13.3
Impact Factor
Theranostics 2025; 15(9):3733-3748. doi:10.7150/thno.104558 This issue Cite
Review
Phenylboronic acid in targeted cancer therapy and diagnosis
Faculty of Chemistry and Technology, University of Split, Ruđera Boškovića 35, 21000 Split, Croatia.
Received 2024-10-2; Accepted 2025-2-11; Published 2025-3-3
Abstract
Over the past few decades, phenylboronic acid (PBA) and its derivatives have gained attention for their biological activity. More recently, they have gained interest for their application in targeted cancer therapy, owing to their ability to selectively and reversibly bind to sialic acids on the cell surface through the formation of boron ester bonds. Initially, the research was focused on the antitumor properties of simple PBA derivatives. However, it has since expanded to include more complex nanomaterials and drug-delivery systems that exploit the unique properties of PBA for enhanced therapeutic efficacy. This paper presents a comprehensive review of the physico-chemical background of PBA-based drugs in cancer therapy, provides insight into recent advancements in PBA-based systems for targeted drug delivery and their role in improving antitumor efficacy, and offers a perspective on future research and development in the field.
Keywords: phenylboronic acid, cancer, drug delivery systems, sialic acid, antitumor activity
1. Introduction
While cancer therapies and diagnostics have undergone revolutionary advancements in the past century, the need for continual improvement remains one of the greatest challenges in medicine today. The concept of "magic bullets" - agents designed to selectively attack cancer cells while at the same time sparing healthy tissues, introduced by the Nobel Laureate Paul Ehrlich, has laid the foundation for modern chemotherapy [1]. Even though numerous advancements in molecular biology, medicinal chemistry, and genetics have been made, the success rate for bringing new cancer drugs to clinical use remains only approximately 5% [2], primarily due to inadequate efficacy and toxicity issues often identified during late-stage clinical trials. Integration of biomarkers into preclinical drug discovery could address these issues and enhance the selection of promising compounds for clinical evaluations. Additionally, contemporary research aims at developing multi-targeted therapies to tackle the complexity of cancer, alongside personalized medicine strategies tailored to individual patients' needs.
In line with these demands, phenylboronic acid (PBA) and its derivatives have recently emerged as promising agents capable of targeting cancer cells and provoking specific biological responses, revealing an unprecedented potential in cancer diagnostics and therapy [3,4]. Understanding the structural interactions of these compounds with cancer-specific biomarkers can enable the design of improved targeted therapies that minimize off-target effects and improve treatment efficacy. This approach facilitates the development of novel diagnostic tools and therapeutic agents that exploit unique cancer cell surface features. Additionally, nanocarriers such as liposomes and micelles incorporating PBA could provide a more personalized approach to cancer therapies, focusing on mechanisms of passive and active targeting for tumor treatment, potentially leading to more effective and less toxic cancer treatments.
This review thoroughly explores the chemistry of PBA in relation to the specific morphology of cancer cells and different types of PBA-based anticancer systems (Figure 1). It provides insight into the evolution of the field from simple PBA-derivatives acting as sensors to highly complex drug delivery systems designed to overcome the challenges of tumor treatments (Figure 2).
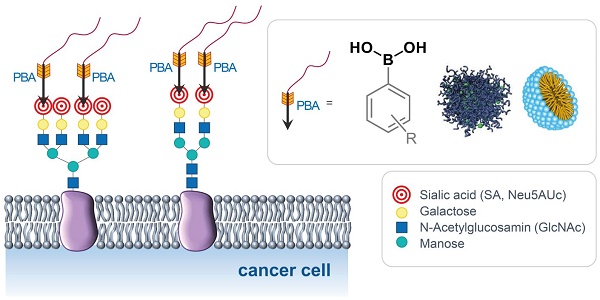
Types of PBA-based systems for targeted cancer therapy and diagnosis.
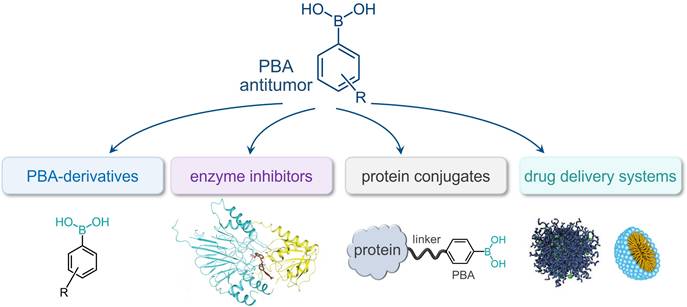
Timeline presenting significant advancements made in PBA-based systems for targeted cancer therapy and diagnosis.
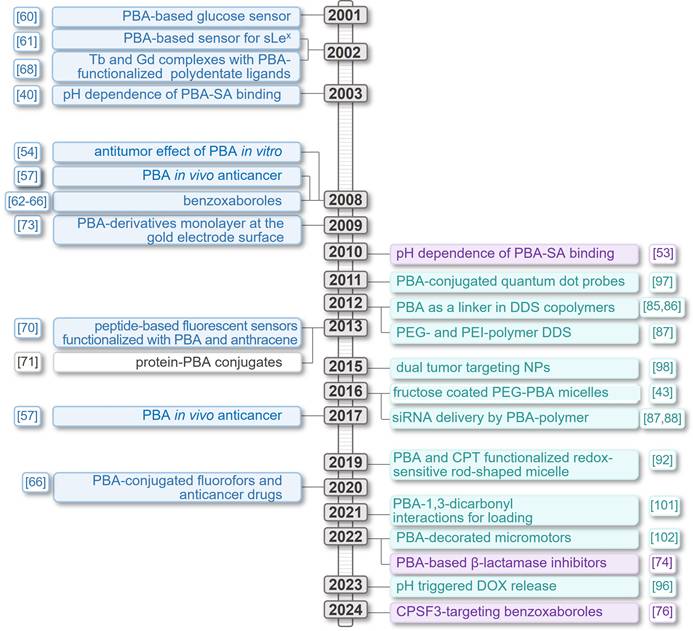
The structure of 5-N-acetylneuraminic acid, Neu5Ac.
2. Chemical basis of the cancer-targeting activity of phenylboronic acid
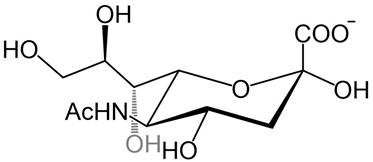
The surfaces of cancer cells exhibit distinct chemical and morphological properties compared to healthy cells, largely due to alterations in their membrane structure. These changes impact cellular function, including cell-cell interactions, apoptosis, immune response, and tumor progression [5-11]. Among other membrane components, glycolipids and glycoproteins on the cell surface represent the first contact of the cell with its surroundings. They are involved in various biological processes such as cellular communication, molecular recognition, and the reception of external signals [10]. Several recent studies showed that many glycans on the surfaces of tumor cells are excessively expressed compared to healthy cells, especially sialic acids (SAs) which stand out due to their special location at the terminal end of glycans in glycolipids and glycoproteins. Sialic acids are anionic monosaccharides with N- and O-substituted derivatives of neuraminic acid and in humans, the dominant one is the 5-N-acetylneuraminic acid (Neu5Ac, Figure 3).
Cancer malignancy and metastasis are often associated with hypersialylation [12-15] and this structural feature of tumor cells can be exploited for the development of new diagnostic and therapeutic tools in which the glycans serve as biomarkers for pathological states and targets for cancer diagnosis and antitumor therapeutics [16-22].
Lectins are compounds in biological systems that exhibit selective binding to sugars of cell-surface glycans. However, their use as potential antitumor agents is impeded by several disadvantages owing to their protein nature: immunogenicity, inflammatory action, cellular toxicity, and mitogenic stimulation, as well as time-consuming and generally expensive large-scale production, relatively low chemical stability, and problematic long-term storage [23]. As an alternative to lectins, a number of smaller non-protein lectin-mimics compounds that overcame the shortcomings of lectins have also demonstrated reversible binding to glycans and have therefore recently attracted much attention, especially compounds based on PBA [24-27].
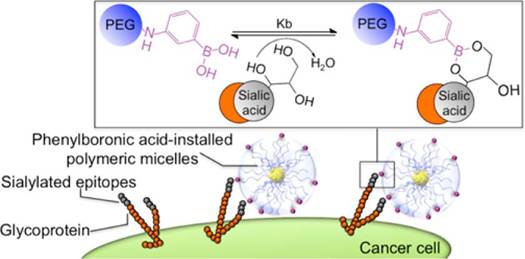
In aqueous solutions at physiological pH values PBA effectively interacts with glycans and binds to 1,2- and 1,3-diol groups by forming five- and six-membered cyclic esters, respectively, as shown in Figure 4. Interestingly, even though this binding occurs through the formation of covalent boronate ester bonds, it is reversible, as well as significantly pH-dependent [4,28-31]. This is the result of the reaction network comprising the two protonation and two esterification equilibria. In general, the boron atom in its uncharged form has sp2-hybridization and forms trigonal planar molecules through the formation of 3 covalent bonds, leaving one of its sp2-orbitals empty. This makes boron compounds Lewis acids, readily available for a reaction with a nucleophile. In this reaction, the two nucleophilic electrons are brought to the boron atom resulting in its negative charge and interconversion to sp3-hybridization state with tetrahedral geometry [32-35]. The hybridization and geometry interconversion are observed also in the acid-base reaction of PBA with the pKa,1 of 8.8, where the planar uncharged form 1 upon nucleophilic reaction with a hydroxy group converts to the tetrahedral and negatively charged form 2 [36,37].
Both of these forms can be subject to esterification by diols and the corresponding esterification reactions are equilibrium processes resulting in the corresponding boronate ester (3, 4) as well as the free PBA forms (1, 2) being present in solution at all times [38]. On the other hand, the acid-base equilibrium of the resulting boronate ester 3 (described by the acidity constant Ka,3) is significantly shifted towards the negatively charged species 4 [39]. Just as the PBA Ka values are 2 to 4 orders of magnitude higher than the Ka of its esters with numerous monosaccharides, the esterification constants for the neutral PBA are 2 to 4 orders of magnitude lower with respect to the negatively charged basic species.
Considering the fine interplay of the four equilibria in the described reaction network, it is not surprising that the overall stability of boronate esters is influenced by several factors: the acidity constants Ka,1 and Ka,3, the esterification constants Ke,1 and Ke,2, pH, and the diol Ka. At low pH PBA exhibits low affinity towards diols, but this affinity increases significantly between pH 4 and 7. In more basic conditions, however, the esterification constants decrease as pH increases. This suggests that the strongest binding of PBA and diols can be achieved at pH ~ 7, but the precise value of the pH at which the binding is the strongest is to a certain extent dependent on the pKa of the diol [38].
In 2003 Kataoka et al. found that the profile of the dependence of the binding strength of PBA and SA on pH significantly differs from the profile for other monosaccharides [40]. More precisely, the binding affinity of PBA towards SA is significantly higher, about 40 times compared to other sugars. Even more, unlike most other saccharides which are the most stable around neutral pH, the stability of the ester formed between PBA and SA increases as the pH decreases (Figure 5). Later, these findings have been further confirmed through several broader studies [40-46]. As a result of this peculiar characteristic, PBA will have higher affinity for the SA on the surface of normal healthy cells in a physiological environment with pH 7.4, while in tumor regions where pH is 6.5 the specific binding will be stronger. In other words, PBA can recognize the overly expressed SA of the tumor cells. This pH sensitivity is particularly advantageous in the context of cancer treatment, since the acidic microenvironment of tumors enhances the selective binding of PBA to cancer cells, facilitating targeted drug activity.
This unusual and highly specific behavior of PBA allows its application as a diol ligand in various contexts, including the development of glucose sensors, use in chromatographic separation of carbohydrates, as protecting groups for diols and amines, and in drug delivery [32,47]. Even more, these properties make PBA a suitable and extremely promising platform for innovative research for the development of systems for rapid detection of disease-related cell-surface glycoproteins and targeted drug delivery. In addition, the selective binding of biologically active PBA-decorated compounds preferably on the surface of tumor cells ensures their direct exposure to the therapeutic and thus a more efficient biological effect [48-53].
As the first step towards medical use, Plopper et al. studied the antitumor effect of PBA in vitro on human prostate and breast cancer lines, comparing it to boric acid [54,55]. They showed that PBA even at micromolar concentrations acted as a selective inhibitor of cell migration and decreased viability, while the same effect was not observed for nontumorigenic cells. This suggested the potential of PBA as anti-metastatic and anti-proliferative agent.
The reaction network of the pH-dependent formation of the boron ester which includes the interconversion of hybridization and geometry of the boron atom.
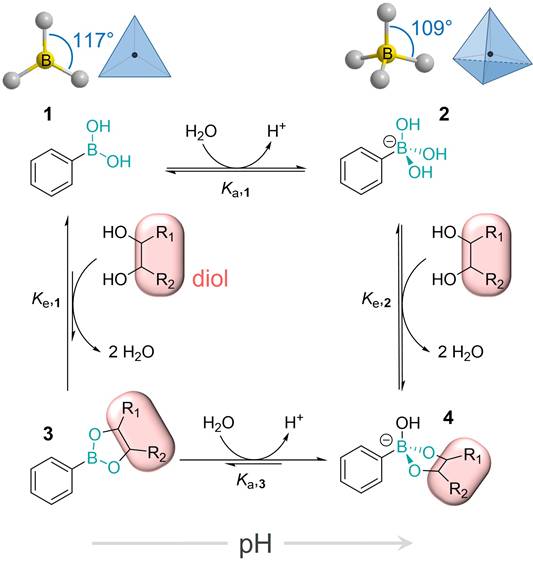
pH dependence of the binding constants for the esterification of a PBA derivative with different diols, namely some common monosaccharides and glycerol. Adapted with permission from [39], copyright 1959 the American Chemical Society.
Moving forward, in 2017, Miloš et al. performed the first in vivo study of the antitumor effects of PBA on mouse mammary adenocarcinoma and squamous carcinoma cells and proved unsubstituted PBA to be highly cytotoxic for several types of tumor cells in mice [56]. Furthermore, three routes of application were considered, and intraperitoneal administration gave the best results, with good toleration by mice, even after repeated large-dose administrations.
Compared to unmodified PBA, the development of PBA derivatives has been driven by the need for improved binding affinity, selectivity, and stability under physiological conditions. These derivatives are often functionalized with electron-withdrawing groups or other modifications to enhance their selective interactions with diols, ensuring better targeting in complex biological environments, which is crucial for increasing the efficacy of targeted therapies and reducing off-target effects [45]. During the last few decades, PBA derivatives have found numerous applications and became the center of interest of modern research in chemistry and related interdisciplinary sciences, biomedicine, analytical sciences, and functional materials [31,57-59].
3. Derivatives of phenylboronic acid in cancer diagnostics and therapeutic interventions
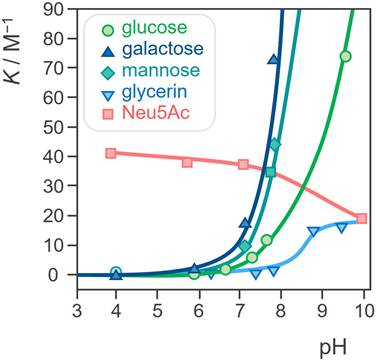
Although various boronic acids have been known for more than a hundred years, it wasn't until the beginning of this century that they started to attract more interest which led to the emergence of new areas of discovery. Firstly, the research revolved mostly around employing the specific properties of PBA for the development of new, efficient, highly sensitive, and selective sensors for saccharides, primarily glucose due to its biochemical importance [60]. Shinkai et al. developed a series of PBA-based glucose sensors [3,4], and later continued to expand their studies to more complex saccharides. In 2002, Yang and coworkers described a fluorescent sensor for sialyl-Lewis X (sLex), a cell surface tetrasaccharide containing SA that plays an important role in cancers and metastasis. The sensor incorporated two PBA moieties for selective binding to the disaccharide and two anthracene moieties to induce a fluorescence response upon binding (Figure 6A) [61]. In the same paper, they demonstrated the efficacy of this sensor in selective labeling of the tumor cells expressing sLeX for fluorescence imaging, while the non-sLeX-expressing cells remained unstained, making this receptor the first synthetic selective cancer diagnostic tool of this type.
A few years later, Hall and coworkers found that benzoxaborole, a cyclic monoester of 2-(hydroxymethyl)phenylboronic acid, unlike PBAs, binds nonreducing saccharides, namely glycopyranosides, in neutral water [62,63]. Soon after, they continued to develop a library of benzoxaborole- and PBA-based receptors for Thomsen-Friedenreich (TF) antigen, a Gal-β-1,3-GalNAc disaccharide which is a known tumor marker expressed in 9 out of 10 cancers [64]. As diboronic acids have a higher affinity for saccharides than simple PBAs [65], they applied the same concept and attached the two boron-containing substituents to the central aminoacids of a polypeptide decorated on one end with a glycol moiety to increase the compounds' solubility (Figure 6B). Utilizing a modular approach, they created a library of receptors that effectively recognize carbohydrates in an aqueous environment. These receptors leverage the unique properties of benzoboroxoles to form reversible complexes with hexopyranosides, enhancing binding affinity through multiple interaction modes, including boronate formation, hydrogen bonding, and hydrophobic interactions. Among them, one receptor showed strong selectivity for the TF-antigen clearly demonstrating the superiority of the bisbenzoxaborole moiety over the boronic acid moiety regarding selective high-affinity binding to pyranoses [23]. It is quite surprising that despite these very promising results, the receptors were never further investigated for their biological activity and especially for their potential antitumor properties.
In addition, some benzoboroxole-derived compounds have been shown to exhibit antiproliferative activity. For example, the Suman group synthesized a series of N-benzoboroxolylureas and N-nitrosoaminobenzoboroxoles derived from aminobenzoboroxoles, several of which showed promise in preliminary cytotoxicity evaluations against human breast cancer and pancreatic cancer cell lines [66].
Structures of A) a fluorescent sLeX sensor by Wang et al. and its use in fluorescent labeling of sLex-expressing cells (bottom) and non-expressing cells (top), and B) a benzoxaborole, PBA, and phenyl substituted TF antigen sensors by Hall et al. Adapted with permission from [61], copyright 2002 Elsevier.
As new insights into the specific biochemical behavior of PBAs emerged in the late 1990s and early 2000s, considerable efforts have been made to exploit their biological activity, and extensive research is currently underway to investigate their potential for medical applications. In the study by Zhang and colleagues in 2020, PBA derivatives were conjugated with dicyanomethylene-4H-pyran fluorophores as well as with the anticancer drug SN-38 [67]. The cellular uptake of PBA-dye conjugate by hepatocellular and lung carcinoma cells was significantly higher than non-PBA-containing counterparts, and PBA-SN-38 conjugates achieved up to 3- to 7-fold increases compared to the cytostatic drug irinotecan. Despite improved delivery, the drug-PBA system did not enhance the anti-proliferative effect against liver trumor cell lines, likely due to the stability of the system and consequent drug inactivation.
The Sporzyński group evaluated the antiproliferative action of a series of PBA and benzoxaborole derivatives for several cancer lines [68]. They noted that the substituent type and position clearly affected the antiproliferative activity of the compound as well as its pKa. The two most active derivatives were identified and used to further study cell cycle arrest and apoptosis induction in ovarian cancer cells. The research further demonstrated that these compounds significantly increase the percentage of cells in the G2/M phase and promote the formation of tetraploid cells, which is a hallmark of mitotic catastrophe. This cell cycle arrest was accompanied by a marked increase in p21 protein levels, which is known to inhibit cyclin-dependent kinases (CDKs), thus preventing cell cycle progression. Interestingly, p21 accumulation was not associated with β-tubulin degradation, indicating that the mechanism of action is distinct from traditional microtubule-targeting agents. Instead, the G2/M arrest resulted in the failure of proper mitotic division, ultimately leading to apoptosis via caspase-3 activation. This phase cycle-specific induction of apoptosis requires further studies to clarify their mechanisms of action and therapeutic potential in cancer treatment.
In the context of PBA derivatives known for targeting SAs on cancer cells, terbium and gadolinium complexes with polydentate ligands functionalized with PBA have also been examined for their tumor-targeting abilities [69,70]. These compounds demonstrated superior effectiveness by combining the covalent binding with SA and electrostatic interaction of the positively charged complex with negatively charged cell surfaces. This dual interaction approach highlights the potential of PBA-functionalized lanthanide complexes for molecular imaging and radiotherapy applications, furthering the use of PBA derivatives in cancer-targeted diagnostics and treatment.
In 2013 Xu et al. presented five peptide-based fluorescent sensors functionalized with PBA and anthracene moiety responsible for fluorescent response, varying in the peptide backbone [71]. These water-soluble and biocompatible compounds were designed for in situ recognition of and targeted fluorescent imaging of cancer cells. Among them, one compound was shown to highly specifically recognize the sLex cell-surface glycan and fluorescently label the targeted cells. The selective recognition of cancer cells is the result of the specific recognition between the specific tripeptide sequence of the sensor and integrins associated with cancer.
A more complex PBA-based system used in cancer diagnosis and therapy was described by Miyahara and coworkers [72,73]. It consisted of a monolayer of PBA derivatives self-assembled at the surface of a gold electrode which was used as a noninvasive potentiometric sensing system for selective detection of tumor metastasis cells by recognizing their surface SA. Even more, this system showed the ability to quantify the degree of metastasis in mice cell suspensions.
Martinelli [74] and Martinelli [75] focused on the solid-phase synthesis and evaluation of PBA-based tumor-targeting MRI (magnetic resonance imaging) contrast agents, with the latter reporting enhanced performance of dimeric analogues. Their study introduced a novel approach to synthesizing paramagnetic macrocycles functionalized with phenylboronic moieties, which hold promise for MRI applications due to their cancer cells targeting ability and local contrast enhancement. The key innovation lies in the solid-phase synthesis strategy employed, which enabled the successful preparation of DOTA-EN-PBA and DOTA-EN-F2PBA ligands (DOTA = 1,4,7,10-tetraazacyclo-dodecane-1,4,7,10-tetraacetic acid, EN = ethylenediamine). Notably, the latter incorporates fluorine substituents to increase the acidity of the boronic moiety, further enhancing its tumor-targeting capabilities. This research represents a significant advancement in the development of MRI contrast agents for precise cancer imaging, offering potential benefits for early detection and diagnosis.
Ultimately, one PBA derivative, 4-boronophenylalanine, is among the agents currently used in clinical trials for boron neutron capture therapy (BNCT). This is a type of radiotherapy used for invasive and localized cancers where first a boron-containing (isotope 10B) and tumor-localizing drug is administered, and then the patient is radiated with epithermal neutrons. Boron atom has a high potential for capturing the neutrons which results in its isotopic decay releasing “high-energy α-particles that kill the cancer cells that have taken up enough 10B”. Currently, the request for new boron-delivering agents that would have lower systemic toxicity and achieve higher concentrations in tumor tissues has led to the development of different systems such as boron-containing nanoparticles (NPs), boronated polyamines, etc. [76].
4. Derivatives of PBA as enzyme inhibitors
PBA derivatives have emerged as potent agents in biomedical research due to their ability to form covalent bonds with biological molecules. This property also enables enzyme inhibition by PBA and of interest is their anticancer potential through their role as proteasome inhibitors. Trippier et al. highlighted the success of bortezomib, a boronic acid-based proteasome inhibitor, which inhibits cancer cell growth by targeting proteasome-mediated protein degradation [49]. Bortezomib's mode of action involves forming covalent bonds with the proteasome, leading to the inhibition of protein degradation, cell cycle arrest, and apoptosis. The success of bortezomib has inspired the development of PBA derivatives, including boronic-chalcone analogs that show strong activity against breast cancer cell lines by inhibiting tubulin polymerization, further underscoring the therapeutic versatility of PBA compounds. This trend toward proteasome targeting continues to be a key focus in cancer therapies, given the crucial role of proteasomes in tumor progression.
Another promising application of PBA derivatives lies in their role as β-lactamase inhibitors, as demonstrated by Zhou et al. [77]. Triazole-substituted PBA derivatives were shown to inhibit KPC-2 β-lactamase, an enzyme responsible for antibiotic resistance. The structure-activity relationship (SAR) studies revealed that the introduction of triazole moieties enhanced enzyme binding affinity and stability. Even more, Xu et al. introduced a series of indolylarylsulfones bearing PBA functionalities as potent HIV-1 non-nucleoside reverse transcriptase inhibitors with remarkable activity against wild-type HIV-1 and several resistant strains [78].
Although the primary focus of these studies was on antibacterial and antiviral activity of PBA derivatives, the findings emphasize their structural versatility and highlight their broader potential in targeting critical enzymes involved in disease pathways. This ability to fine-tune the pKa and binding properties makes PBA derivatives attractive candidates for diverse therapeutic applications, including anticancer therapies where enzyme inhibition plays a crucial role.
An interesting study by the Plopper group showed that PBA acts as an inhibitor of Rho family of GTP-binding proteins RhoA, Rac1, and Cdc42 in metastatic prostate cancer cells cells but not in normal prostate cells [54,55], which also impacts the downstream targets of these proteins. Furthermore, PBA decreased the activity of ROCKII as well as phosphorylation of myosin light chain kinase. Overall, cancer cell growth was arrested and cell migration was inhibited in rapidly proliferating cells.
In addition, a series of benzoxaboroles has been found to induce cancer cell death by targeting CPSF3, an enzyme essential for mRNA processing and transcription termination [79]. These compounds bind to the active site of CPSF3 and act as inhibitors, leading to widespread gene downregulation and eventual cell death.
5. Protein conjugates of PBA
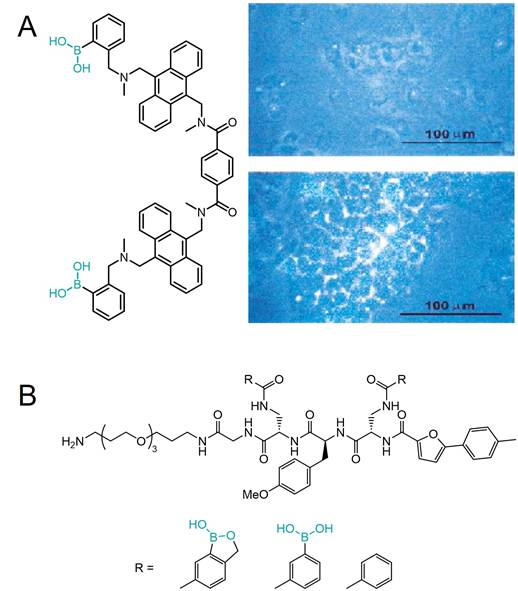
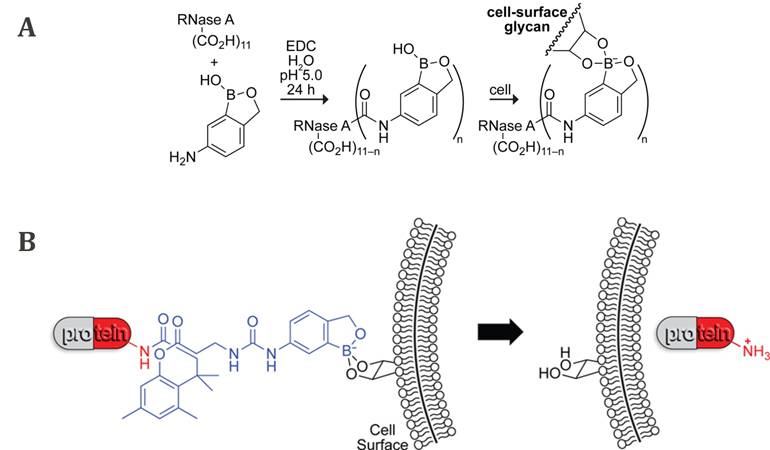
As previously mentioned, the development of new approaches for tumor targeting was linked to research on synthetic lectin mimetics. In this regard, the early attempts to achieve an antitumor effect often involved protein-PBA conjugates, expected to have biological activity similar to lectins. The first example of such bioconjugates was a benzoxaborole linked to a RNase A by an amide bond reported by Raines et al. in 2012 (Figure 7A) [80]. It was shown that the protein affinity for the human erythroleukemia cells increased upon boronation, as well as its cellular uptake. Even more, boronated RNase A inhibited the human erythroleukemia cells proliferation. The recognized potential of boronates as effective lectin mimetics was further exploited in a similarly designed conjugate of the bovine serum albumin (BSA) with PBA [27,81]. The conjugate was deposited on silica beads as well as on a gold chip, and in both cases, efficient binding of sugars and SA on their surface was demonstrated. Although the approach of binding proteins directly to boronic acids via amide bond promotes protein binding to cancer cells and its internalization, unfortunately, the catalytic activity of boronated proteins is significantly reduced compared to their native form [80].
In 2016, Raines et al. proposed an improved strategy using a benzoxaborole-based carrier that binds the proteins through an immolative linker (Figure 7B) [82]. In such a conjugate, the covalent modification of the amino groups of the protein is bio-reversible, since esterases present inside the cell, but not outside, can cleave the bond between the linker and the protein, releasing the native protein into the cell cytosol. Such boronation of the protein chemically inactivates it making it less cytotoxic in the extracellular fluid, and at the same time facilitating its cellular uptake and internalization. Overall, the RNase A conjugate showed a decrease in the IC50 value compared to nonconjugated RNase A of human myelogenous leukemia cells and inhibition of their proliferation.
6. Nanoscale drug delivery systems for cancer therapy based on PBA derivatives
Around the time PBA derivatives were shown to selectively recognize SA, liposomes, micelles, microspheres, and other nanostructures started to gain attention as carriers for diagnostic agents and therapeutics. The use of such drug carriers in biomedicine has several significant advantages compared to the conventional drugs: i) they are biocompatible and enable the transport of poorly soluble compounds to the targeted tissues, ii) they can alter the pharmacokinetics of the drug by ensuring its longer circulation due to the protection from immune system degradation, thus allowing for prolonged release which reduces the overall drug dose needed and minimizes systemic toxicity, iii) they can be designed to deliver drugs specifically to the desired site, increasing bioavailability and concentration in pathological areas, iv) they reduce and even eliminate the unwanted side-effects, v) they can be engineered for controlled drug release over time [83,84]. These beneficial effects combine to significantly enhance the efficiency of the drug. Consequently, such formulations have become increasingly widespread, with the most notable example being COVID-19 mRNA vaccines, where lipid NPs serve as the drug delivery system [85]. Significant efforts have been devoted to developing new formulations, leading to remarkable advances in the field.
6.1. PBA as linker in DDS copolymers
Interestingly, the first use of PBA in drug delivery formulations was not for tumor targeting, but rather as a crosslinker between the polyethylene glycol (PEG) and cholic acids blocks, which self-assemble into micellar copolymers called telodendrimers and thereby increasing their stability [86]. The resulting micelles were loaded with paclitaxel (PAX), a widely used chemotherapeutic drug, and it was shown that the drug release was slow at physiological pH of 7.4, but lower pH triggered the release of PAX enhancing the inhibition of growth of ovarian cancer cells.
A more recent example employing a similar approach was described by Shuai and coworkers [87] who developed another pH-sensitive polymeric drug carrier composed of a hydrophobic and hydrophilic polymer linked by phenylboronic ester, forming an amphiphilic structure that self-assembled into stable PTX-loaded NPs with high encapsulation efficiency. These NPs have excellent drug encapsulation and pH-triggered drug-release capabilities, leading to low systemic toxicity, but high anticancer activity and improved pharmacokinetics compared to free PAX, both in vitro and in vivo.
6.2. PEG- and PEI-based DDS with PBA as the recognition unit
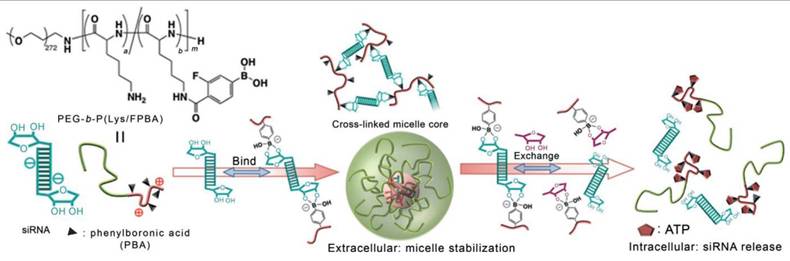
On the other hand, the PBA derivatives can be incorporated into drug delivery systems as moieties designed for efficient and selective recognition of the cancer cells, enabling targeted action. In 2012, Kataoka and colleagues described the first such example of micelles built from the cationic polymer poly(ethylene glycol)-block-poly(L-lysine) whose lysine residues were further modified with a PBA derivative [88]. The resulting polymer formed complexes with siRNA through the formation of a boronic ester bond with riboses of RNA. SiRNA cancer therapy has immense potential, but the lack of safe and effective delivery inhibits its clinical use, and this polymer offered a way to overcome these issues. Interestingly, the presence of ATP at intracellular concentrations triggered siRNA release (Figure 8), resulting in gene silencing of a well-known proto-oncogene in the human renal carcinoma cell line with minimal cytotoxicity.
A) Boronation of RNase A via amide bond and B) protein boronation strategy through an immolative linker (blue) and binding of the conjugates to cell surface glycans followed by internalization of the native protein by Raines et al. Adapted with permission from [80,82], copyright 2012 and 2016 American Chemical Society.
PBA-modified polymer cross-links with siRNA forming a stable micelle that releases siRNA in the presence of intracellular concentrations of ATP by Naito et al. Adapted with permission from [88], copyright 2012 Wiley.
Cellular binding, uptake, and intracellular localization of siRNA in cancer cells. The uptake and binding of siRNA were quantified using flow cytometry. Adapted with permission from [89], copyright 2016 American Chemical Society.
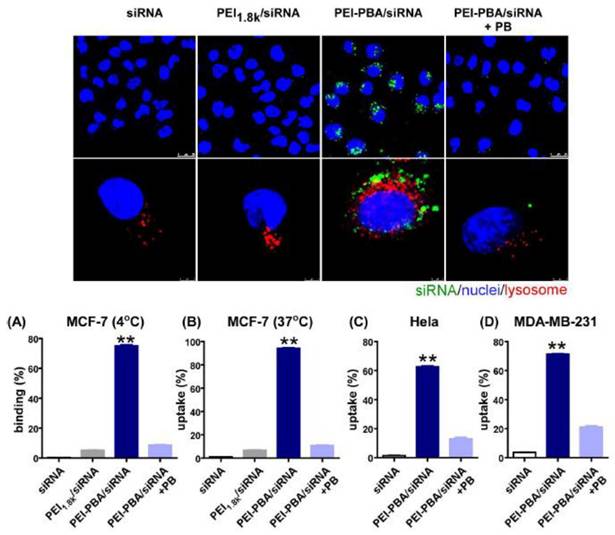
A few years later, Ma et al. offered a similar PBA-based system for siRNA delivery based on PBA-grafted PEI [89]. The nanocomplex formed between the polymer and siRNA had excellent biocompatibility, as well as serum stability, and was resistant to RNases. The PBA-SA interactions led to a dramatic increase in its uptake by cancer cells compared to free siRNA. Even more, the encapsulating polymer promoted the lysosome escape of siRNA which in turn decreased the expression of the target gene in cancer cells and induced tumor apoptosis and cell cycle arrest without causing any significant systemic toxicity or immunotoxicity. The microscopic images depicting cellular binding, uptake, and intracellular localization of siRNA in cancer cells, and the results of flow cytometry for binding and uptake are presented in Figure 9.
PBA decorated micelles loaded with oxaliplatin by Kataoka et al. and their interaction with cancer cells. Adapted with permission from [42], copyright 2013 American Chemical Society.
In 2013, the same group applied a similar approach to prepare micelles made of poly(ethylene glycol)-b-poly(L-glutamic acid), PEG-b-PLGA, with one end functionalized with PBA, as shown in Figure 10 [42]. These micelles were loaded with anticancer drug oxaliplatin and the obtained system demonstrated a higher affinity for SA compared to other common biologically important monosaccharides. Additionally, they showed increased cellular uptake in murine melanoma cells and enhanced antitumor effect compared to micelles lacking the PBA moiety. Furthermore, it was clearly demonstrated that this was a direct result of the interactions between PBA and SA on the surface of cancer cells which enhanced the micelles retention at the tumor giving rise to superior antitumor efficacy both in vitro as well as in vivo.
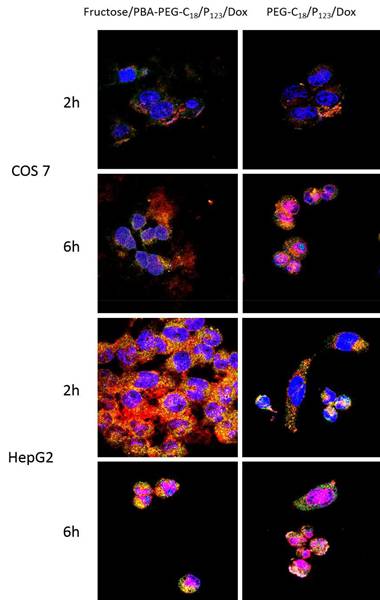
The development of pH-responsive nano-drug delivery systems incorporating PBA represented a notable advancement in the field. In 2016, Cheng, Li et al. took this concept further by introducing a simple yet efficient strategy: coating PBA-terminated polyethylene glycol monostearate and Pluronic P123 (PBA-PEG-C18) mixed micelles with fructose and loading them with DOX [43]. At physiological pH, PBA-PEG-C18 preferentially binds to fructose over SA limiting its interactions with healthy cells and reducing the cytotoxicity of the DOX-loaded micelles toward them, while increasing their targeting ability for tumor cells. In contrast, at the lower pH of the tumor environment, the interactions with SA strengthen and over-compete with fructose, enabling targeted action. This design led to enhanced cellular uptake (Figure 11) by endocytosis and increased cytotoxicity of DOX-loaded micelles.
A similar strategy was employed by Kim et al., who used crosslinked polyethylenimine (PEI) functionalized with PBA and PEI functionalized with galacturonic acid [90]. The resulting cationic polymer gel exhibited stronger interactions with DNA than either component did by itself, suggesting that the polymer decomposition would lead to DNA release. Indeed, a lower pH, intracellular concentrations of ATP, or both triggered the disruption of the crosslinked polymer and the subsequent release of DNA from the previously stable polyplex. The delivery of DNA to the cell nucleus increases gene expression. This approach was used to deliver DNA as a tumor angiogenesis inhibitor, demonstrating that the system effectively targeted tumors and suppressed angiogenesis, highlighting its potential as a vector for therapeutic gene delivery.
In 2016, the Hu group presented an assay to measure SA expression on the surface of cancer cells [91]. They employed PBA-biotin conjugates to selectively recognize the SAs and then attached to them gold NPs conjugated to streptavidin, known for its very high affinity towards biotin to enhance the inductively coupled plasma mass spectrometry (ICPMS) signal. This method demonstrated high selectivity, and competitive experiments provided accurate estimations of SA expression level on the cancer cell surface. Additionally, the assay successfully detected cancer cells, using SA as a biomarker, indicating its potential applicability for biological research and clinical diagnostics.
Xiao, Lu et al. previously described a nano-prodrug consisting of CPT conjugated with gemcitabine through a disulfide bond (CPT-SS-GEM), which showed synergistic antiproliferative efficacy in multiple cancer cell lines [92]. However, the prodrug exhibited insufficient selectivity and tended to self-aggregate and precipitate at higher concentrations, limiting its potential medical application. To address these challenges, they developed a PEG polymer functionalized with PBA at one end and CPT at the other. The resulting polymer co-assembles with CPT-SS-GEM into stable rod-shaped nano-micelles with higher total drug concentration [93]. Both the polymer and CPT-SS-GEM are redox-sensitive prodrugs, allowing them to degrade within the tumor microenvironment, enabling the delivery of two drugs with different mechanisms of action. In vitro experiments against a multidrug-resistant cancer cell line and murine breast cancer cells revealed enhanced cellular internalization and a strong synergistic anticancer effect.
Confocal microscopic images of COS 7 cells at pH 7.4 and HepG2 cells at pH 6.5 showing subcellular distribution of fructose/PBA-PEG-C18/P123/Dox and mPEG-C18/P123/Dox (with stained nucleus and acidic compartments in live cells). Adapted with permission from [43], copyright 2016 American Chemical Society.
In addition to drug delivery systems, Huang and collaborators pioneered the development of PBA-functionalized co-delivery micelles aiming to overcome multidrug resistance in cancer treatment [94]. The micelles consisted of PBA-functionalized PEG-polyglutamic acid polymer with disulfide-bonded CPT and loaded with DOX. The drug ratio resulting in maximal synergistic effect was determined. Additionally, it was shown that the micelles efficiently released the drug in lysosomal conditions, but not in plasma. Also, they demonstrated higher endocytosis and lower drug efflux rates than conventional drugs. Notably, the study suggests that these micelles may act by downregulating specific factors, further emphasizing their potential in combating multidrug resistance. This work represents a significant advancement in cancer therapy, offering a promising strategy to improve drug delivery and efficacy in multidrug-resistant tumors.
In 2022, Zhang introduced linear and star PEG-poly(l-boronophenylalanine) copolymers that self-assembled into micellar NPs [95]. The NPs co-encapsulated antitumor drugs curcumin and sorafenib tosylate and exhibited dual release as a response to H2O2 and acidic environment resulting in greatly enhanced anticancer effect. The relatively easy synthesis of the NPs, robust drug loading and responsive drug release make this system highly interesting for further development of smart cancer nanomedicine.
Li [96] and Wang [97] highlight the potential of PBA-based functional materials in fluorescence imaging and tumor therapy. Wang prepared unimolecular polymer nanomaterials consisting of poly(tert-butyl acrylate) whose arms were functionalized with PBA, DOX and PEG. The incorporation of PBA groups provided specific tumor targeting and high accumulation in tumors, while the presence of a pH-sensitive acylhydrazone linkage between the polymer chain and DOX ensured rapid DOX release in tumor tissues, thus promoting the therapeutic efficacy and enhancing the overall anti-cancer effect.
6.3. Other types of PBA-decorated nanosystems for antitumor therapy
Although most PBA-based DDS used PEG and PEI as drug carriers due to their biocompatibility and the resulting medical applicability, several alternatives emerged.
One of the earliest examples of diagnostic applications was PBA-conjugated quantum dot probes, developed by Duan et al. [98], for effective imaging and tracking of SAs. The study employed single-particle imaging techniques to quantitatively analyze the PBA-SA interactions and demonstrate the average number of PBA groups per QD and their effective targeting capabilities. These findings established PBA-conjugated QD probes as valuable tools for studying glycan dynamics and SA-related biological processes related to sialic acids, with implications for understanding glycosylation in various diseases.
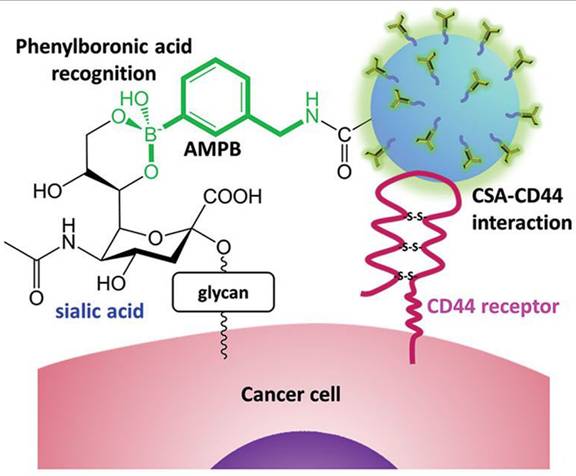
Several years later, Cho, Kim, and coworkers developed NPs composed of biocompatible chondroitin sulfate A (CSA), known for binding to the CD44 receptor expressed in cancer cells, conjugated with Cy5.5 fluorescent dye and deoxycholic acid functionalized with PBA via an amide bond (Figure 12) [99]. The NPs were loaded with doxorubicin (DOX), and a decrease in pH was shown to enhance DOX release. Additionally, the cellular uptake and penetration into human lung adenocarcinoma cells were improved compared to non-PBA-functionalized NPs both in vitro and in vivo. Dual tumor targeting achieved through PBA-SA and CSA-CD44 interactions enhanced cellular uptake leading to suppression of the tumor growth by increasing apoptosis, while maintaining low cytotoxicity to healthy cells.
Tumor targeting of paclitaxel-loaded NPs by Cho, Kim, et al. Adapted with permission from [99], copyright 2015 Wiley.
An interesting advancement was made by Cui, Li, and coworkers who presented an improved fluorescent conjugated polymer modified with PBA for targeted cancer cell imaging [100]. They used SAs as templates during the reprecipitation part of poly(fluorene-alt-benzothiadiazole) NP preparation which were later removed by adjusting the pH. The resulting NPs exhibited improved properties compared to the unmodified NPs, including strong fluorescence and ability to discriminate between cancer cells and normal cells through binding to SAs overexpressed on the surface of cancer cells resulting in their selective staining. The study also demonstrated low cytotoxicity of the NPs, indicating their biocompatibility and potential for use as fluorescent probes for live-cell imaging.
Building upon the exploration of PBA and its derivatives in drug delivery systems, Kang et al. [101] presented a poly(methyl vinyl ether-alt-maleic acid) conjugated with PBA loaded with DOX. The resulting nanocomplex exhibited enhanced anti-tumor activity through tumor-specific targeting in different cancer cells. By targeting the overexpressed Ss, the nanocomplexes enabled precise doxorubicin delivery.
Jung et al. [102] also focused on PBA-functionalized copolypeptides for enhanced anticancer effects through responsive drug release. They prepared PBA-grafted poly(methyl vinyl ether-alt-maleic acid) with a goal to improve the solubility issues of curcumin as a drug. The coordination bonding between 1,3-dicarbonyl compounds and PBA enabled high drug loading capacity of the polymer and pH-responsive drug release mechanism. The platform exhibited promising therapeutic efficacy, showing both chemotherapeutic and anticancer effects.
Finally, in their recent study, Demirbuken et al. [103] introduced artificial motors as a novel approach to drug delivery. Inspired by natural motors, these artificial counterparts have attracted significant interest for their remarkable capabilities and potential applications. Among them, miniaturized nano/micromotors have emerged as versatile tools for (bio)sensing, cargo transport, and drug delivery. The study focused on PTX-conjugated robots featuring a poly(3-aminophenylboronic acid) outer layer, a platinum (Pt)-nickel (Ni) segment, and a Pt catalytic inner layer. These self-functionalized motors exhibited efficient autonomous movement and controlled drug release, particularly under near-infrared (NIR) irradiation. Fabricated through electrochemical methods, the motors demonstrated targeted drug delivery to human breast cancer cells, showcasing pH-dependent behavior. The study highlighted their promising viability and drug release efficiency, underscoring their potential for biomedical applications.
7. Conclusions and future prospects
Cancer remains one of the leading causes of death globally, with nearly 10 million deaths and around 200 million new cases reported annually, according to the World Health Organization (WHO) [104,105]. Conventional treatments, including surgical procedures, radiation therapy, and pharmacological approaches such as chemotherapy, hormone therapy, and targeted biological therapies, are widely employed. However, these methods are often accompanied by significant and severe side effects. This is primarily due to the lack of specificity of many drugs, which, while targeting cancer cells, also inevitably impact healthy cells as they circulate through the body. Consequently, recovery can become prolonged, more challenging, and unpredictable.
Therefore, it is not surprising that the search for targeted diagnostic tools and therapies remains a key focus of cancer research, with PBA-based systems emerging as a highly promising foundation for further developments. PBA derivatives and PBA-functionalized drug delivery systems have shown potential for various biomedical and therapeutic applications [106], primarily due to their unique ability to selectively bind to SA overexpressed on cancer cells surfaces. This specificity also makes the PBA moiety a perfect platform for the development of targeted cancer therapies and diagnostic tools. Over time, PBA-based systems for diagnostics and therapies have evolved from simple derivatives to complex nanomaterials exhibiting improved therapeutic efficacy and reduced off-target effects. This evolution is clearly visible in Figure 2 and emphasizes their important advantages compared to conventional anticancer drugs: targeted drug delivery, increased bioavailability, enhanced therapeutic efficacy, and reduced systemic toxicity. Even more, numerous PBA-based formulations have shown significant potential for use in imaging techniques. Through diverse and innovative approaches, in the last several years, a wide array of very versatile materials and technologies that hold promise in the fight against cancer was developed, translating nature-inspired research into innovative therapeutic strategies with the potential for significant impact in clinical practice. Moreover, although the effect of PBA-derivatives has so far been studied on only several enzymes, the recent emergence of new tools for protein design and structure elucidation recognized with the 2024 Nobel Prize in Chemistry will surely propel research and enable design of specific enzyme-targeting strategies.
As discussed in previous sections, preclinical and clinical trials have offered compelling evidence about the efficiency of PBA-based anticancer agents. However, their primary limitation is their potential toxicity to healthy cells. Since SA is present on both healthy and cancer cell surfaces, PBA-based agents can bind to both. Nevertheless, the binding affinity of PBA towards SA is pH dependent and somewhat lower at the pH of healthy cells than in a more acidic environment of cancer cells. Combined with significantly lower SA expression on healthy cells, this leads to selective targeting of cancer cells and reduced, although not entirely eliminated, impact on non-cancerous tissues. Future research and drug development efforts will likely focus on further increasing this discrepancy and selectivity in order to maximize the therapeutic effect of PBA-based systems while minimizing off-target effects.
The highly promising results of preclinical in vitro and in vivo studies justify the strong interest of the scientific community and lay the groundwork for further development. As this young field continues to rapidly evolve, optimizations of the existing PBA-based systems, as well as development of new strategies to harness the unique properties of PBA, are expected to enhance treatment efficacy and improve outcomes for cancer patients. Modern medicine is shifting towards precision and personalized approaches, so given the exceptional potential of PBA-based drug delivery systems to revolutionize cancer diagnosis and treatment, research is expected to continue expanding through innovations, eventually reaching clinical use. Such advancements could provide groundbreaking personalized cancer therapies, state-of-the-art diagnostic tools, and multifunctional drug delivery systems with profound implications for clinical practices.
Acknowledgements
Dedicated to prof. Mladen Miloš on the occasion of his retirement. The paper is the result of the institutional projects of the Faculty of Chemistry and Technology, University of Split, Croatia, titled: “Antitumor potential of photoactive derivatives of phenylboronic acid”, “NATURELAB: research and innovation in natural chemical compounds” and “Metabolomics in the Development of New Diagnostic Tools”; as well as the project “Photoactive derivatives of phenylboronic acid: synthesis, interactions with sialic acids and biological activity” funded by the Adris Foundation, Croatia.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473-80
2. Collins I, Workman P. New Approaches to Molecular Cancer Therapeutics. Nat Chem Biol. 2006;2:689-700
3. James TD, Samankumara Sandanayake KRA, Iguchi R, Shinkai S. Novel Saccharide-Photoinduced Electron Transfer Sensors Based on the Interaction of Boronic Acid and Amine. J Am Chem Soc. 1995;117:8982-7
4. James TD, Samankumara Sandanayake KRA, Shinkai S. Saccharide Sensing with Molecular Receptors Based on Boronic Acid. Angew Chem Int Ed Engl. 1996;35:1910-22
5. Kondo K, Harada Y, Nakano M. et al. Identification of distinct N-glycosylation patterns on extracellular vesicles from small-cell and non-small-cell lung cancer cells. J Biol Chem. 2022;298:101950
6. Szydlak R, Luty M, Prot VE. et al. Detecting normal and cancer skin cells via glycosylation and adhesion signatures: A path to enhanced microfluidic phenotyping. Biosens Bioelectron. 2024;258:116337
7. Shen C, Zheng B, Chen Z. et al. Identification of prognostic models for glycosylation-related subtypes and tumor microenvironment infiltration characteristics in clear cell renal cell cancer. Heliyon. 2024;10:e27710
8. Zalba S, Ten Hagen TLM. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat Rev. 2017;52:48-57
9. Hilton KLF, Manwani C, Boles JE. et al. The phospholipid membrane compositions of bacterial cells, cancer cell lines and biological samples from cancer patients. Chem Sci. 2021;12:13273-82
10. Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291:2357-64
11. Yamakawa T, Nagai Y. Glycolipids at the cell surface and their biological functions. Trends Biochem Sci. 1978;3:128-31
12. Muripiti V, Ravula V, Batthula S, Banothu J, Gondru R. Phenylboronic acid-derived nanovectors for gene/drug delivery by targeting cell surface glycans. RSC Pharm. 2024;1:403-11
13. Filipsky F, Läubli H. Regulation of sialic acid metabolism in cancer. Carbohydr Res. 2024;539:109123
14. Ghosh M, Hazarika P, Dhanya SJ, Pooja D, Kulhari H. Exploration of sialic acid receptors as a potential target for cancer treatment: A comprehensive review. Int J Biol Macromol. 2024;257:128415
15. Hodgson K, Orozco-Moreno M, Goode EA. et al. Sialic acid blockade inhibits the metastatic spread of prostate cancer to bone. eBioMedicine. 2024;104:105163
16. Lee D-H, Choi S, Park Y, Jin H. Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy. Pharmaceuticals. 2021;14:1053
17. Sun L, Zhang Y, Li W, Zhang J, Zhang Y. Mucin Glycans: A Target for Cancer Therapy. Mol 2023 Vol 28 Page 7033. 2023;28:7033
18. Dube DH, Bertozzi CR. Glycans in cancer and inflammation - Potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477-88
19. Matsumoto A, Cabral H, Sato N, Kataoka K, Miyahara Y. Assessment of Tumor Metastasis by the Direct Determination of Cell Membrane Sialic Acid Expression. Angew Chem Int Ed. 2010;49:5494-7
20. Matsumoto A, Kataoka K, Miyahara Y. New directions in the design of phenylboronate-functionalized polymers for diagnostic and therapeutic applications. Polym J. 2014;46:483-91
21. Berois N, Pittini A, Osinaga E. Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives. Cancers. 2022;14:645
22. Khan T, Cabral H. Abnormal Glycosylation of Cancer Stem Cells and Targeting Strategies. Front Oncol. 2021;11:649338
23. Nakagawa Y, Yukishige I. Molecular architecture and therapeutic potential of lectin mimics. Adv Carbohydr Chem Biochem. 2012;68:1-58
24. Jin S, Cheng Y, Reid S, Li M, Wang B. Carbohydrate recognition by boronolectins, small molecules, and lectins. Med Res Rev. 2010;30:171-257
25. Mazik M. Recent developments in the molecular recognition of carbohydrates by artificial receptors. RSC Adv. 2012;2:2630-42
26. Lacina K, Skládal P, James TD. Boronic acids for sensing and other applications - a mini-review of papers published in 2013. Chem Cent J. 2014;8:1-17
27. Whited J, Rama CK, Sun XL. Synthesis and Evaluation of Protein-Phenylboronic Acid Conjugates as Lectin Mimetics. ACS Omega. 2018;3:13467-73
28. Fang H, Kaur G, Wang B. Progress in boronic acid-based fluorescent glucose sensors. J Fluoresc. 2004;14:481-9
29. Yan J, Springsteen G, Deeter S, Wang B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—it is not as simple as it appears. Tetrahedron. 2004;60:11205-9
30. Bull SD, Davidson MG, Van Den Elsen JMH. et al. Exploiting the reversible covalent bonding of boronic acids: recognition, sensing, and assembly. Acc Chem Res. 2013;46:312-26
31. Sun X, Zhai W, Fossey JS, James TD. Boronic acids for fluorescence imaging of carbohydrates. Chem Commun. 2016;52:3456-69
32. Hall DG, Ed. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. 1st ed. Wiley. 2005
33. Hall DG. Boronic acid catalysis. Chem Soc Rev. 2019;48:3475-96
34. Sharon N. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R-62R
35. António JPM, Russo R, Carvalho CP, Cal PMSD, Gois PMP. Boronic acids as building blocks for the construction of therapeutically useful bioconjugates. Chem Soc Rev. 2019;48:3513-36
36. Edwards JO, Sederstrom RJ. The Thermodynamics of ionization of benzeneboronic acid. J Phys Chem. 1961;65:862-3
37. Martínez-Aguirre MA, Medrano F, Ramírez-Rave S, Yatsimirsky AK. Analysis of the relative stability of trigonal and tetrahedral boronate cyclic esters in terms of boronic acid and diol acidities and the strain release effect. J Phys Org Chem. 2022;35:e4425
38. Springsteen G, Wang B. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58:5291-300
39. Lorand JP, Edwards JO. Polyol Complexes and Structure of the Benzeneboronate Ion. J Org Chem. 1959;24:769-74
40. Otsuka H, Uchimura E, Koshino H, Okano T, Kataoka K. Anomalous Binding Profile of Phenylboronic Acid withN-Acetylneuraminic Acid (Neu5Ac) in Aqueous Solution with Varying pH. J Am Chem Soc. 2003;125:3493-502
41. Djanashvili K, Frullano L, Peters JA. Molecular Recognition of Sialic Acid End Groups by Phenylboronates. Chem - Eur J. 2005;11:4010-8
42. Deshayes S, Cabral H, Ishii T. et al. Phenylboronic Acid-Installed Polymeric Micelles for Targeting Sialylated Epitopes in Solid Tumors. J Am Chem Soc. 2013;135:15501-7
43. Zhao D, Xu J-Q, Yi X-Q. et al. pH-Activated Targeting Drug Delivery System Based on the Selective Binding of Phenylboronic Acid. ACS Appl Mater Interfaces. 2016;8:14845-54
44. Matsumoto A, Stephenson-Brown AJ, Khan T. et al. Heterocyclic boronic acids display sialic acid selective binding in a hypoxic tumor relevant acidic environment. Chem Sci. 2017;8:6165-70
45. Peters JA, Djanashvili K. Exploration and exploitation of the uncommon pH profile of the dynamic covalent interactions between boronic acids and N-acetylneuraminic acids. Coord Chem Rev. 2023;491:215254
46. Peters JA. Interactions between boric acid derivatives and saccharides in aqueous media: Structures and stabilities of resulting esters. Coord Chem Rev. 2014;268:1-22
47. Hall DG, Ed. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials. 1st ed. Wiley. 2011
48. Trippier PC, McGuigan C. Boronic acids in medicinal chemistry: anticancer, antibacterial and antiviral applications. MedChemComm. 2010;1:183
49. Trippier PC, McGuigan C, Balzarini J. Phenylboronic-Acid-Based Carbohydrate Binders as Antiviral Therapeutics: Monophenylboronic Acids. Antivir Chem Chemother. 2010;20:249-57
50. Trippier PC, Balzarini J, McGuigan C. Phenylboronic-Acid-Based Carbohydrate Binders as Antiviral Therapeutics: Bisphenylboronic Acids. Antivir Chem Chemother. 2011;21:129-42
51. Windsor IW, Palte MJ, Lukesh JC, Gold B, Forest KT, Raines RT. Sub-picomolar Inhibition of HIV-1 Protease with a Boronic Acid. J Am Chem Soc. 2018;140:14015-8
52. Ghosh AK, Xia Z, Kovela S. et al. Potent HIV-1 Protease Inhibitors Containing Carboxylic and Boronic Acids: Effect on Enzyme Inhibition and Antiviral Activity and Protein-Ligand X-ray Structural Studies. ChemMedChem. 2019;14:1863-72
53. Hai W, Pu S, Wang X. et al. Poly(3,4-ethylenedioxythiophene) Bearing Pyridylboronic Acid Group for Specific Recognition of Sialic Acid. Langmuir. 2020;36:546-53
54. Bradke TM, Hall C, Carper SW, Plopper GE. Phenylboronic acid selectively inhibits human prostate and breast cancer cell migration and decreases viability. Cell Adhes Migr. 2008;2:153-60
55. McAuley EM, Bradke TA, Plopper GE. Phenylboronic acid is a more potent inhibitor than boric acid of key signaling networks involved in cancer cell migration. Cell Adhes Migr. 2011;5:382-6
56. Marasovic M, Ivankovic S, Stojkovic R, Djermic D, Galic B, Milos M. In vitro and in vivo antitumour effects of phenylboronic acid against mouse mammary adenocarcinoma 4T1 and squamous carcinoma SCCVII cells. J Enzyme Inhib Med Chem. 2017;32:1299-304
57. Wu W, Zhou T, Berliner A, Banerjee P, Zhou S. Glucose-Mediated Assembly of Phenylboronic Acid Modified CdTe/ZnTe/ZnS Quantum Dots for Intracellular Glucose Probing. Angew Chem Int Ed. 2010;49:6554-8
58. Williams GT, Kedge JL, Fossey JS. Molecular Boronic Acid-Based Saccharide Sensors. ACS Sens. 2021;6:1508-28
59. Lamping S, Otremba T, Ravoo BJ. Carbohydrate-Responsive Surface Adhesion Based on the Dynamic Covalent Chemistry of Phenylboronic Acid- and Catechol-Containing Polymer Brushes. Angew Chem Int Ed. 2018;57:2474-8
60. Yang W, He H, Drueckhammer DG. Computer-Guided Design in Molecular Recognition: Design and Synthesis of a Glucopyranose Receptor This work was supported by the National Institutes of Health (grant DK5523402). Angew Chem Int Ed Engl. 2001;40:1714-8
61. Yang W, Gao S, Gao X. et al. Diboronic acids as fluorescent probes for cells expressing sialyl lewis X. Bioorg Med Chem Lett. 2002;12:2175-7
62. Bérubé M, Dowlut M, Hall DG. Benzoboroxoles as Efficient Glycopyranoside-Binding Agents in Physiological Conditions: Structure and Selectivity of Complex Formation. J Org Chem. 2008;73:6471-9
63. Davis AP. Sticking to sugars. Nature. 2010;464:169-70
64. Pal A, Bérubé M, Hall DG. Design, Synthesis, and Screening of a Library of Peptidyl Bis(Boroxoles) as Oligosaccharide Receptors in Water: Identification of a Receptor for the Tumor Marker TF-Antigen Disaccharide. Angew Chem Int Ed. 2010;49:1492-5
65. James TD, Sandanayake KRAS, Shinkai S. A Glucose-Selective Molecular Fluorescence Sensor. Angew Chem Int Ed Engl. 1994;33:2207-9
66. Suman P, Patel BP, Kasibotla AV, Solano LN, Jonnalagadda SC. Synthesis and evaluation of functionalized aminobenzoboroxoles as potential anti -cancer agents. J Organomet Chem. 2015;798:125-31
67. Zhang Q, Che R, Lu W. Enhanced cellular uptake efficiency of DCM probes or SN38 conjugating with phenylboronic acids. Bioorg Med Chem. 2020;28:115377
68. Psurski M, Łupicka-Słowik A, Adamczyk-Woźniak A, Wietrzyk J, Sporzyński A. Discovering simple phenylboronic acid and benzoxaborole derivatives for experimental oncology - phase cycle-specific inducers of apoptosis in A2780 ovarian cancer cells. Invest New Drugs. 2019;37:35-46
69. Djanashvili K, Koning GA, Van Der Meer AJGM, Wolterbeek HTh, Peters JA. Phenylboronate 160Tb complexes for molecular recognition of glycoproteins expressed on tumor cells. Contrast Media Mol Imaging. 2007;2:35-41
70. Geninatti Crich S, Alberti D, Szabo I, Aime S, Djanashvili K. MRI Visualization of Melanoma Cells by Targeting Overexpressed Sialic Acid with a GdIII-dota-en-pba Imaging Reporter. Angew Chem Int Ed. 2013;52:1161-4
71. Xu X-D, Cheng H, Chen W-H, Cheng S-X, Zhuo R-X, Zhang X-Z. In situ recognition of cell-surface glycans and targeted imaging of cancer cells. Sci Rep. 2013;3:2679
72. Matsumoto A, Cabral H, Sato N, Kataoka K, Miyahara Y. Assessment of tumor metastasis by the direct determination of cellmembrane sialic acid expression. Angew Chem - Int Ed. 2010;49:5494-7
73. Matsumoto A, Sato N, Kataoka K, Miyahara Y. Noninvasive Sialic Acid Detection at Cell Membrane by Using Phenylboronic Acid Modified Self-Assembled Monolayer Gold Electrode. J Am Chem Soc. 2009;131:12022-3
74. Martinelli J, Jiménez-Juárez R, Alberti D, Geninatti Crich S, Djanashvili K. Solid-phase synthesis and evaluation of tumour-targeting phenylboronate-based MRI contrast agents. Org Biomol Chem. 2020;18:7899-906
75. Martinelli J, Tei L, Geninatti Crich S, Alberti D, Djanashvili K. Towards Enhanced MRI Performance of Tumor-Specific Dimeric Phenylboronic Contrast Agents. Molecules. 2021;26:1730
76. Malouff TD, Seneviratne DS, Ebner DK. et al. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front Oncol. 2021;11:601820
77. Zhou J, Stapleton P, Xavier-Junior FH. et al. Triazole-substituted phenylboronic acids as tunable lead inhibitors of KPC-2 antibiotic resistance. Eur J Med Chem. 2022;240:114571
78. Xu S, Song S, Sun L. et al. Indolylarylsulfones bearing phenylboronic acid and phenylboronate ester functionalities as potent HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg Med Chem. 2022;53:116531
79. Tao Y, Budhipramono A, Huang J. et al. Anticancer benzoxaboroles block pre-mRNA processing by directly inhibiting CPSF3. Cell Chem Biol. 2024;31:139-149.e14
80. Ellis GA, Palte MJ, Raines RT. Boronate-Mediated Biologic Delivery. J Am Chem Soc. 2012;134:3631-4
81. Narla SN, Pinnamaneni P, Nie H, Li Y, Sun X-L. BSA-boronic acid conjugate as lectin mimetics. Biochem Biophys Res Commun. 2014;443:562-7
82. Andersen KA, Smith TP, Lomax JE, Raines RT. Boronic Acid for the Traceless Delivery of Proteins into Cells. ACS Chem Biol. 2016;11:319-23
83. Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers long-circulating? Adv Drug Deliv Rev. 1995;16:141-55
84. Torchilin VP. Passive and Active Drug Targeting: Drug Delivery to Tumors as an Example. In: Schäfer-Korting M, Ed. Drug Delivery. Berlin, Heidelberg: Springer Berlin Heidelberg. 2010:3-53 (Handbook of Experimental Pharmacology; vol. 197)
85. Wilson B, Geetha KM. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J Drug Deliv Sci Technol. 2022;74:103553
86. Li Y, Xiao W, Xiao K. et al. Well-Defined, Reversible Boronate Crosslinked Nanocarriers for Targeted Drug Delivery in Response to Acidic pH Values and cis-Diols. Angew Chem Int Ed. 2012;51:2864-9
87. Chen W, Xie W, Zhao G, Shuai Q. Efficient pH-Responsive Nano-Drug Delivery System Based on Dynamic Boronic Acid/Ester Transformation. Molecules. 2023;28:4461
88. Naito M, Ishii T, Matsumoto A, Miyata K, Miyahara Y, Kataoka K. A Phenylboronate-Functionalized Polyion Complex Micelle for ATP-Triggered Release of siRNA. Angew Chem Int Ed. 2012;51:10751-5
89. Ji M, Li P, Sheng N. et al. Sialic Acid-Targeted Nanovectors with Phenylboronic Acid-Grafted Polyethylenimine Robustly Enhance siRNA-Based Cancer Therapy. ACS Appl Mater Interfaces. 2016;8:9565-76
90. Kim J, Lee YM, Kim H, Park D, Kim J, Kim WJ. Phenylboronic acid-sugar grafted polymer architecture as a dual stimuli-responsive gene carrier for targeted anti-angiogenic tumor therapy. Biomaterials. 2016;75:102-11
91. Zhang X, Chen B, He M, Zhang Y, Peng L, Hu B. Boronic acid recognition based-gold nanoparticle-labeling strategy for the assay of sialic acid expression on cancer cell surface by inductively coupled plasma mass spectrometry. The Analyst. 2016;141:1286-93
92. Xu Y, Huang Y, Zhang X, Lu W, Yu J, Liu S. Carrier-free Janus nano-prodrug based on camptothecin and gemcitabine: Reduction-triggered drug release and synergistic in vitro antiproliferative effect in multiple cancer cells. Int J Pharm. 2018;550:45-56
93. Xu Y, Huang Y, Lu W, Liu S, Xiao Y, Yu J. 4-Carboxyphenylboronic acid-decorated, redox-sensitive rod-shaped nano-micelles fabricated through co-assembling strategy for active targeting and synergistic co-delivery of camptothecin and gemcitabine. Eur J Pharm Biopharm. 2019;144:193-206
94. Huang Y, He Y, Xia X, Quan H, Yu J. Phenylboronic acid-functionalized co-delivery micelles with synergistic effect and down-regulation of HIF-1alpha to overcome multidrug resistance. J Drug Deliv Sci Technol. 2021;62:102346
95. Zhang Q, Liu Y, Fei Y. et al. Phenylboronic Acid-Functionalized Copolypeptides: Facile Synthesis and Responsive Dual Anticancer Drug Release. Biomacromolecules. 2022;23:2989-98
96. Li S, Hou X, Ma Y, Wang Z. Phenylboronic-acid-based Functional Chemical Materials for Fluorescence Imaging and Tumor Therapy. ACS Omega. 2022;7:2520-32
97. Wang C, Tang Y, Yuan Y, Li C, Wu W, Jiang X. Phenylboronic acid-modified nanoscale multi-arm polymers for tumor-targeted therapy. Biomater Sci. 2023;11:4281-8
98. Liu A, Peng S, Soo JC, Kuang M, Chen P, Duan H. Quantum Dots with Phenylboronic Acid Tags for Specific Labeling of Sialic Acids on Living Cells. Anal Chem. 2011;83:1124-30
99. Lee J, Chung S, Cho H, Kim D. Phenylboronic Acid-Decorated Chondroitin Sulfate A-Based Theranostic Nanoparticles for Enhanced Tumor Targeting and Penetration. Adv Funct Mater. 2015;25:3705-17
100. Liu R, Cui Q, Wang C, Wang X, Yang Y, Li L. Preparation of Sialic Acid-Imprinted Fluorescent Conjugated Nanoparticles and Their Application for Targeted Cancer Cell Imaging. ACS Appl Mater Interfaces. 2017;9:3006-15
101. Kang B-Y, Hur W, Kim SM. et al. Phenylboronic acid conjugated to doxorubicin nanocomplexes as an anti-cancer drug delivery system in hepatocellular carcinoma. Nanomedicine Nanotechnol Biol Med. 2021;34:102389
102. Jung S, Jiang L, Zhao J. et al. Clathrin light chain-conjugated drug delivery for cancer. Bioeng Transl Med. 2023;8:e10273
103. Demirbuken SE, Karaca GY, Kaya HK. et al. Paclitaxel-conjugated phenylboronic acid-enriched catalytic robots as smart drug delivery systems. Mater Today Chem. 2022;26:101172
104. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/cancer
105. International Agency for Research on Cancer. https://gco.iarc.fr
106. Lan T, Guo Q. Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application. Nanotechnol Rev. 2019;8:548-61
Author contact
![]() Corresponding author: e-mail: marina.tranfic-bakichr.
Corresponding author: e-mail: marina.tranfic-bakichr.
 Global reach, higher impact
Global reach, higher impact