13.3
Impact Factor
Theranostics 2025; 15(9):3961-3978. doi:10.7150/thno.107406 This issue Cite
Review
Mechanisms and novel therapeutic roles of bitter taste receptors in diseases
Department of Pharmaceutics, School of Pharmacy, Shenyang Pharmaceutical University, No. 103, Wenhua Road, Shenyang, 110016, P. R. China
# These authors contributed to the work equally.
Received 2024-11-22; Accepted 2025-1-30; Published 2025-3-3
Abstract
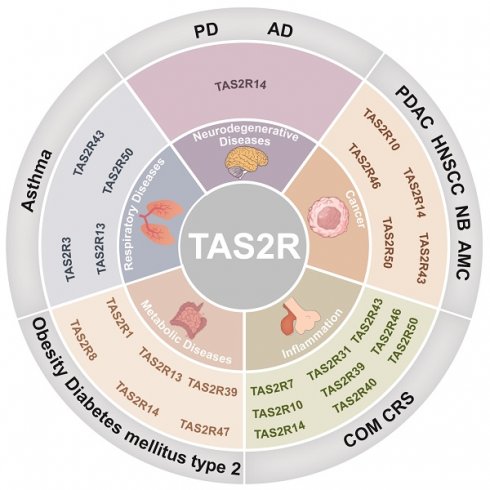
Bitter taste receptors (TAS2R) are expressed in the oral cavity, intestine, airways, and vascular smooth muscle, where they regulate physiological processes, including immune responses. However, the activation of TAS2R triggers signaling pathways that influence inflammation, metabolism, and cell proliferation, suggesting their potential as therapeutic targets for diseases such as Alzheimer's disease, Parkinson's disease, asthma, and cancer. Bitter compounds capable of activating TAS2R have shown potential in modulating these pathways, presenting novel opportunities for drug development. This review examines the expression of TAS2R across diverse tissues, their complex physiological roles, and potential therapeutic applications, including disease management and targeted drug delivery.
Keywords: TAS2R, Signaling mechanism, Extraoral expression, Disease management, Drug delivery
1. Introduction
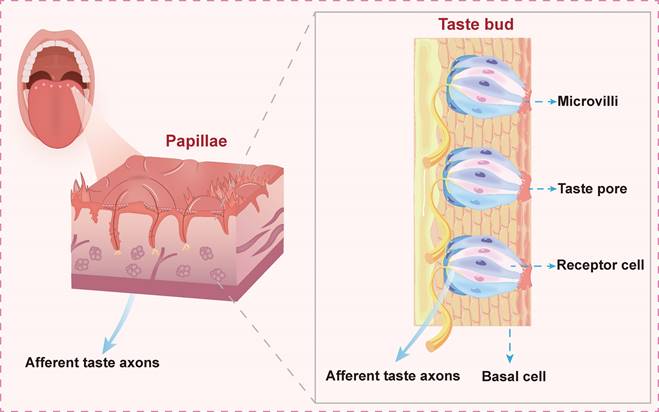
Taste receptors, including ion channels and G protein-coupled receptors (GPCRs), detect sour, bitter, salty, sweet, and umami flavors. It was initially identified as a taste receptor expressed in small populations of specialized epithelial cells on the tongue (Figure 1), these receptors are now recognized for their broader roles beyond oral taste perception. Their detection functions extend throughout the digestive tract, including the intestinal epithelium, respiratory tract, and gums, where they sense various metabolites involved in interactions between mucosal surfaces and microorganisms [1]. For instance, isolated chemosensory cells (SCCs) and tuft cells expressing taste-signaling proteins are present in several mucous membranes. SCCs can detect allergens, bacteria, noxious stimuli, viruses, driving avoidance behavior, antimicrobial responses, and neuroinflammation in the airway. Similarly, tuft cells in the intestine sense helminth infections and bacterial dysregulation, triggering a type II immune response characterized by tissue remodeling. In the gums, SCCs detect disease, evoke innate immune responses, and release antimicrobial compounds in the epithelium, thereby regulating the composition of the microbiome [2].
Taste cells can be categorized into three distinct types based on morphology and function: Type I, Type II, and Type III. These specialized cells are responsible for detecting the five basic tastes. The sensation of "bitter" is mediated by specific GPCRs expressed in certain Type II cells [3]. TAS2R, which are GPCRs, are encoded by approximately 27 to 51 genes. These receptors are localized on the surface of taste buds and exhibit a characteristic structure comprising seven transmembrane helices formed by a single polypeptide chain [4, 5]. To date, 25 TAS2R genes have been identified in humans, with their genomic locations mapped to chromosomes 5p15, 7q31, and 12p13. Some TAS2R, such as TAS2R10, TAS2R14, and TAS2R46, can recognize multiple compounds, while others demonstrate strict specificity for a single bitter compound [6].
Schematic illustration of taste bud organization on the tongue. Lingual papillae housing taste buds are shown with structural components including microvilli, taste pores, and receptor cells, which mediate signal transduction through gustatory afferent nerve fibers to central processing centers.
TAS2R is expressed in various tissues and cells throughout the human body, including the gastrointestinal tract, skin, tuft cells and tumor cells. The activation of TAS2R in tuft cells can elicit a type 2 immune response effective in treating parasitic infections [7]. Additionally, TAS2R may aid in managing obesity by repairing intestinal barrier integrity, upregulating GLP-1 release, and promoting tuft cell proliferation [8]. In keratinocytes, TAS2R function as intracellular “gatekeepers” facilitating the excretion of harmful substances via ABCB1, enhancing our understanding of their role in non-gustatory tissues and laying the groundwork for novel drugs to bolster the skin's defense against harmful substances [9]. Furthermore, TAS2R's involvement in regulating tumor cell apoptosis signifies their significant research value [10, 11]. In this review, we provide a detailed overview of the signaling mechanisms of TAS2R and explore their roles in tissues and cells outside the oral cavity. TAS2R are expressed in a wide array of tissues, and this broad tissue distribution suggests that TAS2R could play a crucial role in modulating various physiological processes. Consequently, TAS2R represents a promising target for the development of novel therapeutic strategies.
2. Signaling mechanism of TAS2R
2.1 TAS2R-PDE-cAMP pathways
TAS2R activation follows dual signaling mechanisms through G-protein-coupled pathways, inducing rapid second messenger changes. In one pathway, bitter compounds such as cycloheximide bind to TAS2R, activating the G protein's α-subunit. This activation reduces intracellular cAMP levels via phosphodiesterase stimulation [12]. Consequently, cAMP-dependent ion channels are inactivated, triggering the release of intracellular calcium stores, increasing cytosolic Ca²⁺ concentration, and causing membrane depolarization.
2.2 TAS2R-PLCβ2-IP3 Q pathways
Another mechanism involves the binding of bitter compounds to TAS2R, which activates the β-γ subunit of the gustatory G protein. This activation induces the synthesis of phospholipase Cβ2 (PLCβ2) through inositol 1,4,5-triphosphate (IP3), leading to the release of intracellular Ca²⁺. The increased Ca²⁺ levels depolarize bitter taste receptor cells, prompting neurotransmitter release [13]. Interestingly, studies on Gustducin-deficient mice suggest alternative pathways, where specific bitter substances directly stimulate TAS2R, bypassing Gustducin and activating ion channels [14].
2.3 The role of multiple signal transduction pathways in TAS2R
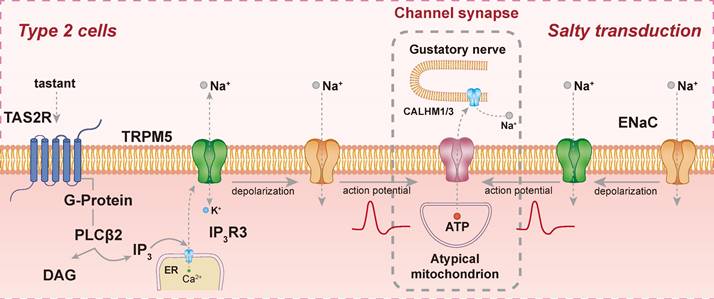
Certain substances, such as quinine, can close K⁺ channels, resulting in the depolarization of TAS2R-expressing cells [15]. Additionally, bitter compounds can activate G proteins and PLCβ2 by binding to TAS2R, thereby elevating intracellular Ca²⁺ concentrations and leading to the activation of TRPM4 and TRPM5, resulting in Na⁺ influx [13]. These findings highlight the existence of multiple transduction pathways for a single substance, revealing interdependent mechanisms and shared signaling components (Figure 2).
3. Extra-oral role of TAS2R
Initially, TAS2R was believed to help animals avoid toxic compounds such as strychnine and nicotine [16]. When a bitter compound binds to TAS2R, it activates the receptors, initiating a sensory response. The chords tympani and glossopharyngeal nerves transmit this signal to the brain for processing [3]. However, not all toxic compounds taste bitter, and a bitter taste does not always signify toxicity [4]. Further research has revealed that TAS2R is expressed beyond the oral cavity, including in the intestine, airways, urinary tract, vascular and respiratory smooth muscle, nervous system, and thyroid gland. TAS2R at these diverse sites performs distinct regulatory roles in various physiological processes (Table 1).
Signal transduction pathways of TAS2R. Bitter substances bind to TAS2R, activating PLCβ2 to produce IP3 and DAG, leading to Ca²⁺ release and TRPM5 activation, which causes depolarization and action potential generation. Salty stimuli modulate ENaC channel activity, directly depolarizing taste cells to trigger action potentials. Synaptic transmission is mediated by CALHM1/3 channels, facilitating neurotransmitter release to gustatory afferent neurons.
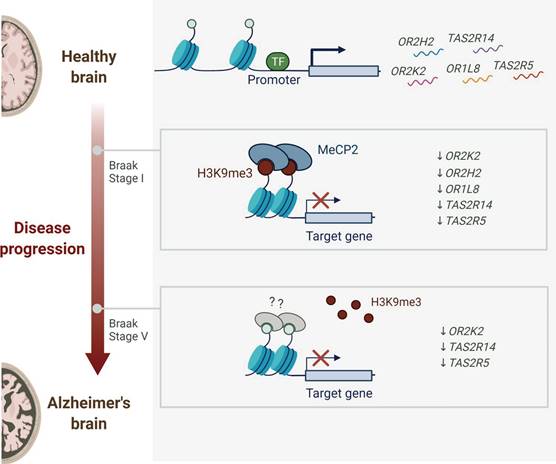
Dynamic alterations in receptor expression during Alzheimer's disease progression. Healthy brains constitutively express olfactory receptors (OR2H2, OR1L8) and bitter taste receptors (TAS2R14, TAS2R5). Early Braak stages (I-V) exhibit progressive downregulation of OR2K2, OR2H2, OR1L8, TAS2R14, and TAS2R5, with persistent OR2K2 suppression observed in advanced neuropathological stages. Adapted with permission from [71], copyright 2023, Springer.
Distribution and expression of TAS2R in the normal human system
| TAS2R | Localization in the human body | Method | Ref. |
|---|---|---|---|
| TAS2R1 | Immune system, Cardio-vascular system, Excretory system, Nervous system, Endocrine system, Respiratory system, Skin | RT-PCR | [17, 18] |
| TAS2R3 | Immune system, Endocrine system, Cardio-vascular system, Respiratory system, Reproductive system, Nervous system, Digestive system, Skin | ELCLIA | [19, 20] |
| TAS2R4 | Immune system, Endocrine system, Cardio-vascular system, Respiratory system, Reproductive system, Nervous system, Digestive system, Excretory system Skin | IHC | [21, 22] |
| TAS2R5 | Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Excretory system, Nervous system, Digestive system, Skin | RT-PCR | [23-25] |
| TAS2R7 | Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Excretory system, Nervous system, Skin | IFC | [26-28] |
| TAS2R8 | Immune system, Endocrine system, Cardio-vascular system, Excretory system, Nervous system, Skin | ChiP | [29-31] |
| TAS2R9 | Immune system, Endocrine system, Cardio-vascular system, Excretory system, Nervous system, Skin | [32] | |
| TAS2R10 | Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Excretory system, Nervous system, Digestive system, Skin | WB | [31, 33-36] |
| TAS2R13 | Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Excretory system, Nervous system, Skin | IFC | [37] |
| TAS2R14 | Immune system, Respiratory system, Cardio-vascular system, Endocrine system, Reproductive system, Excretory system, Nervous system, Skin | RT-qPCR | [38-40] |
| TAS2R16 | Respiratory system, Endocrine system, Excretory system, Cardio-vascular system, Nervous system, Skin | IFC | [41, 42] |
| TAS2R19 | Immune system, Reproductive system, Cardio-vascular system, Excretory system, Skin | RT-PCR | [43, 44] |
| TAS2R20 | Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Excretory system, Nervous system, Digestive system, Skin | RT-qPCR | [45] |
| TAS2R30 | Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Excretory system, Digestive system, Skin | RT-qPCR | [46, 47] |
| TAS2R31 | Immune system, Endocrine system, Cardio-vascular system, Excretory system, Skin | RT-qPCR | [48, 49] |
| TAS2R38 | Respiratory system, Immune system, Endocrine system, Cardio-vascular system, Excretory system, Nervous system, Adipose tissue, Digestive system, Skin | RT-PCR | [50-53] |
| TAS2R39 | Respiratory system, Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Excretory system, Nervous system, Skin | RT-PCR | [45, 54] |
| TAS2R40 | Immune system, Endocrine system, Cardio-vascular system, Excretory system, Nervous system, Skin | RT-qPCR | [49, 55] |
| TAS2R41 | Immune system, Cardio-vascular system, Endocrine system, Excretory system, Nervous system, Skin | RT-qPCR | [56] |
| TAS2R42 | Respiratory system, Immune system, Endocrine system, Reproductive system, Cardio-vascular system, Excretory system, Nervous system, Skin | PCR | [29, 46] |
| TAS2R43 | Respiratory system, Cardio-vascular system, Immune system, Endocrine system, Reproductive system, Nervous system, Excretory system, Digestive system, Skin | RT-PCR, WB, IFC | [57, 58] |
| TAS2R44 | Nervous system | RT-PCR | [59] |
| TAS2R45 | Respiratory system, Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Nervous system, Excretory system, Skin | RT-qPCR | [60] |
| TAS2R46 | Respiratory system, Immune system, Endocrine system, Cardio-vascular system, Nervous system, Skin | RT-qPCR | [61] |
| TAS2R47 | Respiratory system | RT-PCR | [62, 63] |
| TAS2R49 | Immune System, Endocrine System, Cardiovascular System, Reproductive System, Excretory System, Nervous System, Digestive System, Skin, Respiratory System | RT-qPCR | [64] |
| TAS2R50 | Respiratory system, Immune system, Endocrine system, Cardio-vascular system, Reproductive system, Nervous system, Skin | RT-qPCR | [64] |
| TAS2R60 | Immune system, Cardio-vascular system, Nervous system, Skin | qPCR | [65] |
3.1 Role of TAS2R in neurodegenerative diseases
Brain disorders pose a significant global healthcare challenge, with drug delivery across the blood-brain barrier remaining a critical obstacle. Characteristic features of central nervous system (CNS) disorders include protein misfolding and cell death, often aggravated by neuroinflammation and oxidative stress [66]. Therein, Alzheimer's disease (AD) and Parkinson's disease (PD) are two of the most common neurodegenerative diseases worldwide, typically characterized by progressive neuronal loss and functional decline.
3.1.1 Role of TAS2R in Alzheimer's disease
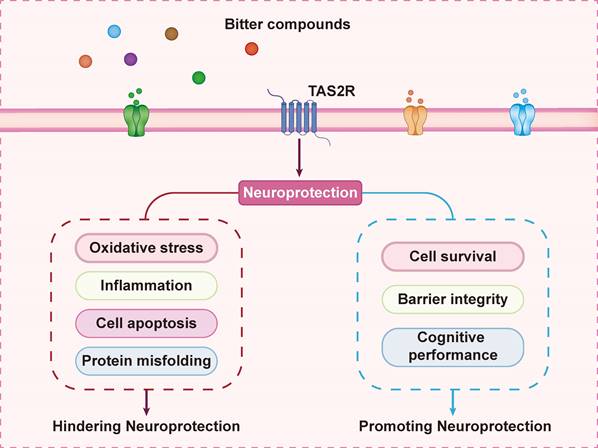
AD, one of the most widespread neurodegenerative disorders, progresses to Alzheimer's dementia, characterized by the progressive impairment of cognition and functional capacity, ultimately compromising patient independence [67]. Increasing evidence suggests that genetic, cellular, and circuitry dysregulation contribute to both the cellular pathology and cognitive deficits observed in AD [68]. Neuroinflammation plays a pivotal role in AD pathogenesis, with microglia, the resident innate immune cells of the CNS, serving a key role in this process. Extracellular β-amyloid plaques interact with receptors for advanced glycation end products (RAGE) on microglia, leading to their overactivation and the subsequent release of pro-inflammatory factors. This heightened inflammatory state can alter brain energy metabolism, impair neuronal network function, and compromise the integrity of the blood-brain barrier [69]. Pathophysiological changes involving alterations in specific proteins lead to dysfunction across various cellular pathways, including mitochondrial dysfunction, excitotoxicity, synaptic dysfunction, damage to protein degradation systems, endoplasmic reticulum (ER) stress, DNA damage, inflammation and increased levels of reactive oxygen species (ROS) due to cell cycle re-entry [70]. These changes contribute to the development of AD. Furthermore, Alves et al. [71] reported a correlation between TAS2R expression modulation and AD progression, as shown in Figure 3. TAS2R genes, along with other AD-associated genes, were observed to be progressively downregulated from the early stages of AD, with continued decline as the disease advanced. This indicates that TAS2R may serve as both a potential biomarker and a therapeutic target, warranting further investigation. However, the precise role of TAS2R in AD pathophysiology remains poorly understood, emphasizing the need for further studies to elucidate the mechanisms linking its expression to disease progression.
3.1.2 Role of TAS2R in Parkinson's disease
PD is the second most prevalent neurodegenerative disorder after AD, presenting as a distinct clinical syndrome [72]. As in AD, a key molecular pathway in PD involves α-synuclein aggregation, which triggers NLRP3 inflammasome activation. The fibrillation of α-synuclein has been shown to induce a delayed yet robust activation of the NLRP3 inflammasome in primary mouse microglia [73].
Alterations in TAS2R expression have been observed in PD patients [74, 75], with downstream signaling pathways involved in inflammatory responses suggesting potential therapeutic applications. NF-κB and the NLRP3 inflammasome are central regulators of inflammation in human cells, contributing to both neuroinflammation and TAS2R signaling dysregulation. NF-κB and the NLRP3 inflammasome are central regulators of inflammation in human cells, contributing to both neuroinflammation and TAS2R signaling dysregulation. NF-κB, a key regulator of inflammatory transcription networks, controls the expression of genes involved in immune responses, inflammation, and oxidative stress following exposure to pathogens, cytokines, and reactive species. IL-1β, a pro-inflammatory cytokine, may be produced due to TAS2R signaling defects and can activate NF-κB via Toll-like receptor pathways [76]. In a 2011 study, Nisha Singh et al. [77] discovered TAS2R expression in various regions of the rat brain. RT-PCR analysis confirmed the expression of TAS2R4, TAS2R48, and TAS2R107 in the brainstem, cerebellum, cortex, and ventral nucleus. TAS2R activation has been linked to memory improvement by reducing IL-1β and TNF-α levels and mitigating LPS-induced neuroinflammation, oxidative stress, and apoptosis [78].
Certain TAS2R agonists have been shown to suppress NLRP3 and NF-κB signaling, suggesting their potential as therapeutic agents to mitigate neuroinflammation in CNS disorders. Accordingly, bitter receptor agonists have been demonstrated to partially downregulate inflammatory pathways by inhibiting NLRP3, NF-κB, and related oxidative stress markers (Figure 4). Efforts are underway to identify new therapeutic strategies for neuroinflammatory diseases using cannabinoids, phenols, and endogenous anti-inflammatory cytokines [79]. TAS2R regulates multiple biological processes initiated by bitter taste ligands in the brain, and bitter compounds are emerging as promising candidates for CNS disease treatment. Further research is required to elucidate the transport mechanisms of bitter compounds across the blood-brain barrier, as their limited bioavailability remains a challenge.
3.2 The role of TAS2R in chronic otitis media
Chronic otitis media (COM), a persistent ear, nose, and throat disorder, significantly affects individuals' health. It remains prevalent both domestically and internationally, being a primary cause of hearing loss [80]. Key aspects of its pathogenesis involve mucosal hyperplasia and leukocyte infiltration in the middle ear, conditions that typically resolve with bacterial clearance through apoptosis. Activating innate immune receptors during the inflammatory phase leads to the recruitment of intracellular transcription factors (e.g. NF-κB, AP-1), which regulate the inflammatory response and influence the proliferation of tissue cell lineages [81].
To assess the presence of TAS2R in the middle ear and its potential link with COM, Kaufman et al. [82] recruited 84 volunteers, detecting TAS2R expression in all participants' middle ear samples. The rs1376251 allele of TAS2R50 was identified as being associated with chronic otitis media. However, while the study identified an association, further investigation is required to determine causality and clarify the mechanisms through which TAS2R50 and its alleles contribute to COM pathophysiology. A deeper understanding of these mechanisms could support the development of TAS2R-targeted therapies aimed at modulating inflammatory responses and improving outcomes for patients with COM.
3.3 Role of TAS2R in nasal inflammation
Chronic rhinosinusitis (CRS) is a complex upper respiratory disease characterized by persistent bacterial or fungal infections due to impaired mucociliary clearance [83]. Traditionally, primary CRS has been classified into two main categories: CRS with nasal polyps and CRS without nasal polyps, with eosinophilic sinusitis and allergic fungal sinusitis identified as additional subtypes [84]. Allergic rhinitis (AR) is an immunoglobulin E (IgE)-mediated inflammatory condition triggered by allergen exposure. Upon contact with an allergen, the immune system generates IgE, which binds to mast cells and basophils. Upon re-exposure, allergen binding to IgE triggers a Type I hypersensitivity reaction, leading to histamine release and the activation of immune cells and cytokines involved in nasal mucosal inflammation [85]. Common symptoms include sneezing, nasal congestion, itching, and a runny nose, affecting over 400 million people worldwide [86]. Inflammatory nasal diseases like CRS and AR lack a definitive cure and significantly impact patients' quality of life, increase healthcare costs, and reduce productivity [87]. In recent years, research has highlighted the role of taste receptor signaling in modulating mucosal immunity, positioning this pathway as a promising therapeutic target for respiratory diseases. Early studies identified TRPM5, a transient receptor potential ion channel, as crucial for bitter taste perception and highly expressed in the mouse nasal cavity [88]. Expanding on this, Dr. Henry P. Barham discovered that TAS2R46, TAS2R14, and TAS2R4, along with downstream components such as α-Gustducin, PLCβ2, and TRPM5, are expressed in human nasal structures, including the inferior and middle turbinate and the nasal septum [89]. Cilia, the first line of defense against inhaled pathogens, play a critical role in airway protection. TAS2R are expressed on active airway cilia, where they initiate innate immune responses by enhancing ciliary beating frequency (CBF) through guanylyl cyclase and protein kinase G activation, ultimately improving mucosal clearance [90]. The TAS2R14 agonist DPD enhances both CBF and nitric oxide production at the air-liquid interface (ALI) in the nasal cavity, inhibiting bacterial growth and biofilm formation [91].
Recently, studies by Freund et al. and Hariri et al. further demonstrated the expression of TAS2R4, TAS2R14, and TAS2R16 in sinus cilia, with activation by quinolone derivatives such as 2-heptyl-3-hydroxy-4-quinolone and 4-hydroxy-2-heptylquinolone [92, 93]. Notably, certain TAS2R, such as TAS2R39, are upregulated in response to inflammatory cytokines, including IL-3, IL-5, IL-10, and TGF-β. Bitter compounds can induce nasal mucosal constriction, with similar effects observed in rat models. However, the upregulation of several TAS2R during inflammation suggests a dual role, contributing to both nasal mucosal contraction and the pathogenesis of inflammatory diseases [94, 95]. TAS2R plays a crucial role in CRS and AR, as their activation enhances ciliary activity, aiding pathogen clearance. However, the involvement of TAS2R in inflammatory cytokine regulation warrants further investigation to clarify their complex roles and therapeutic potential in inflammatory nasal diseases.
Multifaceted neuroprotection mediated by TAS2R receptors. TAS2R signaling orchestrates neuronal protection through dual mechanisms: (1) suppressing oxidative stress, neuroinflammation, apoptotic signaling, and protein misfolding pathologies; (2) enhancing cell survival pathways and integrity while improving cognitive function.
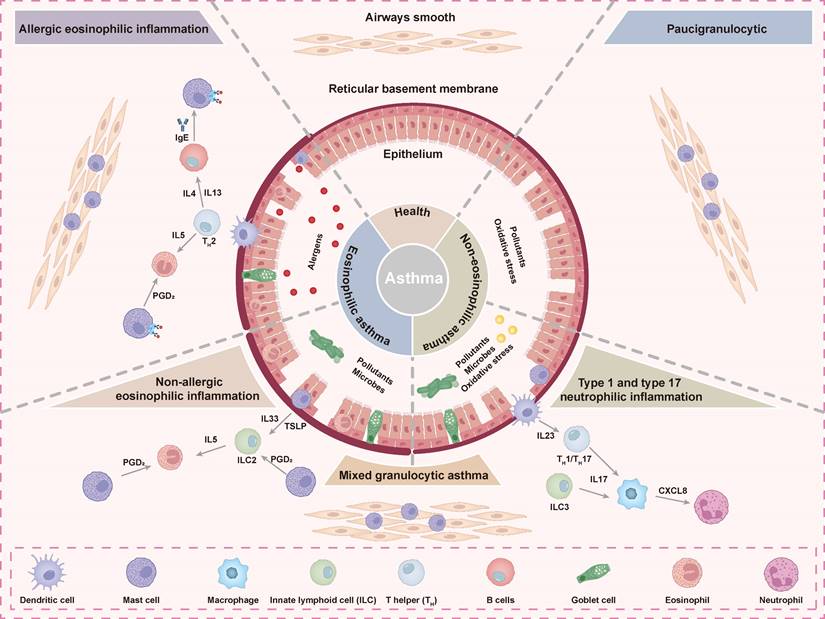
Mechanisms and characteristic pathological features of the immunopathology of asthma. Asthma includes allergic eosinophilic, non-allergic eosinophilic, mixed granulocytic, and type 1 and type 17 neutrophilic phenotypes. Each phenotype is linked to specific cell types and mediators. Asthmatic airways show changes in epithelial cells, basement membrane, and smooth muscle compared to healthy airways.
3.4 Role of TAS2R in asthma
Asthma is a chronic respiratory syndrome characterized by airway inflammation, nonspecific airway hyperresponsiveness, and reversible airway obstruction, with symptoms including wheezing and shortness of breath [96, 97]. Bronchial asthma involves multiple inflammatory factors and immune cells and can be classified into paucigranulocytic asthma (PGA), eosinophilic asthma (EA), mixed granulocytic asthma (MGA), and neutrophilic asthma (NA) based on distinct inflammatory profiles in induced sputum (Figure 5) [98]. Among these, eosinophils are key inflammatory cells and can be further categorized into allergic and non-allergic subtypes [99]. Asthma results from complex interactions between genetic and environmental factors. Exacerbations often involve airway remodeling, characterized by airway smooth muscle (ASM) thickening, excessive mucus secretion, and mucosal angiogenesis, which collectively contribute to airway stiffening and irreversible airway obstruction [100]. Current treatments, such as β2-agonists and corticosteroids, focus on bronchoconstriction relief but have limited efficacy against airway remodeling due to β2-agonist a number of adverse effects [23]. Therefore, the identification of novel therapeutic targets is crucial for addressing airway remodeling in asthma. Recent studies have identified TAS2R as promising therapeutic targets because of their expression on ASM cells and their capacity to induce ASM relaxation and airway dilation upon activation [101].
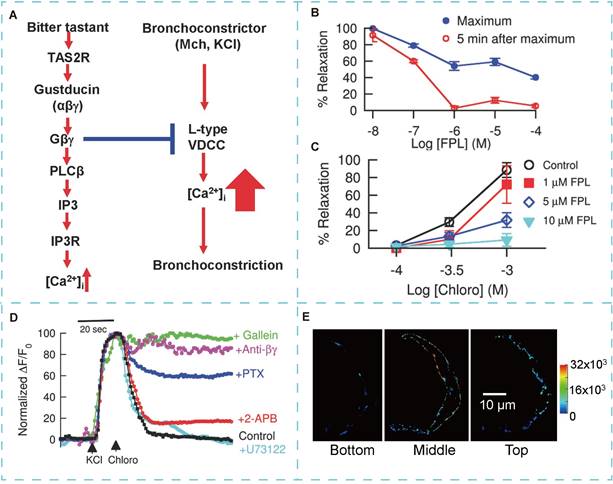
Deshpande et al. [102] demonstrated that TAS2R is expressed in the ASM of the human lung. The expression of TAS2R in ASM renders them potential drug targets for asthma treatment. Studies have shown that bitter taste receptor agonists induce relaxation and dilation of isolated ASM, with effects three times more pronounced than those induced by beta-agonists. Recently, TAS2R agonists have emerged as potential direct bronchodilators, among the 25 TAS2R isoforms, three—TAS2R10, TAS2R14, and TAS2R31—are notably overexpressed in ASM. Unlike β2 receptors, TAS2R-induced airway dilation occurs independently of cAMP increase. This suggests that the mechanism of TAS2R-induced airway relaxation is not mediated through an elevation in cAMP levels [103]. Upon TAS2R activation by bitter substances, the associated G-protein subunit Gαt dissociates from the TAS2R-Gαt/Gβγ complex. The released Gαt binds to the acetylcholine receptor (AchR), competitively inhibiting the function of the Gq protein coupled to AchR. This prevents calcium signaling initiated by AchR activation, resulting in ASM relaxation [101]. TAS2R agonism enhances cofilin activation, a key regulatory protein of the actin cytoskeleton, leading to ASM relaxation [106]. Additionally, TAS2R activation increases local Ca2+ concentration, triggering ASM relaxation by activating large-conductance Ca2+-activated K+ channels (BKCa) [102]. Furthermore, Zhang et al. [104] demonstrated that bitter substances induce bronchodilation by reversing the calcium concentration increase caused by bronchoconstrictors (Figure 6).
A key feature of airway remodeling in asthma is the proliferation of ASM, manifested as both hypertrophy and hyperplasia. A significant number of patients with severe asthma exhibit ASM proliferation. The drugs currently used to treat asthma range from beta-agonists to corticosteroids, with protein kinase A (PKA) playing a pivotal role in mediating the effects of beta-agonists on ASM function. However, PKA activity induced by beta-agonists in ASM cells appears to be desensitized to beta2-adrenoceptors, rendering beta-agonist-based antimitogenic drugs relatively weak and with limited impact on remodeling. Pawan Sharma et al. [105] utilized a TAS2R agonist to pre-treat airway smooth muscle (ASM) cells isolated from both healthy controls and asthmatic patients. This treatment demonstrated a dose-dependent suppression of ASM proliferation, indicating a significant anti-mitogenic effect of TAS2R agonists. Specifically, TAS2R agonists were found to block the downstream signaling of phosphatidylinositol 3-kinase and reduce the phosphorylation of growth factor-stimulated protein kinase B (Akt), without inhibiting the supply of phosphatidylinositol 3,4,5-trisphosphate. Collectively, TAS2R agonists suppress ASM proliferation in a dose-dependent manner, demonstrating significant anti-mitogenic effects. This suggests the existence of new receptor targets and signaling pathways that could mitigate airway remodeling and bronchoconstriction in asthma. These findings highlight TAS2R as a promising therapeutic target for asthma, potentially providing an alternative to current treatments.
The basis of TAS2R induced bronchodilation. (A) A model for TAS2R signaling and bitter tastant-induced bronchodilation. (B) FPL 64176 (FPL) dose-dependently reversed chloro-induced bronchodilation in Mch precontracted airways. (C) FPL dose-dependently inhibited chloro-induced bronchodilation of KCl-precontracted airways. (D) Representative recordings of changes in [Ca2+]. (E) Cellular distribution of TAS2R107 in three planes of an isolated mouse ASM cell. Adapted with permission from [104], copyright 2013, Public Library of Science.
3.5 The role of TAS2R in metabolic diseases
3.5.1 Diabetes mellitus type 2
The aetiopathology of type 2 diabetes mellitus (T2DM) is characterized by insulin resistance, initially coupled with hyperinsulinemia, followed by a progressive decline in the insulin-producing capacity of pancreatic beta cells. In addition, dysregulation of incretin hormones, lipolysis, hyperinsulinemia, enhanced renal glucose reabsorption, and disrupted central appetite regulation are key factors in the pathophysiology of T2DM [106]. Incretin hormones, secreted following food intake, are crucial for glucose homeostasis. Mammalian TAS2R, along with sensitizing molecules, is expressed in the intestinal mucosa, where it activates intestinal TAS2R to stimulate the secretion of glucagon-like peptide-1 (GLP-1) and other gut hormones by intestinal endocrine cells [107]. Bitter melon extract (BME), whose bitter taste suggests TAS2R activation, has been extensively studied for its anti-diabetic properties. The constituents of bitter melon activate TAS2R, inducing the secretion of various hormones, notably GLP-1, by intestinal endocrine cells. GLP-1, a critical hormone, has emerged as a promising target for anti-diabetic drug development due to its role in regulating blood glucose levels through various mechanisms, including the modulation of insulin production. Upon TAS2R activation in intestinal endocrine cells, the β and γ subunits dissociate from the α subunit of the trimeric G proteins. This dissociation triggers the activation of phospholipase Cβ2 (PLCβ2), which catalyzes the production of diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) from phosphatidylinositide at the cell membrane. IP3 binds to its receptor on the endoplasmic reticulum (ER) membrane, leading to Ca2+ release from the ER, thereby increasing the intracellular [Ca2+]. The subsequent increase in intracellular [Ca2+] leads to GLP-1 secretion by intestinal endocrine cells through mechanisms that remain to be fully elucidated [108]. GLP-1 interacts with its receptor in the β and γ cells of the pancreas (GLP-1R) to stimulate the biosynthesis and release of insulin and somatostatin respectively, thereby contributing to the regulation of blood glucose levels [109]. TAS2R activation initiates a cascade of intracellular events, including the dissociation of G protein subunits, activation of PLCβ2, production of DAG and IP3, and release of Ca2+ from the ER, culminating in GLP-1 secretion. GLP-1 then interacts with its receptor in pancreatic cells to regulate the release of insulin and somatostatin, contributing to the control of blood glucose levels. These findings underscore the potential of TAS2R agonists as therapeutic targets for T2DM.
3.5.2 Obesity
Obesity, which affects approximately one-third of the global population, is increasingly recognized as a significant public health issue. Obesity is believed to be associated with altered gene expression in taste buds [110]. As a sensory system, taste is the most intuitive indicator of our readiness to ingest food. Therefore, any alteration in this system can influence food intake and, consequently, body weight [111]. The question of whether fat can be considered the "sixth taste" in humans has long been debated [112]. New evidence supports the existence and function of TAS2R in various non-oral tissues. Specifically, TAS2R in gastrointestinal endocrine cells is involved in controlling appetite and regulating gut hormones that influence hunger and food intake [113]. This indicates that region-selective targeting of TAS2R holds potential as a promising strategy for obesity treatment.
3.5.2.1 Inhibition of ghrelin secretion
Gastrin, a potent appetite stimulant secreted by the stomach and consisting of 28 amino acids, targets the hypothalamus and brainstem. Gastrin signals hunger to the nervous system through ghrelin receptors, stimulating appetite and food intake while promoting the use of carbohydrates as a fuel source. Gastrin impedes fat utilization, contributing to weight gain; it also inhibits glucose-induced insulin secretion and stimulates gastrointestinal motility [114-117]. In contrast, peptide YY (PYY), cholecystokinin (CCK), and GLP-1 influence a range of processes in the central nervous system, including the stimulation and inhibition of POMC/α-MSH neurons, gastrointestinal motility, and gastric emptying. These hormones induce a feeling of fullness by modulating food intake. Gastrin acts antagonistically to PYY, CCK, and GLP-1 signaling, amplifying appetite and enhancing food consumption by stimulating orexin activity, while suppressing the release of these gut-derived peptide hormones, ultimately contributing to obesity [118]. Studies have demonstrated that subjects significantly reduce food intake after consuming a microencapsulated bitter ingredient (EBI) [119]. Immunofluorescence studies reveal that gastrin co-localizes with taste complex G proteins. Bitter taste receptor agonists inhibit gastrin and motilin secretion via the taste G protein α-Gustducin, which in turn increases GLP-1, PYY, and CCK secretion, thereby reducing appetite [120]. TAS2R agonists inhibit the secretion of gastrin and motilin, thereby increasing the secretion of GLP-1, PYY, and CCK, which in turn helps reduce appetite. This underscores the potential of targeting TAS2R as a therapeutic strategy for obesity and related metabolic disorders.
3.5.2.2 Regulate gastrointestinal function
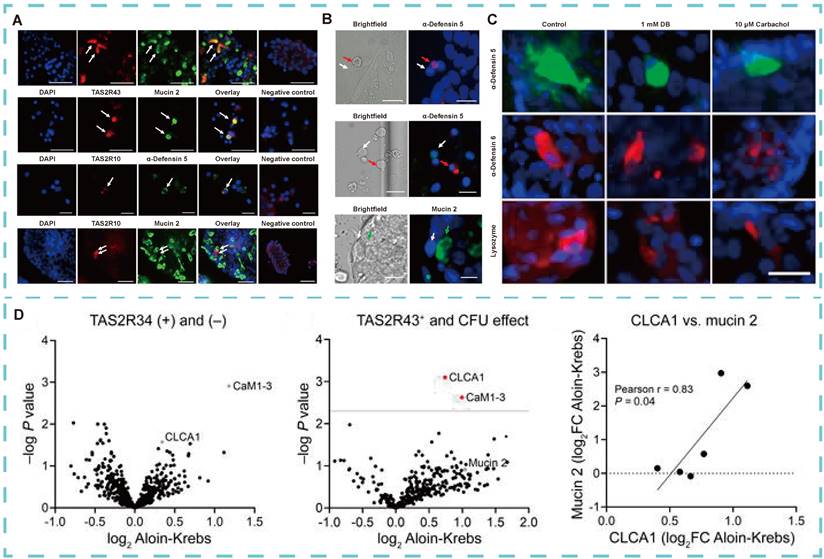
Dysbiosis of the gut microbiota is recognized as a major contributor to obesity [121]. Enteroendocrine cells (EECs), goblet cells, and Paneth cells regulate food intake and the secretion of hunger hormones in mice [34, 122]. These cells are located in distinct regions of the gut, with Paneth cells residing at the base of the intestinal villi within the crypts. The crypts are tubular structures that form depressions in the intestinal wall, and their depth is inversely correlated with nutrient absorption capacity. Bitter compounds stimulate jejunal crypts, particularly in obese individuals, triggering the release of antimicrobial peptides. These peptides regulate obesity by protecting against pathogenic infections, preventing commensal bacterial antigen translocation, and modulating the gut microbiota [123], Notably, peptides such as alpha-defensin 5 and regenerating islet-derived protein 3 alpha (REG3A), along with innate immune factors like mucins and chemokines, are released in response to E. coli exposure. To confirm the physiological role of TAS2R in the human intestine, Kathrin I. Liszt's team [57] found that Paneth cells co-localize with TAS2R10 and TAS2R43, whereas goblet cells co-localize only with TAS2R43 in the jejunal crypts of obese individuals (Figure 7A). Stimulation of these crypts with bitter compounds induces the release of antimicrobial peptides and proteins (Figure 7B-D), thereby playing a role in obesity regulation.
Role of TAS2R in human intestinal physiology. (A) Typical double fluorescence images of jejunal crypts in obese subjects. (B) Identification of immunostaining of cells in primary crypt foci of obese patients and the effect of bitter compound DB of intracellular Ca2+ changes. (C) Quantification of the effect of time-dependent protein expression in Paneth cells of lean and obese individuals. (D) Proteomic analysis of supernatant of jejunal crypts in obese patients after stimulation with bittering agents. Adapted with permission from [57], copyright 2021, American Society for Clinical Investigation.
Additionally, TAS2R expressed in gastrointestinal smooth muscle cells plays a critical role in regulating gut motility. Activation of TAS2R by bitter compounds enhances gastrointestinal motility, induces smooth muscle contraction, delays gastric emptying, and reduces inter-digestive gastric motility. These effects are mediated by Ca2+ elevation and ERK phosphorylation in human gastric smooth muscle cells (hGSMC), leading to earlier satiety and reduced nutrient intake. Furthermore, studies have identified pathways through which bitter compounds influence food intake by modulating the expression of GDF15, ADM2, and LDLR. The upregulation of these molecules following bitter compound ingestion triggers a conditioned taste aversion response to food stress, contributing to anorexic and weight-reducing effects. In summary, the activation of TAS2R in the gastrointestinal tract, which enhances gut motility and influences hunger signaling, presents a potential strategy for weight control [57].
3.5.2.3 Reduce lipid accumulation
Kimura et al. [124] identified the expression of TAS2R108, TAS2R126, TAS2R135, TAS2R137, and TAS2R143 in murine adipose tissue, though the function of TAS2R in this tissue remains unclear. The team investigated the role of TAS2R in adipocytes through experiments analyzing gene expression changes in 3T3-L1 cells following bitter compound stimulation, along with TAS2R gene overexpression in preadipocytes. Their findings revealed that TAS2R gene expression was elevated in white adipose tissue (WAT) following exposure to bitter compounds or feeding in mice. An increase in TAS2R expression was also observed after the induction of 3T3-L1 adipocyte differentiation, bitter stimulation, and serum starvation. These findings suggest a potential role for TAS2R in 3T3-L1 adipocytes. Overexpression of TAS2R108 and TAS2R126 reduced the proliferation and differentiation of 3T3-L1 preadipocytes [125], whereas both overexpression and knockdown of TAS2R106 decreased lipid accumulation in adipocytes and reduced the expression of adipogenic genes. Although the specific functions of TAS2R remain to be fully elucidated, these findings suggest that TAS2R may play a significant role in regulating adipocyte function and lipid metabolism, thus offering a potential target for modulating adiposity and related metabolic processes.
3.6 The therapeutic potential of TAS2R as a target for preterm labor intervention
Current research suggests that TAS2R plays a crucial role in modulating physiological and pathological processes. The widespread expression of TAS2R across diverse tissues underscores their potential as promising molecular targets for drug delivery strategies. Therefore, exploring the expression and function of TAS2R is crucial for developing more effective and precise drug delivery systems. Preterm birth (PTB), defined as birth before 37 weeks of gestation, is a leading cause of infant illness and death worldwide. Each year, 15 million preterm births result in 1 million neonatal deaths. PTB affects 1 in 10 pregnancies in the U.S., impacting 500,000 newborns each year, and is associated with 70% of fetal health issues [126]. Therefore, identifying innovative molecular targets that facilitate uterine relaxation, alongside the development of new classes of efficacious and safe tocolytics, is essential for advancing the management and treatment of PTB. Zheng et al. [127] demonstrated that activating bitter taste receptors exerts a potent relaxing effect on the contracted myometrium by inhibiting uterotonic-induced calcium (Ca²⁺) signaling pathways. Their studies revealed that this relaxation mechanism surpasses the efficacy of current tocolytic agents in preventing preterm births in two clinically relevant mouse models. Furthermore, activating the TAS2R signaling system in myometrial cells induces profound relaxation of the myometrium precontracted by a broad spectrum of contractile agonists. These findings suggest that targeting TAS2R is an ideal strategy for PTB treatment and highlight the potential for developing a novel class of tocolytics targeting the TAS2R family, offering a promising avenue for improved treatment and prevention of PTB. In summary, TAS2R is emerging as a key modulator of various physiological and pathological processes. These findings underscore the potential of TAS2R as a novel class of therapeutic targets, offering a promising avenue for developing more effective and precise drug delivery systems to address significant health challenges.
3.7 Role of TAS2R in cancer
3.7.1 Head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) primarily affects regions such as the nasal cavity, sinuses, oral cavity, pharynx, and larynx [128]. It is one of the most prevalent cancers, characterized by a high incidence and low survival rate. In addition to regional surgery, conventional cytotoxic chemotherapy, and radiotherapy, treatment options remain limited [129], highlighting the need for the exploration of targeted therapies. To determine whether TAS2R in HNSCC cells are functional, Carey et al. [130] evaluated various bitter compounds, assessing the responses elicited by agonists in calcium-loaded HNSCC cells. The findings demonstrated that bitter substances activate TAS2R-mediated Ca2+ influx, which triggers mitochondrial depolarization, caspase activation, and apoptosis. Buffering the nuclear Ca2+ elevation was shown to attenuate cysteine activation, indicating that TAS2R can induce apoptosis in vitro to inhibit HNSCC proliferation. Furthermore, an increase in the expression levels of TAS2R within HNSCC, as mapped within the cancer genome, was found to be significantly correlated with improved overall survival. The study suggests that TAS2R may serve as a therapeutic target to stimulate apoptosis and facilitate tumor-microbiome crosstalk in HNSCC, representing a potential biomarker for predicting outcomes and guiding therapeutic decisions (Figure 8).
3.7.2 Pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC), the most common subtype of pancreatic cancer (PC) [131], is a highly aggressive and lethal malignancy. PDAC arises when pancreatic cells acquire abnormal DNA mutations, enabling uncontrolled growth and division, which leads to tumor formation [132, 133]. Due to the lack of characteristic early-stage symptoms and its aggressive nature, many PDAC patients are diagnosed at an advanced stage, when the cancer has already spread. Consequently, chemotherapy and radiotherapy remain the primary treatment options [134]. PDAC exhibits multiple mechanisms of resistance to various drugs, which limit the effectiveness of these treatments. Understanding drug resistance at the molecular level is crucial for identifying novel therapeutic targets to restore drug efficacy and overcome chemotherapy resistance. This underscores the potential of bitter compounds and their receptors in addressing these challenges. Louisa Stern [135] was the first to report the expression and function of TAS2R10 in PDAC tissues and cell lines. Moreover, caffeine, a known ligand for TAS2R10, was found to sensitize tumor cells to the effects of two standard chemotherapeutic agents: gemcitabine and 5-fluorouracil. Knockdown of TAS2R10 in the BxPC-3 cell line diminished the efficacy of caffeine-induced sensitization. A plausible mechanism is that caffeine inhibits Akt phosphorylation by activating TAS2R10, leading to downregulation of ABCG2 expression. ABCG2 is a critical mediator of multidrug resistance, enabling cellular resilience against various chemotherapies. In summary, TAS2R10 functions in PDAC by downregulating chemoresistance in tumor cells, potentially improving patient outcomes. Additionally, Hung et al. [136] designed a TAS2R9-targeted liposome and demonstrated its ability to bind to TAS2R9 recombinant protein in a proof-of-concept drug delivery experiment. The study reports and validates TAS2R9 expression in pancreatic cancer-associated fibroblasts (CAFs), showing that TAS2R9 is a feasible target for therapy. The results show that TAS2R9 is a targeted receptor for HTTIPKV and a novel selective marker for CAFs (Figure 9). Higher liposome accumulation was observed in the targeted group compared to control liposomes, as shown using FMT system software. This indicated increased total drug exposure, and imaging via confocal microscopy revealed evident binding of liposomes to CAFs. In contrast to systemic administration, the average tumor volume in mice treated with targeted liposomes was significantly lower than in untreated mice. Collectively, these results show that the targeted liposome can effectively target tumor cells and restrain tumor development. These results suggest that TAS2R may play a significant role in drug delivery.
3.7.3 Neuroblastoma
Neuroblastoma (NB), a malignancy originating from neural crest cells, is the most prevalent extracranial solid tumor in pediatric populations and can arise anywhere within the sympathetic nervous system [137]. Unfortunately, current treatment regimens for high-risk neuroblastoma yield poor success rates, leaving survivors burdened with numerous long-term side effects from treatment [138]. NB cells exhibit characteristics similar to cancer stem cells (CSCs), facilitating both tumorigenesis and metastasis. According to SEO [31], TAS2R8 and TAS2R10 are expressed in NB cells, where they play crucial roles in reducing stemness, migration, and invasion of these cancer cells. Similarly, ectopic transfection of these TAS2R in BE (2) C cells induced synaptic elongation, downregulated CSC markers (such as DLK1, CD133, Notch, and Sox2), and suppressed tumorigenicity. Moreover, overexpression of TAS2R suppresses cell migration, invasion, and matrix metalloproteinase activity. Notably, TAS2R also reduces the expression of hypoxia-inducible factor-1α, a pivotal regulator of tumor metastasis, along with its downstream targets, including vascular endothelial growth factor and glucose transporter-1 [139]. Collectively, these findings indicate that TAS2R can target CSCs by inhibiting cancer susceptibility traits and NB cell invasion, thereby highlighting their therapeutic potential in NB. This emphasizes the need for further investigation into the expression and function of TAS2R in NB and other diseases to facilitate the development of more effective and precise drug delivery systems.
3.7.4 Acute myeloid leukemia
Acute myeloid leukemia (AML), a rare but highly aggressive hematological malignancy, is associated with a high mortality rate [140]. Its pathogenesis is driven by epigenetic dysregulation, resulting from reciprocal translocations and mutations in transcriptional regulators and chromatin remodeling factors. These alterations lead to bone marrow differentiation arrest and an enhanced capacity for malignant self-renewal [141]. In the complex interplay between AML cells and their microenvironment, membrane receptors initiate intracellular signals, enabling cells to respond to external stimuli and activate specific signaling pathways. Valentina et al. [142] characterized TAS2R expression in AML cells. Gene expression analysis revealed that activation of TAS2R by the prototypical agonist benzoic acid diamide significantly affected a range of genes involved in key AML processes. These molecular findings were supported by functional assays, which demonstrated that denatonium inhibited AML cell proliferation by inducing cell cycle arrest in the G0/G1 phase and triggering apoptosis through caspase cascade activation. Additionally, diammonium exposure reduced AML cell motility and migration, while simultaneously impairing cellular respiration by decreasing glucose uptake and oxidative phosphorylation. In conclusion, these findings extend previous observations of TAS2R expression in cancer cells to hematological malignancies, highlighting the role of TAS2R in the extrinsic regulation of leukemic cell functions. These emphasize the need for further investigation into the expression and function of TAS2R in AML and other diseases to develop more effective and precise drug delivery systems, offering a promising avenue for improving treatment and prevention of this devastating disease.
3.7.5 Other cancers
TAS2R also plays significant roles in the pathophysiology and potential treatment of cancers such as breast, ovarian, and prostate cancer. Breast cancer remains a leading cause of cancer-related mortality among women, with approximately 26% of diagnoses annually and contributing to 14% of cancer-related deaths [10]. Several therapeutic approaches are available for breast cancer treatment, including surgery, radiotherapy, chemotherapy, endocrine therapy, targeted therapy, and immunotherapy [143]. However, these strategies often cause significant side effects, contribute to drug resistance, and demonstrate limited efficacy in certain cases, leading to suboptimal therapeutic outcomes. Singh et al. [10] reported reduced TAS2R4 expression and elevated TAS2R14 expression in breast cancer tissues. The activation of TAS2R4 and TAS2R14 was shown to suppress tumor cell migration and proliferation while promoting apoptosis through the MAPK/ERK1/2 and Gα12/13-RhoGTPase pathways, as well as GPCR transactivation. These findings suggest that TAS2R activation can inhibit tumor progression by modulating intracellular signaling pathways, with minimal adverse effects and a lower likelihood of drug resistance. Consequently, TAS2R represents a promising target for breast cancer therapy due to its multifaceted anti-tumor effects.
Role of TAS2R in Head and Neck Squamous Cell Carcinoma. (A) Quantitative reverse transcription PCR of TAS2R. (B) Bitter (T2R) agonists cause mitochondrial depolarization of HNSCC. (C) Bitter (T2R) agonists decrease cellular metabolism in HNSCC. Adapted with permission from [130], copyright 2021, John Wiley & Sons.
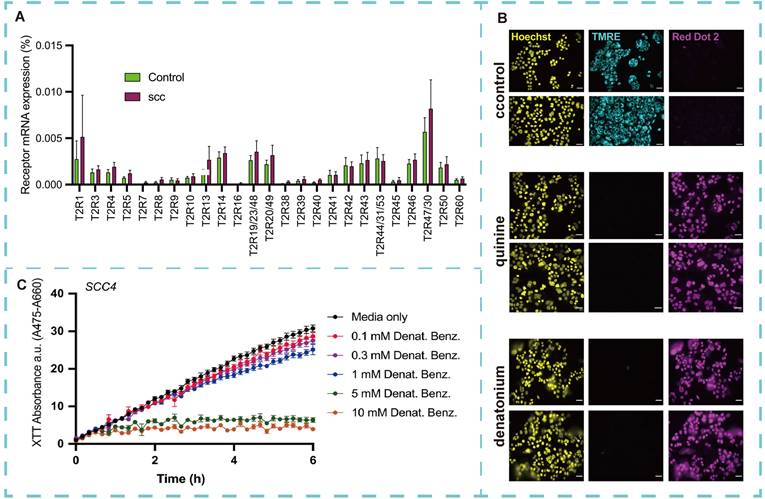
TAS2R9 is a viable molecular target for Pancreatic Ductal Adenocarcinoma. (A) Schematic of HTTIPKV liposome. (B) Schematic of mouse study design. (C) FMT images at 24 h post-liposome injection. (D) Average tumor volumes following different treatments in mice. Adapted with permission from [136], copyright 2023, MDPI.
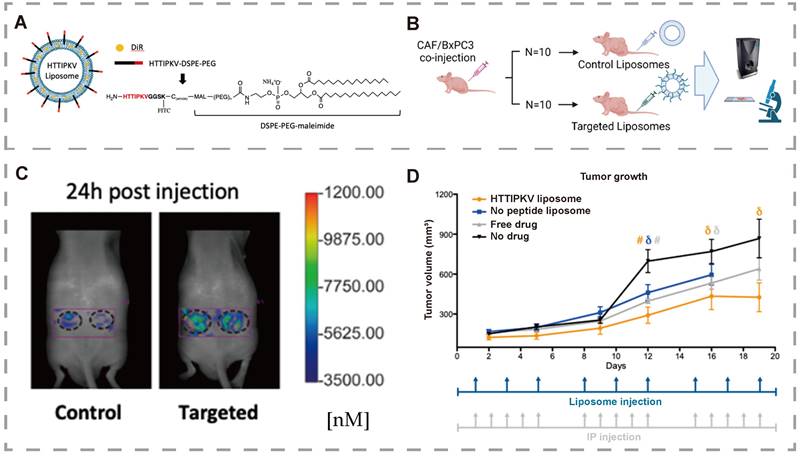
Prostate cancer ranks as the second most common malignancy among men and is the second leading cause of cancer-related deaths in this population [144]. Patients with early-stage localized prostate cancer generally have a favorable prognosis, with a 10-year survival rate of 99%. However, metastatic prostate cancer drastically reduces the five-year survival rate to approximately 30%. High-grade serous ovarian carcinoma (HGSOC) is the most lethal gynecological malignancy worldwide, responsible for the majority of ovarian cancer-related deaths, with a five-year survival rate near 30% [145]. These poor survival rates emphasize the need for novel therapeutic strategies for both prostate and ovarian cancer. Martin et al. [11] examined TAS2R expression in human epithelial ovarian and prostate cancer cells using qPCR analysis. Compared to the benign prostatic hyperplasia cell line (BPH1), most TAS2R genes were significantly downregulated in prostate cancer cells. Furthermore, noscapine treatment induced significant apoptosis in ovarian cancer cells. These findings suggest that TAS2R activation by bitter compounds can trigger apoptosis in tumor cells, highlighting TAS2R as a promising target for novel anticancer therapies.
4. TAS2R receptor agonists
Following the discovery of TAS2R, numerous bitter compounds have been screened for their interaction with cognate receptors, including drugs and food components from both natural and synthetic sources. Several bitter compounds have been identified as agonists for specific TAS2R isomers. Thousands of these compounds are well-documented and cataloged in existing databases. Among the diverse types of bitter compounds, polyphenols are particularly prominent [146]. Based on this principle, we conducted a preliminary literature review to summarize the TAS2R targeted by various common bitter compounds and outline their physiological roles (Table 2).
5. Conclusion and outlook
Significant progress has been made in elucidating the expression of TAS2R beyond the oral cavity and their diverse physiological roles. These receptors extend beyond taste perception and toxic substance aversion, influencing multiple physiological functions. However, the mechanisms through which TAS2R modulate disease states, including osteomyelitis, cancer, and type 2 diabetes mellitus (T2DM), remain insufficiently explored. TAS2R can both suppress and exacerbate inflammation, underscoring the need for deeper insights into their dual roles in disease pathophysiology. While bitter taste perception is a well-established TAS2R function, their involvement in broader physiological processes remains poorly defined. The association between TAS2R polymorphisms and human lifespan presents a compelling avenue for further research. A major challenge is the scarcity of well-characterized TAS2R agonist formulations, as most available drugs exhibit mild bitterness, with limited investigation into their impact on patient adherence.
Common bitter receptor agonists
| Compound | TAS2R | Main function | Ref |
|---|---|---|---|
| Chinese gentian | TAS2R1 | Inhibit fat production and reduce daily energy intake | [119, 147] |
| Quinine | TAS2R4, 7,10,14, 31,39,40,43,46 | Regulate lipogenesis and treat chronic sinusitis | [148, 149] |
| Chloroquine | TAS2R3 | Relieve respiratory discomfort | [150] |
| 1,10-phenanthroline | TAS2R5 | Causes relaxation of airway smooth muscle | [23] |
| Diammonium benzoate | TAS2R8, 13,47 | Regulate metabolism | [151] |
| Caffeine | TAS2R10,14,43,46 | Induce gastric parietal cells to secrete gastric acid and down-regulate drug resistance of PDAC cells | [135, 152] |
| Epicatechin | TAS2R14, 39 | Enhance satiety and reduce food intake. | [153] |
| Saccharin | TAS2R31, 43 | Can dilate bronchi. | [154] |
| Quercetin | TAS2R14 | Play a neuroprotective role. | [155] |
| Naringenin | TAS2R4, 14 | Bronchiectasis, anti-inflammatory, anti-tumor | [156-158] |
| Andrographolide | TAS2R50 | Antioxidant and anti-inflammatory. | [159] |
| Diphenhydramine | TAS2R14 | Central nervous depressant | [91] |
| Vanillin | TAS2R14, 20,39 | Enhance appetite and improve the composition of the gastrointestinal microbiota. | [45] |
| Piperine | TAS2R14 | Stimulation of glucagon-like peptide-1 secretion. | [33] |
TAS2R regulate various physiological functions, including hormone secretion, gastrointestinal (GI) motility, and neural activation. Activation of these receptors stimulates neurohormone release, modulating food intake through peripheral effects on GI motility and central orexigenic signaling. These findings suggest that TAS2R could be targeted for obesity management, highlighting taste receptor modulation as a novel strategy for appetite control. TAS2R agonists have also been investigated for their signaling mechanisms and pharmacological effects, revealing expression in extraoral tissues such as myometrial and bone cells, as well as in cancers like pancreatic ductal adenocarcinoma (PDAC). This suggests their potential not only as therapeutic targets but also as biomarkers for disease progression. The characterization of numerous TAS2R agonists with favorable safety profiles in phase I clinical trials for metabolic and inflammatory diseases further supports their potential for broader therapeutic applications.
In summary, TAS2R exhibit diverse physiological roles extending well beyond taste perception. Emerging evidence suggests they should be considered not only as taste receptors but also as promising therapeutic targets for multiple diseases. This potential is particularly evident in respiratory diseases like asthma, where TAS2R activation has demonstrated airway relaxation and improved lung function. However, the full therapeutic potential of TAS2R remains to be elucidated. Further investigation is necessary to clarify TAS2R signaling mechanisms, receptor specificity, and potential side effects, along with their relevance in treating diseases beyond the respiratory system. Continued research is essential to fully unlock the therapeutic potential of TAS2R, paving the way for the development of targeted treatments across multiple conditions.
Acknowledgements
This work was grateful to the financial support from the National Natural Science Foundation of China (82304425), the project of Liaoning Province Department of Education (JYTQN2023321, LJ212410163025), and the Doctoral Start-up Foundation of Liaoning Province (2024-BS-071).
Author contributions
Aiyang Tong: writing—original draft, visualization, writing—review and editing. Hongyu Yang: investigation, writing—review and editing. Xiaohui Yu: investigation, visualization. Dongkai Wang: writing—review and editing, visualization. Jian Guan: writing—review and editing. Ming Zhao: conceptualization, project administration. Ji Li: conceptualization, project administration, writing—review and editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dong H, Liu J, Zhu J, Zhou Z, Tizzano M, Peng X. et al. Oral Microbiota-Host Interaction Mediated by Taste Receptors. Front Cell Infect Microbiol. 2022;12:802504
2. Xi R, Zheng X, Tizzano M. Role of Taste Receptors in Innate Immunity and Oral Health. J Dent Res. 2022;101:759-68
3. Behrens M, Lang T. Extra-Oral Taste Receptors—Function, Disease, and Perspectives. Front Nutr. 2022;9:881177
4. Wooding SP, Ramirez VA, Behrens M. Bitter taste receptors: Genes, evolution and health. Evol Med Public Health. 2021;9:431-47
5. Feng P, Wang H, Liang X, Dong X, Liang Q, Shu F. et al. Relationships between Bitter Taste Receptor Gene Evolution, Diet, and Gene Repertoire in Primates. Genome Biol Evol. 2024;16:evae104
6. Lang T, Di Pizio A, Risso D, Drayna D, Behrens M. Activation Profile of TAS2R2, the 26th Human Bitter Taste Receptor. Mol Nutr Food Res. 2023;67:e2200775
7. Luo X-C, Chen Z-H, Xue J-B, Zhao D-X, Lu C, Li Y-H. et al. Infection by the parasitic helminthTrichinella spiralisactivates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc Natl Acad Sci U S A. 2019;116:5564-9
8. Sun S, Yang Y, Xiong R, Ni Y, Ma X, Hou M. et al. Oral berberine ameliorates high-fat diet-induced obesity by activating TAS2Rs in tuft and endocrine cells in the gut. Life Sci. 2022;311:121141
9. Mori S, Nakamura N, Fuchigami A, Yoshimoto S, Sakakibara M, Ozawa T. et al. Intracellular TAS2Rs act as a gatekeeper for the excretion of harmful substances via ABCB1 in keratinocytes. FASEB BioAdv. 2024;6:424-41
10. Singh N, Shaik FA, Myal Y, Chelikani P. Chemosensory bitter taste receptors T2R4 and T2R14 activation attenuates proliferation and migration of breast cancer cells. Mol Cell Biochem. 2020;465:199-214
11. Martin LTP, Nachtigal MW, Selman T, Nguyen E, Salsman J, Dellaire G. et al. Bitter taste receptors are expressed in human epithelial ovarian and prostate cancers cells and noscapine stimulation impacts cell survival. Mol Cell Biochem. 2018;454:203-14
12. Talmon M, Pollastro F, Fresu LG. The Complex Journey of the Calcium Regulation Downstream of TAS2R Activation. Cells. 2022;11:3638
13. Richter P, Andersen G, Kahlenberg K, Mueller AU, Pirkwieser P, Boger V. et al. Sodium-Permeable Ion Channels TRPM4 and TRPM5 are Functional in Human Gastric Parietal Cells in Culture and Modulate the Cellular Response to Bitter-Tasting Food Constituents. J Agric Food Chem. 2024;72:4906-17
14. Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90:822S-5S
15. Yuan G, Jing Y, Wang T, Fernandes VS, Xin W. The bitter taste receptor agonist-induced negative chronotropic effects on the Langendorff-perfused isolated rat hearts. Eur J Pharmacol. 2020;876:173063
16. Belloir C, Karolkowski A, Thomas A, Menin R, Briand L. Modulation of bitter taste receptors by yeast extracts. Food Res Int. 2024;190:114596
17. Stoeger V, Holik A-K, Hölz K, Dingjan T, Hans J, Ley JP. et al. Bitter-Tasting Amino Acids l-Arginine and l-Isoleucine Differentially Regulate Proton Secretion via T2R1 Signaling in Human Parietal Cells in Culture. J Agric Food Chem. 2020;68:3434-44
18. Upadhyaya J, Pydi SP, Singh N, Aluko RE, Chelikani P. Bitter taste receptor T2R1 is activated by dipeptides and tripeptides. Biochem Biophys Res Commun. 2010;398:331-5
19. Choi J-H, Lee J, Yang S, Lee EK, Hwangbo Y, Kim J. Genetic variations in TAS2R3 and TAS2R4 bitterness receptors modify papillary carcinoma risk and thyroid function in Korean females. Sci Rep. 2018;8:15004
20. Mikołajczyk-Stecyna J, Malinowska AM, Chmurzynska A. Polymorphism of TAS2R3, TAS2R5, TAS2R19, and TAS2R50 genes and bitter food intake frequency inelderly woman. Acta Sci Pol Technol Aliment. 2020;19:109-22
21. Zhu H, Liu L, Ren L, Chen J, Peng L, Shi C. et al. Bitter receptor member TAS2R4 may have neurobiological function beyond acting as a bitter receptor. Acta Biochim Biophys Sin. 2020;52:460-2
22. Sterneder S, Stoeger V, Dugulin CA, Liszt KI, Di Pizio A, Korntheuer K. et al. Astringent Gallic Acid in Red Wine Regulates Mechanisms of Gastric Acid Secretion via Activation of Bitter Taste Sensing Receptor TAS2R4. J Agric Food Chem. 2021;69:10550-61
23. Kim D, An SS, Lam H, Leahy JW, Liggett SB. Identification and Characterization of Novel Bronchodilator Agonists Acting at Human Airway Smooth Muscle Cell TAS2R5. ACS Pharmacol Transl Sci. 2020;3:1069-75
24. Grau-Bové C, Grau-Bové X, Terra X, Garcia-Vallve S, Rodríguez-Gallego E, Beltran-Debón R. et al. Functional and genomic comparative study of the bitter taste receptor family TAS2R: Insight into the role of human TAS2R5. FASEB J. 2022;36:e22175
25. Jalševac F, Descamps-Solà M, Grau-Bové C, Segú H, Auguet T, Avilés-Jurado FX. et al. Profiling bitter taste receptors (TAS2R) along the gastrointestinal tract and their influence on enterohormone secretion. Gender- and age-related effects in the colon. Front Endocrinol (Lausanne). 2024;15:1436580
26. Wang Y, Zajac AL, Lei W, Christensen CM, Margolskee RF, Bouysset C. et al. Metal Ions Activate the Human Taste Receptor TAS2R7. Chem Senses. 2019;44:339-47
27. Behrens M, Redel U, Blank K, Meyerhof W. The human bitter taste receptor TAS2R7 facilitates the detection of bitter salts. Biochem Biophys Res Commun. 2019;512:877-81
28. Chen J-G, Ping N-N, Liang D, Li M-Y, Mi Y-N, Li S. et al. The expression of bitter taste receptors in mesenteric, cerebral and omental arteries. Life Sci. 2016;170:16-24
29. Kojima T, Maeda T, Suzuki A, Yamamori T, Kato Y. Intracellular zinc-dependent TAS2R8 gene expression through CTCF activation. Biomed Res. 2020;41:217-25
30. Fotsing JR, Darmohusodo V, Patron AP, Ching BW, Brady T, Arellano M. et al. Discovery and Development of S6821 and S7958 as Potent TAS2R8 Antagonists. J Med Chem. 2020;63:4957-77
31. Seo Y, Kim Y-S, Lee KE, Park TH, Kim Y. Anti-cancer stemness and anti-invasive activity of bitter taste receptors, TAS2R8 and TAS2R10, in human neuroblastoma cells. PLoS One. 2017;12:e0176851
32. Nolden AA, Hayes JE, Feeney EL. Variation in TAS2R receptor genes explains differential bitterness of two common antibiotics. Front Genet. 2022;13:960154
33. Huang T-T, Gu P-P, Zheng T, Gou L-S, Liu Y-W. Piperine, as a TAS2R14 agonist, stimulates the secretion of glucagon-like peptide-1 in the human enteroendocrine cell line Caco-2. Food Funct. 2021;13:242-54
34. Wang Q, Liszt KI, Deloose E, Canovai E, Thijs T, Farré R. et al. Obesity alters adrenergic and chemosensory signaling pathways that regulate ghrelin secretion in the human gut. FASEB J. 2019;33:4907-20
35. Li Q, Shan X, Yuan Y, Ye W, Fang X. Shegan-Mahuang decoction ameliorates cold-induced asthma via regulating the proliferation and apoptosis of airway smooth muscle cells through TAS2R10: An in vivo and in vitro study. J Ethnopharmacol. 2024;334:118504
36. Navarro-Dorado J, Climent B, López-Oliva ME, Pilar Martínez M, Hernández-Martín M, Agis-Torres Á. et al. The bitter taste receptor (TAS2R) agonist denatonium promotes a strong relaxation of rat corpus cavernosum. Biochem Pharmacol. 2023;215:115754
37. Dmitrzak-Weglarz M, Szczepankiewicz A, Rybakowski J, Kapelski P, Bilska K, Skibinska M. et al. Transcriptomic profiling as biological markers of depression - A pilot study in unipolar and bipolar women. World J Biol Psychiatry. 2021;22:744-56
38. Taher S, Borja Y, Cabanela L, Costers VJ, Carson-Marino M, Bailes JC. et al. Cholecystokinin, gastrin, cholecystokinin/gastrin receptors, and bitter taste receptor TAS2R14: trophoblast expression and signaling. Am J Physiol Regul Integr Comp Physiol. 2019;316:R628-R39
39. Duarte AC, Rosado T, Costa AR, Santos J, Gallardo E, Quintela T. et al. The bitter taste receptor TAS2R14 regulates resveratrol transport across the human blood-cerebrospinal fluid barrier. Biochem Pharmacol. 2020;177:113953
40. Ni K, Che B, Gu R, Wang C, Xu H, Li H. et al. BitterDB database analysis plus cell stiffness screening identify flufenamic acid as the most potent TAS2R14-based relaxant of airway smooth muscle cells for therapeutic bronchodilation. Theranostics. 2024;14:1744-63
41. Malovini A, Accardi G, Aiello A, Bellazzi R, Candore G, Caruso C. et al. Taste receptors, innate immunity and longevity: the case of TAS2R16 gene. Immun Ageing. 2019;16:5
42. Kriauciunas A, Gedvilaite G, Bruzaite A, Zekonis G, Razukevicius D, Liutkeviciene R. Generalised Periodontitis: Examining TAS2R16 Serum Levels and Common Gene Polymorphisms (rs860170, rs978739, rs1357949). Biomedicines. 2024;12:319
43. Deng M, Hida N, Yamazaki T, Morishima R, Kato Y, Fujita Y. et al. Comparison of Bitterness Intensity between Prednisolone and Quinine in a Human Sensory Test Indicated Individual Differences in Bitter-Taste Perception. Pharmaceutics. 2022;14:2454
44. Purnell PR, Addicks BL, Zalzal HG, Shapiro S, Wen S, Ramadan HH. et al. Single Nucleotide Polymorphisms in Chemosensory Pathway Genes GNB3, TAS2R19, and TAS2R38 Are Associated with Chronic Rhinosinusitis. Int Arch Allergy Immunol. 2019;180:72-8
45. Morini G, Winnig M, Vennegeerts T, Borgonovo G, Bassoli A. Vanillin Activates Human Bitter Taste Receptors TAS2R14, TAS2R20 and TAS2R39. Front Nutr. 2021;8:683627
46. Shaw L, Mansfield C, Colquitt L, Lin C, Ferreira J, Emmetsberger J. et al. Personalized expression of bitter 'taste' receptors in human skin. PLoS One. 2018;13:e0205322
47. Kang W, Wang Y, Li J, Xie W, Zhao D, Wu L. et al. TAS2R supports odontoblastic differentiation of human dental pulp stem cells in the inflammatory microenvironment. Stem Cell Res Ther. 2022;13:374
48. Li Y, Liu B, Connolly ID, Kakusa BW, Pan W, Nagpal S. et al. Brief Report: Recurrently Mutated Genes Differ between Leptomeningeal and Solid Lung Cancer Brain Metastases. J Thorac Oncol. 2018;13:1022-7
49. Grassin-Delyle S, Salvator H, Mantov N, Abrial C, Brollo M, Faisy C. et al. Bitter Taste Receptors (TAS2Rs) in Human Lung Macrophages: Receptor Expression and Inhibitory Effects of TAS2R Agonists. Front Physiol. 2019;10:1267
50. Douglas JE, Lin C, Mansfield CJ, Arayata CJ, Cowart BJ, Spielman AI. et al. Tissue-Dependent Expression of Bitter Receptor TAS2R38 mRNA. Chem Senses. 2019;44:33-40
51. Melis M, Errigo A, Crnjar R, Pes GM, Tomassini Barbarossa I. TAS2R38 bitter taste receptor and attainment of exceptional longevity. Sci Rep. 2019;9:18047
52. Risso D, Carmagnola D, Morini G, Pellegrini G, Canciani E, Antinucci M. et al. Distribution of TAS2R38 bitter taste receptor phenotype and haplotypes among COVID-19 patients. Sci Rep. 2022;12:1-7
53. Yilmaz G, Eyigor H, Gur OE, Kalkan T, Gur N, Selcuk OT. et al. The role of TAS2R38 genotype in surgical outcomes and culturable bacteria in chronic rhinosinusitis with or without nasal polyps. Rhinology. 2023;61:54-60
54. Jalševac F, Terra X, Rodríguez-Gallego E, Beltran-Debón R, Blay MT, Pinent M. et al. The Hidden One: What We Know About Bitter Taste Receptor 39. Front Endocrinol (Lausanne). 2022;13:854718
55. Dunkel A, Hofmann T, Di Pizio A. In Silico Investigation of Bitter Hop-Derived Compounds and Their Cognate Bitter Taste Receptors. J Agric Food Chem. 2020;68:10414-23
56. Wang Q-C, Wang Z-Y, Xu Q, Li R-B, Zhang G-G, Shi R-Z. Exploring the Role of Epicardial Adipose Tissue in Coronary Artery Disease From the Difference of Gene Expression. Front Physiol. 2021;12:605811
57. Liszt KI, Wang Q, Farhadipour M, Segers A, Thijs T, Nys L. et al. Human intestinal bitter taste receptors regulate innate immune responses and metabolic regulators in obesity. J Clin Invest. 2021;132:e144828
58. Liszt KI, Ley JP, Lieder B, Behrens M, Stöger V, Reiner A. et al. Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc Natl Acad Sci U S A. 2017;114:E6260-E9
59. Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M. et al. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 2004;24:10260-5
60. Roudnitzky N, Risso D, Drayna D, Behrens M, Meyerhof W, Wooding SP. Copy Number Variation in TAS2R Bitter Taste Receptor Genes: Structure, Origin, and Population Genetics. Chem Senses. 2016;41:649-59
61. Xu W, Wu L, Liu S, Liu X, Cao X, Zhou C. et al. Structural basis for strychnine activation of human bitter taste receptor TAS2R46. Science. 2022;377:1298-304
62. Tuzim K, Korolczuk A. An update on extra-oral bitter taste receptors. J Transl Med. 2021;19:440
63. Clark AA, Dotson CD, Elson AE, Voigt A, Boehm U, Meyerhof W. et al. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015;29:164-72
64. Tiroch J, Sterneder S, Di Pizio A, Lieder B, Hoelz K, Holik A-K. et al. Bitter Sensing TAS2R50 Mediates the trans-Resveratrol-Induced Anti-inflammatory Effect on Interleukin 6 Release in HGF-1 Cells in Culture. J Agric Food Chem. 2021;69:13339-49
65. de Jesus VC, Mittermuller B-A, Hu P, Schroth RJ, Chelikani P. Genetic variants in taste genes play a role in oral microbial composition and severe early childhood caries. iScience. 2022;25:105489
66. Dash UC, Bhol NK, Swain SK, Samal RR, Nayak PK, Raina V. et al. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm Sin B. 2024: S2211383524004040.
67. Kverno K. New Treatment Aimed at Preventing Alzheimer's Dementia. J Psychosoc Nurs Ment Health Serv. 2022;60:11-4
68. Kosyreva AM, Sentyabreva AV, Tsvetkov IS, Makarova OV. Alzheimer's Disease and Inflammaging. Brain Sci. 2022;12:1237
69. Li Y, Xia X, Wang Y, Zheng JC. Mitochondrial dysfunction in microglia: a novel perspective for pathogenesis of Alzheimer's disease. J Neuroinflammation. 2022;19:248
70. Morén C, deSouza RM, Giraldo DM, Uff C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. Int J Mol Sci. 2022;23:9328
71. Alves VC, Figueiro-Silva J, Ferrer I, Carro E. Epigenetic silencing of OR and TAS2R genes expression in human orbitofrontal cortex at early stages of sporadic Alzheimer's disease. Cell Mol Life Sci. 2023;80:196
72. Zhu B, Yin D, Zhao H, Zhang L. The immunology of Parkinson's disease. Semin Immunopathol. 2022;44:1-14
73. Su Q, Ng WL, Goh SY, Gulam MY, Wang L-F, Tan E-K. et al. Targeting the inflammasome in Parkinson's disease. Front Aging Neurosci. 2022;14:957705
74. Garcia-Esparcia P, Schlüter A, Carmona M, Moreno J, Ansoleaga B, Torrejón-Escribano B. et al. Functional genomics reveals dysregulation of cortical olfactory receptors in Parkinson disease: novel putative chemoreceptors in the human brain. J Neuropathol Exp Neurol. 2013;72:524-39
75. Vascellari S, Melis M, Cossu G, Melis M, Serra A, Palmas V. et al. Genetic variants of TAS2R38 bitter taste receptor associate with distinct gut microbiota traits in Parkinson's disease: A pilot study. Int J Biol Macromol. 2020;165:665-74
76. Welcome MO, Mastorakis NE. The taste of neuroinflammation: Molecular mechanisms linking taste sensing to neuroinflammatory responses. Pharmacol Res. 2021;167:105557
77. Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun. 2011;406:146-51
78. Duarte AC, Costa AR, Gonçalves I, Quintela T, Preissner R, Santos CRA. The druggability of bitter taste receptors for the treatment of neurodegenerative disorders. Biochem Pharmacol. 2022;197:114915
79. Gianchecchi E, Fierabracci A. Insights on the Effects of Resveratrol and Some of Its Derivatives in Cancer and Autoimmunity: A Molecule with a Dual Activity. Antioxidants. 2020;9:91
80. Jotic AD, Opankovic AM, Radin ZZ, Cvorovic L, Vujovic KRS, Krejovic-Trivic SB. et al. Symptoms of depression, anxiety and stress in patients with chronic otitis media. PLoS One. 2022;17:e0270793
81. Leichtle A, Kurabi A, Leffers D, Därr M, Draf CS, Ryan AF. et al. Immunomodulation as a Protective Strategy in Chronic Otitis Media. Front Cell Infect Microbiol. 2022;12:826192
82. Kaufman AC, Colquitt L, Ruckenstein MJ, Bigelow DC, Eliades SJ, Xiong G. et al. Bitter Taste Receptors and Chronic Otitis Media. Otolaryngol Head Neck Surg. 2021;165:290-9
83. Marin C, Hummel T, Liu Z, Mullol J. Chronic Rhinosinusitis and COVID-19. J Allergy Clin Immunol Pract. 2022;10:1423-32
84. Grayson JW, Cavada M, Harvey RJ. Clinically relevant phenotypes in chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2019;48:23
85. Zhang Y, Lan F, Zhang L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy. 2022;77:3309-19
86. Mao D, He Z, Li L, Lei Y, Xiao M, Zhang H. et al. Recent Progress in Traditional Chinese Medicines and Their Mechanism in the Treatment of Allergic Rhinitis. J Healthc Eng. 2022;2022:3594210
87. Chen J, Larson ED, Anderson CB, Agarwal P, Frank DN, Kinnamon SC. et al. Expression of Bitter Taste Receptors and Solitary Chemosensory Cell Markers in the Human Sinonasal Cavity. Chem Senses. 2019;44:483-95
88. Kaske S, Krasteva G, König P, Kummer W, Hofmann T, Gudermann T. et al. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49
89. Barham HP, Cooper SE, Anderson CB, Tizzano M, Kingdom TT, Finger TE. et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. 2013;3:450-7
90. Jeruzal-Świątecka J, Fendler W, Pietruszewska W. Clinical Role of Extraoral Bitter Taste Receptors. Int J Mol Sci. 2020;21:E5156
91. Kuek LE, McMahon DB, Ma RZ, Miller ZA, Jolivert JF, Adappa ND. et al. Cilia Stimulatory and Antibacterial Activities of T2R Bitter Taste Receptor Agonist Diphenhydramine: Insights into Repurposing Bitter Drugs for Nasal Infections. Pharmaceuticals. 2022;15:452
92. Hariri BM, McMahon DB, Chen B, Freund JR, Mansfield CJ, Doghramji LJ. et al. Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J Biol Chem. 2017;292:8484-97
93. Freund JR, Mansfield CJ, Doghramji LJ, Adappa ND, Palmer JN, Kennedy DW. et al. Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling. J Biol Chem. 2018;293:9824-40
94. Piskadło-Zborowska K, Stachowiak M, Sarnowska E, Jowik R, Dżaman K. Assessment of the effect of inflammatory changes and allergic reaction on TAS2R38 receptor expression in patients with chronic sinusitis (CRS). Otolaryngol Pol. 2020;74:17-23
95. Kook JH, Kim HK, Kim HJ, Kim KW, Kim TH, Kang KR. et al. Increased expression of bitter taste receptors in human allergic nasal mucosa and their contribution to the shrinkage of human nasal mucosa. Clin Exp Allergy. 2016;46:584-601
96. Ahn JY, Choi BS. Application of a Cold Dry Air Provocation Test in Pediatric Patients with Asthma. Children. 2022;9:920
97. McIntyre A, Busse WW. Asthma exacerbations: the Achilles heel of asthma care. Trends Mol Med. 2022;28:1112-27
98. Bai Y, Zhou Q, Fang Q, Song L, Chen K. Inflammatory Cytokines and T-Lymphocyte Subsets in Serum and Sputum in Patients with Bronchial Asthma and Chronic Obstructive Pulmonary Disease. Med Sci Monit. 2019;25:2206-10
99. Lee J-H, Kim T-B. Eosinophil granule proteins: what they can tell us about asthma. Thorax. 2022;77:532-3
100. Joseph C, Tatler AL. Pathobiology of Airway Remodeling in Asthma: The Emerging Role of Integrins. J Asthma Allergy. 2022;15:595-610
101. Zhou Y-W, Sun J, Wang Y, Chen C-P, Tao T, Ma M. et al. Tas2R activation relaxes airway smooth muscle by release of Gαt targeting on AChR signaling. Proc Natl Acad Sci U S A. 2022;119:e2121513119
102. Deshpande DA, Wang WCH, McIlmoyle EL, Robinett KS, Schillinger RM, An SS. et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299-304
103. Kim D, Cho S, Castaño MA, Panettieri RA, Woo JA, Liggett SB. Biased TAS2R Bronchodilators Inhibit Airway Smooth Muscle Growth by Downregulating Phosphorylated Extracellular Signal-regulated Kinase 1/2. Am J Respir Cell Mol Biol. 2019;60:532-40
104. Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 2013;11:e1001501
105. Sharma P, Panebra A, Pera T, Tiegs BC, Hershfeld A, Kenyon LC. et al. Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2015;310:L365-L76
106. Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. The Lancet. 2022;400:1803-20
107. Sternini C, Rozengurt E. Bitter taste receptors as sensors of gut luminal contents. Nat Rev Gastroenterol Hepatol. 2025;22:39-53
108. Schmitz EA, Takahashi H, Karakas E. Structural basis for activation and gating of IP3 receptors. Nat Commun. 2022;13:1408
109. Granata A, Maccarrone R, Anzaldi M, Leonardi G, Pesce F, Amico F. et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15:sfac069
110. Archer N, Shaw J, Cochet-Broch M, Bunch R, Poelman A, Barendse W. et al. Obesity is associated with altered gene expression in human tastebuds. Int J Obesity. 2019;43:1475-84
111. Cancello R, Micheletto G, Meta D, Lavagno R, Bevilacqua E, Panizzo V. et al. Expanding the role of bitter taste receptor in extra oral tissues: TAS2R38 is expressed in human adipocytes. Adipocyte. 2020;9:7-15
112. Şeref B, Yıldıran H. A new perspective on obesity: perception of fat taste and its relationship with obesity. Nutr Rev. 2025: nuae028.
113. Lela L, Carlucci V, Kioussi C, Choi J, Stevens JF, Milella L. et al. Humulus lupulus L.: Evaluation of Phytochemical Profile and Activation of Bitter Taste Receptors to Regulate Appetite and Satiety in Intestinal Secretin Tumor Cell Line (STC-1 Cells). Mol Nutr Food Res. 2024;68:e2400559
114. Cervone DT, Lovell AJ, Dyck DJ. Regulation of adipose tissue and skeletal muscle substrate metabolism by the stomach-derived hormone, ghrelin. Curr Opin Pharm. 2020;52:25-32
115. Zhang C-S, Wang L-X, Wang R, Liu Y, Song L-M, Yuan J-H. et al. The Correlation Between Circulating Ghrelin and Insulin Resistance in Obesity: A Meta-Analysis. Front Physiol. 2018;9:1308
116. Abizaid A. Stress and obesity: The ghrelin connection. J Neuroendocrinol. 2019;31:e12693
117. Rubinić I, Kurtov M, Likić R. Novel Pharmaceuticals in Appetite Regulation: Exploring emerging gut peptides and their pharmacological prospects. Pharmacol Res Perspect. 2024;12:e1243
118. van Loenen MR, Geenen B, Arnoldussen IAC, Kiliaan AJ. Ghrelin as a prominent endocrine factor in stress-induced obesity. Nutr Neurosci. 2020;25:1413-24
119. Mennella I, Fogliano V, Ferracane R, Arlorio M, Pattarino F, Vitaglione P. Microencapsulated bitter compounds (from Gentiana lutea) reduce daily energy intakes in humans. Br J Nutr. 2016;116:1841-50
120. Janssen S, Laermans J, Verhulst P-J, Thijs T, Tack J, Depoortere I. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A. 2011;108:2094-9
121. Geng J, Ni Q, Sun W, Li L, Feng X. The links between gut microbiota and obesity and obesity related diseases. Biomed Pharmacother. 2022;147:112678
122. Steensels S, Depoortere I. Chemoreceptors in the Gut. Annu Rev Physiol. 2018;80:117-41
123. Bisht V, Das B, Hussain A, Kumar V, Navani NK. Understanding of probiotic origin antimicrobial peptides: a sustainable approach ensuring food safety. npj Science of Food. 2024;8:67
124. Kimura S, Kato E. TAS2R expression profile in brown adipose, white adipose, skeletal muscle, small intestine, liver and common cell lines derived from mice. Gene Rep. 2020;20:100763
125. Kimura S, Tsuruma A, Kato E. Taste 2 Receptor Is Involved in Differentiation of 3T3-L1 Preadipocytes. Int J Mol Sci. 2022;23:8120
126. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B. et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013 10 S2
127. Zheng K, Lu P, Delpapa E, Bellve K, Deng R, Condon JC. et al. Bitter taste receptors as targets for tocolytics in preterm labor therapy. FASEB J. 2017;31:4037-52
128. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92
129. Elmusrati A, Wang J, Wang C-Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int J Oral Sci. 2021;13:24
130. Carey RM, McMahon DB, Miller ZA, Kim T, Rajasekaran K, Gopallawa I. et al. T2R bitter taste receptors regulate apoptosis and may be associated with survival in head and neck squamous cell carcinoma. Mol Oncol. 2021;16:1474-92
131. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. The Lancet. 2020;395:2008-20
132. Moore A, Donahue T. Pancreatic Cancer. JAMA. 2019;322:1426
133. Hu ZI, O'Reilly EM. Therapeutic developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2024;21:7-24
134. Shah A, Ganguly K, Rauth S, Sheree SS, Khan I, Ganti AK. et al. Unveiling the resistance to therapies in pancreatic ductal adenocarcinoma. Drug Resist Updat. 2024;77:101146
135. Stern L, Giese N, Hackert T, Strobel O, Schirmacher P, Felix K. et al. Overcoming chemoresistance in pancreatic cancer cells: role of the bitter taste receptor T2R10. J Cancer. 2018;9:711-25
136. Hung J, Perez SM, Dasa SSK, Hall SP, Heckert DB, Murphy BP. et al. A Bitter Taste Receptor as a Novel Molecular Target on Cancer-Associated Fibroblasts in Pancreatic Ductal Adenocarcinoma. Pharmaceuticals (Basel). 2023;16:389
137. Y KN, Arjunan A, Maigandan D, Dharmarajan A, Perumalsamy LR. Advances and challenges in therapeutic resistant biomarkers of neuroblastoma: A comprehensive review. Biochim Biophys Acta. 2024;1879:189222
138. Zanotti S, Decaesteker B, Vanhauwaert S, De Wilde B, De Vos WH, Speleman F. Cellular senescence in neuroblastoma. Br J Cancer. 2022;126:1529-38
139. Costa AR, Duarte AC, Costa-Brito AR, Gonçalves I, Santos CRA. Bitter taste signaling in cancer. Life Sci. 2023;315:121363
140. Shimony S, Stahl M, Stone RM. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:502-26
141. Fennell KA, Bell CC, Dawson MA. Epigenetic therapies in acute myeloid leukaemia: where to from here? Blood. 2019;134:1891-901
142. Salvestrini V, Ciciarello M, Pensato V, Simonetti G, Laginestra MA, Bruno S. et al. Denatonium as a Bitter Taste Receptor Agonist Modifies Transcriptomic Profile and Functions of Acute Myeloid Leukemia Cells. Front Oncol. 2020;10:1225
143. Luque-Bolivar A, Pérez-Mora E, Villegas VE, Rondón-Lagos M. Resistance and Overcoming Resistance in Breast Cancer. Breast Cancer (Dove Med Press). 2020;12:211-29
144. Zhang Y, Liu C, Yang Y, Ren H, Ren T, Huang Y. et al. TRIB3 inhibition by palbociclib sensitizes prostate cancer to ferroptosis via downregulating SOX2/SLC7A11 expression. Cell Death Discov. 2024;10:1-10
145. Battistini C, Kenny HA, Zambuto M, Nieddu V, Melocchi V, Decio A. et al. Tumor microenvironment-induced FOXM1 regulates ovarian cancer stemness. Cell Death Dis. 2024;15:370
146. Soares S, Silva MS, García-Estevez I, Groβmann P, Brás N, Brandão E. et al. Human Bitter Taste Receptors Are Activated by Different Classes of Polyphenols. J Agric Food Chem. 2018;66:8814-23
147. Choi R-Y, Nam S-J, Lee H-I, Lee J, Leutou AS, Ri Ham J. et al. Gentiopicroside isolated from Gentiana scabra Bge. inhibits adipogenesis in 3T3-L1 cells and reduces body weight in diet-induced obese mice. Bioorg Med Chem Lett. 2019;29:1699-704
148. Ning X, He J, Shi Xe, Yang G. Regulation of Adipogenesis by Quinine through the ERK/S6 Pathway. Int J Mol Sci. 2016;17:504
149. Workman AD, Maina IW, Brooks SG, Kohanski MA, Cowart BJ, Mansfield C. et al. The Role of Quinine-Responsive Taste Receptor Family 2 in Airway Immune Defense and Chronic Rhinosinusitis. Front Immunol. 2018;9:624
150. Bouazza B, Ramdani I, Chahed R. Chloroquine and COVID-19: role as a bitter taste receptor agonist? New Microbes New Infect. 2021;40:100843
151. Brial F, Matsuda F, Gauguier D. Diet dependent impact of benzoate on diabetes and obesity in mice. Biochimie. 2021;194:35-42
152. Richter P, Sebald K, Fischer K, Behrens M, Schnieke A, Somoza V. Bitter Peptides YFYPEL, VAPFPEVF, and YQEPVLGPVRGPFPIIV, Released during Gastric Digestion of Casein, Stimulate Mechanisms of Gastric Acid Secretion via Bitter Taste Receptors TAS2R16 and TAS2R38. J Agric Food Chem. 2022;70:11591-602
153. Turner A, Veysey M, Keely S, Scarlett CJ, Lucock M, Beckett EL. Genetic Variation in the Bitter Receptors Responsible for Epicatechin Detection Are Associated with BMI in an Elderly Cohort. Nutrients. 2021;13:1-14
154. Lasconi C, Pifferi S, Hernandez-Clavijo A, Merigo F, Cecchini MP, Gonzalez-Velandia KY. et al. Bitter tastants and artificial sweeteners activate a subset of epithelial cells in acute tissue slices of the rat trachea. Sci Rep. 2019;9:8834
155. Grewal AK, Singh TG, Sharma D, Sharma V, Singh M, Rahman MH. et al. Mechanistic insights and perspectives involved in nfeuroprotective action of quercetin. Biomed Pharmacother. 2021;140:111729
156. Ni K, Guo J, Bu B, Pan Y, Li J, Liu L. et al. Naringin as a plant-derived bitter tastant promotes proliferation of cultured human airway epithelial cells via activation of TAS2R signaling. Phytomedicine. 2021;84:153491
157. Escribano-Ferrer E, Queralt Regué J, Garcia-Sala X, Boix Montañés A, Lamuela-Raventos RM. In Vivo Anti-inflammatory and Antiallergic Activity of Pure Naringenin, Naringenin Chalcone, and Quercetin in Mice. J Nat Prod. 2019;82:177-82
158. Stabrauskiene J, Kopustinskiene DM, Lazauskas R, Bernatoniene J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines. 2022;10:1686
159. Hossain R, Quispe C, Herrera-Bravo J, Beltrán JF, Islam MT, Shaheen S. et al. Neurobiological Promises of the Bitter Diterpene Lactone Andrographolide. Oxid Med Cell Longev. 2022;2022:3079577
Author contact
![]() Corresponding authors: Ji Li*(syphulijicom); Ming Zhao* (syphuzmcom); Jian Guan* (guanjian2621com).
Corresponding authors: Ji Li*(syphulijicom); Ming Zhao* (syphuzmcom); Jian Guan* (guanjian2621com).
 Global reach, higher impact
Global reach, higher impact