Lancet Oncol. 2024;25:e545-80
3. Pei Z, Lei H, Cheng L. Bioactive inorganic nanomaterials for cancer theranostics. Chem Soc Rev. 2023;52:2031-81
4. Xu R, Zhang S, Wang P. et al. Nanozyme-based strategies for efficient theranostics of brain diseases. Coord Chem Rev. 2024;501:215519
5. Roy S, Hasan I, Guo B. Recent advances in nanoparticle-mediated antibacterial applications. Coord Chem Rev. 2023;482:215075
6. Chabanova E, Logager V, Moller JM, Dekker H, Barentsz J, Thomsen HS. Imaging Liver Metastases with a New Oral Manganese-Based Contrast Agent. Acad Radiol. 2006;13:827-32
7. Sterenczak KA, Meier M, Glage S. et al. Longitudinal MRI contrast enhanced monitoring of early tumour development with manganese chloride (MnCl2) and superparamagnetic iron oxide nanoparticles (SPIOs) in a CT1258 based in vivo model of prostate cancer. BMC Cancer. 2012;12:1-11
8. Rainu SK, Ramachandran RG, Parameswaran S, Krishnakumar S, Singh N. Advancements in Intraoperative Near-Infrared Fluorescence Imaging for Accurate Tumor Resection: A Promising Technique for Improved Surgical Outcomes and Patient Survival. ACS Biomater Sci Eng. 2023;9:5504-26
9. Stabile A, Giganti F, Rosenkrantz AB. et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol. 2019;17:41-61
10. Cabella C, Crich SG, Corpillo D. et al. Cellular labeling with Gd(III) chelates: only high thermodynamic stabilities prevent the cells acting as 'sponges' of Gd3+ ions. Contrast Media Mol Imaging. 2006;1:23-9
11. Dutta S, Baruah S, Dutta P. Gadolinium Dilemma: Navigate Water Contamination in the Face of Indispensable Medical Advancements. LNNS. 2024;1022:489-501
12. Perazella MA. Gadolinium-Contrast Toxicity in Patients with Kidney Disease: Nephrotoxicity and Nephrogenic Systemic Fibrosis. Curr. Drug Saf. 2008;3:67-75
13. -Gancedo V, Geraldes CFGC. Rational Design of Magnetic Nanoparticles as T1-T2 Dual-Mode MRI Contrast Agents. Molecules. 2024;29:1352
14. Ashoor M, Khorshidi A. Improving signal-to-noise ratio by maximal convolution of longitudinal and transverse magnetization components in MRI: application to the breast cancer detection. Med Biol Eng Comput. 2024;62:941-954
15. Sun Y, Cao D, Pillai JJ. et al. Rapid imaging of intravenous gadolinium-based contrast agent (GBCA) entering ventricular cerebrospinal fluid (CSF) through the choroid plexus in healthy human subjects. Fluids Barriers CNS. 2024;21:1-15
16. He M, Wang M, Xu T. et al. Reactive oxygen species-powered cancer immunotherapy: Current status and challenges. J Control Release. 2023;356:623-48
17. Gallo J, Alam IS, Lavdas I, Wylezinska-Arridge M, Aboagye EO, Long NJ. RGD-targeted MnO nanoparticles as T1 contrast agents for cancer imaging - the effect of PEG length in vivo. J Mater Chem B. 2014;2:868-76
18. Li B, Gu Z, Kurniawan N. et al. Manganese-Based Layered Double Hydroxide Nanoparticles as a T1-MRI Contrast Agent with Ultrasensitive pH Response and High Relaxivity. Adv Mater. 2017;29:1700373
19. Huang G, Zhang KL, Chen S. et al. Manganese-iron layered double hydroxide: a theranostic nanoplatform with pH-responsive MRI contrast enhancement and drug release. J Mater Chem B. 2017;5:3629-33
20. Chen Y, Yin Q, Ji X. et al. Manganese oxide-based multifunctionalized mesoporous silica nanoparticles for pH-responsive MRI, ultrasonography and circumvention of MDR in cancer cells. Biomaterials. 2012;33:7126-37
21. Lu C, Dong P, Pi L. et al. Hydroxyl-PEG-Phosphonic Acid-Stabilized Superparamagnetic Manganese Oxide-Doped Iron Oxide Nanoparticles with Synergistic Effects for Dual-Mode MR Imaging. Langmuir. 2019;35:9474-82
22. Brito B, Price TW, Gallo J, Bañobre-López M, Stasiuk GJ. Smart magnetic resonance imaging-based theranostics for cancer. Theranostics. 2021;11:8706
23. Gao L, Yu J, Liu Y. et al. Tumor-penetrating Peptide Conjugated and Doxorubicin Loaded T1-T2 Dual Mode MRI Contrast Agents Nanoparticles for Tumor Theranostics. Theranostics. 2018;8:92
24. Li J, Wu C, Hou P, Zhang M, Xu K. One-pot preparation of hydrophilic manganese oxide nanoparticles as T1 nano-contrast agent for molecular magnetic resonance imaging of renal carcinoma in vitro and in vivo. Biosens Bioelectron. 2018;102:1-8
25. Terreno E, Sanino A, Carrera C. et al. Determination of water permeability of paramagnetic liposomes of interest in MRI field. J Inorg Biochem. 2008;102:1112-9
26. Guillet-Nicolas R, Laprise-Pelletier M, Nair MM. et al. Manganese-impregnated mesoporous silica nanoparticles for signal enhancement in MRI cell labelling studies. Nanoscale. 2013;5:11499-511
27. Gallo J, Alam IS, Lavdas I, Wylezinska-Arridge M, Aboagye EO, Long NJ. RGD-targeted MnO nanoparticles as T 1 contrast agents for cancer imaging - the effect of PEG length in vivo. J Mater Chem B. 2014;2:868-76
28. Wei R, Liu K, Zhang K, Fan Y, Lin H, Gao J. Zwitterion-Coated Ultrasmall MnO Nanoparticles Enable Highly Sensitive T1-Weighted Contrast-Enhanced Brain Imaging. ACS Appl Mater Interfaces. 2022;14:3784-91
29. Liu K, Liu C, Xia J. The r1 relaxivity and T1 imaging properties of dendrimer-based manganese and gadolinium chelators in magnetic resonance imaging. Front Bioeng Biotechnol. 2022;10:1004414
30. Roy S, Gu J, Xia W, Mi C, Guo B. Advancements in manganese complex-based MRI agents: Innovations, design strategies, and future directions. Drug Discov Today. 2024;29:104101
31. Lv M, Chen M, Zhang R. et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966-79
32. Fan H, Guo Z. Tumor microenvironment-responsive manganese-based nanomaterials for cancer treatment. Coord Chem Rev. 2023;480:215027
33. Zhao Z, Dong S, Liu Y. et al. Tumor Microenvironment-Activable Manganese-Boosted Catalytic Immunotherapy Combined with PD-1 Checkpoint Blockade. ACS Nano. 2022;16:20400-18
34. Yang Y, Wu H, Liu B, Liu Z. Tumor microenvironment-responsive dynamic inorganic nanoassemblies for cancer imaging and treatment. Adv Drug Deliv Rev. 2021;179:114004
35. Cao Y, Li Y, Ren C, Yang C, Hao R, Mu T. Manganese-based nanomaterials promote synergistic photo-immunotherapy: green synthesis, underlying mechanisms, and multiple applications. J Mater Chem B. 2024;12:4097-117
36. Nofiele JT, Czarnota GJ, Cheng HLM. Noninvasive manganese-enhanced magnetic resonance imaging for early detection of breast cancer metastatic potential. Mol Imaging. 2014 p:13
37. Wu M, Liao Y, Guo D. et al. Manganese-based nanomaterials in diagnostics and chemodynamic therapy of cancers: new development. RSC Adv. 2024;14:14722-41
38. Wang J, Wang H, Ramsay IA. et al. Manganese-Based Contrast Agents for Magnetic Resonance Imaging of Liver Tumors: Structure-Activity Relationships and Lead Candidate Evaluation. J Med Chem. 2018;61:8811-24
39. Qian X, Han X, Yu L, Xu T, Chen Y. Manganese-Based Functional Nanoplatforms: Nanosynthetic Construction, Physiochemical Property, and Theranostic Applicability. Adv Funct Mater. 2020;30:1907066
40. Sun X, Zhou X, Shi X. et al. Strategies for the development of metalloimmunotherapies. Nat Biomed Eng. 2024;8:1073-91
41. Deng Z, Xi M, Zhang C. et al. Biomineralized MnO2 Nanoplatforms Mediated Delivery of Immune Checkpoint Inhibitors with STING Pathway Activation to Potentiate Cancer Radio-Immunotherapy. ACS Nano. 2023;17:4495-4506
42. Huang P, Tang Q, Li M. et al. Manganese-derived biomaterials for tumor diagnosis and therapy. J Nanobiotechnology. 2024;22:1-33
43. Choi KY, Liu G, Lee S, Chen X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nanoscale. 2012;4:330-342
44. Pant K, Sedláček O, Nadar RA. et al. Radiolabelled Polymeric Materials for Imaging and Treatment of Cancer: Quo Vadis? Adv Healthc Mater. 2017;6:1601115
45. Guo Y, Wang Z, Shi X, Shen M, Engi S. Engineered cancer cell membranes: An emerging agent for efficient cancer theranostics. Exploration. 2022;2:20210171
46. Zhang Y, Yang H, Daohe W. et al. Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration. 2021;1:20210115
47. Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064-1079
48. Indoria S, Singh V, Hsieh M-F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int J Pharm. 2020;582:119314
49. Peng H, Liu X, Wang G. et al. Polymeric multifunctional nanomaterials for theranostics. J Mater Chem B. 2015;3:6856
50. Barhoum A, Altintas Z, Devi KSS, Forster RJ. Electrochemiluminescence biosensors for detection of cancer biomarkers in biofluids: Principles, opportunities, and challenges. Nano Today. 2023;50:101874
51. Chen Q, Wen J, Li H, Xu Y, Liu F, Sun S. Recent advances in different modal imaging-guided photothermal therapy. Biomaterials. 2016;106:144-66
52. Li X, Yue R, Guan G, Zhang C, Zhou Y, Song G. Recent development of pH-responsive theranostic nanoplatforms for magnetic resonance imaging-guided cancer therapy. Exploration. 2023;3:20220002
53. Jiang S, Li X, Zhang F. et al. Manganese Dioxide-Based Nanocarrier Delivers Paclitaxel to Enhance Chemotherapy against Orthotopic Glioma through Hypoxia Relief. Small Methods. 2022;6:2101531
54. Sui X, Chen R, Wang Z. et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838
55. Wu CP, Hsieh CH, Wu YS. The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol Pharm. 2011;8:1996-2011
56. Jung W, Asaduddin M, Yoo D. et al. Noninvasive ROS imaging and drug delivery monitoring in the tumor microenvironment. Biomaterials. 2024;310:122633
57. Zhang J, Ning L, Huang J, Zhang C, Pu K. Activatable molecular agents for cancer theranostics. Chem Sci. 2020;11:618-630
58. Li X, Sun Y, Ma L, Liu G, Wang Z. The Renal Clearable Magnetic Resonance Imaging Contrast Agents: State of the Art and Recent Advances. Molecules. 2020;25:5072
59. Liu X, Rong P. Recent Advances of Manganese-Based Hybrid Nanomaterials for Cancer Precision Medicine. Front Oncol. 2021;11:707618
60. Niazi M, Alizadeh E, Zarebkohan A. et al. Advanced Bioresponsive Multitasking Hydrogels in the New Era of Biomedicine. Adv Funct Mater. 2021;31:2104123
61. Zheng R, Guo J, Cai X. et al. Manganese complexes and manganese-based metal-organic frameworks as contrast agents in MRI and chemotherapeutics agents: Applications and prospects. Colloids Surf B Biointerfaces. 2022;213:112432
62. Li Y, Song K, Cao Y, Peng C, Yang G. Keratin-Templated Synthesis of Metallic Oxide Nanoparticles as MRI Contrast Agents and Drug Carriers. ACS Appl Mater Interfaces. 2018;10:26039-26045
63. Pan J, Wang Y, Pan H. et al. Mimicking Drug-Substrate Interaction: A Smart Bioinspired Technology for the Fabrication of Theranostic Nanoprobes. Adv Funct Mater. 2017;27:1603440
64. Zhou C, Hou S, Huang C, Jia N. A Mn-doped calcium phosphate nanoparticle-based multifunctional nanocarrier for targeted drug delivery and cellular MR imaging. J Nanopart Res. 2022;24:17
65. Shenoy RUK, Rama A, Govindan I, Naha A. The purview of doped nanoparticles: Insights into their biomedical applications. OpenNano. 2022;8:100070
66. Chu D, Qu H, Huang X. et al. Manganese Amplifies Photoinduced ROS in Toluidine Blue Carbon Dots to Boost MRI Guided Chemo/Photodynamic Therapy. Small. 2024;20:2304968
67. Tang Z, Liu Y, He M, Bu W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angewandte Chemie. 2019;131:958-68
68. Liang S, Liao G, Zhu W, Zhang L. Manganese-based hollow nanoplatforms for MR imaging-guided cancer therapies. Biomater Res. 2022;26:40824-022 -00275-5
69. Ruan J, Qian H. Recent Development on Controlled Synthesis of Mn-Based Nanostructures for Bioimaging and Cancer Therapy. Adv Ther (Weinh). 2021;4:2100018
70. Lin L-S, Song J, Song L. et al. Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO2-Based Nanoagent to Enhance Chemodynamic Therapy. Angewandte Chemie. 2018;130:4996-5000
71. He T, Qin X, Jiang C. et al. Tumor pH-responsive metastable-phase manganese sulfide nanotheranostics for traceable hydrogen sulfide gas therapy primed chemodynamic therapy. Theranostics. 2020;10:2453-2462
72. Zhou Y, Shu G, Luo Y. et al. Achieving Complete Tumor Clearance: A Minimalist Manganese Hydrogel for Magnetic Resonance Imaging-Guided Synergetic Microwave Ablation and Chemodynamic Therapy. Adv Healthc Mater. 2024;13:2303268
73. Wang X, Li C, Qian J, Lv X. et al. NIR-II Responsive Hollow Magnetite Nanoclusters for Targeted Magnetic Resonance Imaging-Guided Photothermal/Chemo-Therapy and Chemodynamic Therapy. Small. 2021;17:2100794
74. Shi Y, Shi Y, Wang Z. et al. Glucose-responsive mesoporous prussian blue nanoprobes coated with ultrasmall gold and manganese dioxide for magnetic resonance imaging and enhanced antitumor therapy. Chem Eng J. 2023;453:139885
75. MacDonald TD, Liu TW, Zheng G. An MRI-Sensitive, Non-Photobleachable Porphysome Photothermal Agent. Angewandte Chemie. 2014;126:7076-9
76. Yin C, Zhang H, Sun B. et al. Remarkable Suppression of Vibrational Relaxation in Organic Semiconducting Polymers by Introducing a Weak Electron Donor for Improved NIR-II Phototheranostics. Adv Funct Mater. 2021;31:2106575
77. Jing L, Liang X, Li X. et al. Mn-porphyrin Conjugated Au Nanoshells Encapsulating Doxorubicin for Potential Magnetic Resonance Imaging and Light Triggered Synergistic Therapy of Cancer. Theranostics. 2014;4:858-71
78. Ma G, Zhang X, Zhao K. et al. Polydopamine Nanostructure-Enhanced Water Interaction with pH-Responsive Manganese Sulfide Nanoclusters for Tumor Magnetic Resonance Contrast Enhancement and Synergistic Ferroptosis-Photothermal Therapy. ACS Nano. 2024;18:3369-81
79. Liu Z, Zhang S, Lin H. et al. Theranostic 2D ultrathin MnO2 nanosheets with fast responsibility to endogenous tumor microenvironment and exogenous NIR irradiation. Biomaterials. 2018;155:54-63
80. Jin L, Liu J, Tang Y. et al. MnO 2-Functionalized Co-P Nanocomposite: A New Theranostic Agent for pH-Triggered T 1 /T 2 Dual-Modality Magnetic Resonance Imaging-Guided Chemo-photothermal Synergistic Therapy. ACS Appl Mater Interfaces. 2017;9:41648-41658
81. Huang Y, Ouyang W, Lai Z. et al. Nanotechnology-enabled sonodynamic therapy against malignant tumors. Nanoscale Adv. 2024;6:1974-91
82. Sun L, Cao Y, Li W. et al. Perovskite-Type Manganese Vanadate Sonosensitizers with Biodegradability for Enhanced Sonodynamic Therapy of Cancer. Small. 2023;19:2300101
83. Yang M, Ren W, Cui H. et al. Ginsenoside Rk1-Loaded Manganese-Doped Hollow Titania for Enhancing Tumor Sonodynamic Therapy via Upregulation of Intracellular Reactive Oxygen Species. ACS Appl Mater Interfaces. 2023;15:20800-10
84. Gong F, Cheng L, Yang N. et al. Ultrasmall Oxygen-Deficient Bimetallic Oxide MnWO X Nanoparticles for Depletion of Endogenous GSH and Enhanced Sonodynamic Cancer Therapy. Adv Mater. 2019;31:1900730
85. Wang D, Cheng D-B, Ji L. et al. Precise magnetic resonance imaging-guided sonodynamic therapy for drug-resistant bacterial deep infection. Biomaterials. 2021;264:120386
86. Wang K, Du Y, Zhang Z. et al. Fluorescence image-guided tumour surgery. Nat Rev Bioeng. 2023;1:161-79
87. Zhuang D, Zhang H, Hu G. et al. Recent development of contrast agents for magnetic resonance and multimodal imaging of glioblastoma. J. Nanobiotechnology. 2022;20:284
88. Chen Y, Chen H, Sun Y. et al. Multifunctional Mesoporous Composite Nanocapsules for Highly Efficient MRI-Guided High-Intensity Focused Ultrasound Cancer Surgery. Angew Chem Int Ed Engl. 2011;50:12505-9
89. Zhang M, Xing L, Ke H. et al. MnO2-Based Nanoplatform Serves as Drug Vehicle and MRI Contrast Agent for Cancer Theranostics. ACS Appl Mater Interfaces. 2017;9:11337-11344
90. Cai X, Zhu Q, Zeng Y, Zeng Q, Chen X, Zhan Y. Manganese oxide nanoparticles as mri contrast agents in tumor multimodal imaging and therapy. Int J Nanomedicine. 2019;14:8321-44
91. Li Y, Niu P, Han Z. et al. Gadolinium-Manganese-Based Nanoplatform Reverses Radiotherapy Resistant Factors for Radiotherapy Sensitization and Computed Tomography/Magnetic Resonance Dual-Modal Imaging. Small Struct. 2024;5:2400033
92. Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS Nano. 2016;10:633-647
93. Guo C, Sun L, Cai H. et al. Gadolinium-Labeled Biodegradable Dendron-Hyaluronic Acid Hybrid and Its Subsequent Application as a Safe and Efficient Magnetic Resonance Imaging Contrast Agent. ACS Appl Mater Interfaces. 2017;9:23508-19
94. Mamidi N, Delgadillo RMV, Sustaita AO, Lozano K, Yallapu MM. Current nanocomposite advances for biomedical and environmental application diversity. Med Res Rev. 2024 p: 1-53
95. Liu J, Zhang W, Kumar A. et al. Acridine Orange Encapsulated Mesoporous Manganese Dioxide Nanoparticles to Enhance Radiotherapy. Bioconjug Chem. 2020;31:82-92
96. Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45:6597-626
97. Arnaut LG, Pereira MM. Overcoming the challenges of infrared photosensitizers in photodynamic therapy: the making of redaporfin. Chem Commun. 2023;59:9457-68
98. Sarbadhikary P, George BP, Abrahamse H. Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer. Theranostics. 2021;11:9054-88
99. Yin Z, Ji Q, Wu D. et al. H2O2-Responsive Gold Nanoclusters @ Mesoporous Silica @ Manganese Dioxide Nanozyme for 'off/On' Modulation and Enhancement of Magnetic Resonance Imaging and Photodynamic Therapy. ACS Appl Mater Interfaces. 2021;13:14928-37
100. Kálmán FK, Nagy V, Váradi B. et al. Mn(II)-Based MRI Contrast Agent Candidate for Vascular Imaging. J Med Chem. 2020;63:6057-65
101. Zhang K, Qi C, Cai K. Manganese-Based Tumor Immunotherapy. Adv Mater. 2023;35:2205409
102. Sun X, Zhang Y, Li J. et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat Nanotechnol. 2021;16:1260-70
103. Yang H, Yang S, Guo Q, Sheng J, Mao Z. ATP-Responsive Manganese-Based Bacterial Materials Synergistically Activate the cGAS-STING Pathway for Tumor Immunotherapy. Adv Mater. 2024;36:2310189
104. He Q, Zheng R, Ma J, Zhao L, Shi Y, Qiu J. Responsive manganese-based nanoplatform amplifying cGAS-STING activation for immunotherapy. Biomater Res. 2023;27:1-16
105. Li X, Khorsandi S, Wang Y. et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat Nanotechnol. 2022;17:891-9
106. Cai J, Jiang S, Liao J. et al. Manganese-doped biostimulatory nanoneedle for MRI-visual bispecific antibody gene delivery and immunosuppression reversal as a cancer immunotherapy strategy. Chem Eng J. 2023;462:1385-8947
107. Ding B, Zheng P, Jiang F. et al. MnOx Nanospikes as Nanoadjuvants and Immunogenic Cell Death Drugs with Enhanced Antitumor Immunity and Antimetastatic Effect. Angew Chem Int Ed Engl. 2020;59:16381-4
108. Neelanjana Bag, Souravi Bardhan, Shubham Roy. et al. Nanoparticle Mediated Stimuli Responsive Antibacterial Therapy. Biomater Sci. 2023;11:1994-2019
109. Huang H, Ali A, Liu Y. et al. Advances in image-guided drug delivery for antibacterial therapy. Adv Drug Deliv Rev. 2023;192:114634
110. Roy S, Roy J, Guo B. Nanomaterials as multimodal photothermal agents (PTAs) against 'Superbugs'. J Mater Chem B. 2023;11:2287-306
111. Zhu C, Ma Q, Gong L. et al. Manganese-based multifunctional nanoplatform for dual-modal imaging and synergistic therapy of breast cancer. Acta Biomater. 2022;141:429-39
112. Li Y, Zhang P, Tang W. et al. Bright, Magnetic NIR-II Quantum Dot Probe for Sensitive Dual-Modality Imaging and Intensive Combination Therapy of Cancer. ACS Nano. 2022;16:8076-8094
113. Tian H, Wang G, Sang W. et al. Manganese-phenolic nanoadjuvant combines sonodynamic therapy with cGAS-STING activation for enhanced cancer immunotherapy. Nano Today. 2022;43:101405
114. Zhang W, Lu L, Zhu Z. et al. A Manganese-Based Nanodriver Coordinates Tumor Prevention and Suppression through STING Activation in Glioblastoma. Adv Healthc Mater. 2024;13:2400421
115. Luo G, Li X, Lin J. et al. Multifunctional Calcium-Manganese Nanomodulator Provides Antitumor Treatment and Improved Immunotherapy via Reprogramming of the Tumor Microenvironment. ACS Nano. 2023;17:15449-65
116. Deng Z, Xi M, Zhang C. et al. Biomineralized MnO2 Nanoplatforms Mediated Delivery of Immune Checkpoint Inhibitors with STING Pathway Activation to Potentiate Cancer Radio-Immunotherapy. ACS Nano. 2023;17:4495-506
117. Zheng SJ, Yang M, Luo JQ. et al. Manganese-Based Immunostimulatory Metal-Organic Framework Activates the cGAS-STING Pathway for Cancer Metalloimmunotherapy. ACS Nano. 2023;17:15905-17
118. Ding J, He Z, Zhai Y. et al. Advances in metal-based nano drugs and diagnostic probes for tumor. Coord Chem Rev. 2024;501:215594
119. Zairov RR, Akhmadeev BS, Fedorenko S V, Mustafina AR. Recent progress in design and surface modification of manganese nanoparticles for MRI contrasting and therapy. Chem Eng J. 2023;459:141640
120. Baranyai Z, Carniato F, Nucera A. et al. Defining the Conditions for the Development of the Emerging Class of Fe III -Based MRI Contrast Agents. Chem Sci. 2021;12:11138-11145
121. Li J, Zhao Z, Feng J, Gao J, Chen Z. Understanding the metabolic fate and assessing the biosafety of MnO nanoparticles by metabonomic analysis. Nanotechnology. 2013 24 455102
122. Wang X, Xu L, Ren Z. et al. A novel manganese chelated macromolecular MRI contrast agent based on O-carboxymethyl chitosan derivatives. Colloids Surf B Biointerfaces. 2019;183:110452
123. Lucchini RG, Christopher Martin J, Doney BC. From Manganism to Manganese-Induced Parkinsonism: A Conceptual Model Based on the Evolution of Exposure. Neuromolecular Med. 2009;11:311-321
124. Gunter TE, Gerstner B, Lester T. et al. An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays. Toxicol Appl Pharmacol. 2010;249:65-75
125. Chen P, Totten M, Zhang Z. et al. Iron and manganese-related CNS toxicity: mechanisms, diagnosis and treatment. Expert Rev Neurother. 2019;19:243-60
126. Schrantz N, Blanchard DA, Mitenne F, Auffredou MT, Vazquez A, Leca G. Manganese induces apoptosis of human B cells: caspase-dependent cell death blocked by Bcl-2. Cell Death Differ. 1999;6:445-53
127. Zhang J, Cao R, Cai T. et al. The role of autophagy dysregulation in manganese-induced dopaminergic neurodegeneration. Neurotox Res. 2013;24:478-90
128. Zou Y, Zheng C, Fei W. et al. Targeted GSH-exhausting and hydroxyl radical self-producing manganese-silica nanomissiles for MRI guided ferroptotic cancer therapy Targeted GSH-exhausting and hydroxyl radical self-producing manganese-silica nanomissiles for MRI guided ferroptotic cancer therapy. Nanoscale. 2020;12:16738-16754
129. Korupalli C, Kuo CC, Getachew G. et al. Multifunctional manganese oxide-based nanocomposite theranostic agent with glucose/light-responsive singlet oxygen generation and dual-modal imaging for cancer treatment. J Colloid Interface Sci. 2023;643:373-84
130. Chevallier P, Walter A, Garofalo A. et al. Tailored biological retention and efficient clearance of pegylated ultra-small MnO nanoparticles as positive MRI contrast agents for molecular imaging. J Mater Chem B. 2014;2:1779-90
131. Pan D, Schmieder AH, Wickline SA, Lanza GM. Manganese-based MRI contrast agents: past, present, and future. Tetrahedron. 2011;67:8431-44
132. Zhao Y, Pan Y, Zou K. et al. Biomimetic manganese-based theranostic nanoplatform for cancer multimodal imaging and twofold immunotherapy. Bioact Mater. 2023;19:237-50
133. Fu H, Yang L, Zeng Y. et al. Black Phosphorus-Loaded Manganese Nanosystem Embedded in Thermosensitive Polymer Gels as an Injectable Platform for Osteosarcoma Therapy. ACS Appl Nano Mater. 2024;7:10963-73
134. Koretsky AP, Silva AC. Manganese-enhanced magnetic resonance imaging (MEMRI). NMR Biomed. 2004;17:527-31
135. Du R, Zhao Z, Cui J, Li Y. Manganese-Based Nanotheranostics for Magnetic Resonance Imaging-Mediated Precise Cancer Management. Int J Nanomedicine. 2023;18:6077-99
Author contact
![]() Corresponding authors: Lingyan Zhang (18819818005com); Bing Guo (guobing2020edu.cn).
Corresponding authors: Lingyan Zhang (18819818005com); Bing Guo (guobing2020edu.cn).
 Global reach, higher impact
Global reach, higher impact