13.3
Impact Factor
Theranostics 2025; 15(7):2700-2719. doi:10.7150/thno.108309 This issue Cite
Review
Recent advances in the mechanisms, current treatment status, and application of multifunctional biomaterials for radiation-induced skin injury
Institute of Rocket Force Medicine, State Key Laboratory of Trauma and Chemical Poisoning, Third Military Medical University (Army Medical University), Gaotanyan Road Street, Shapingba district, Chongqing 400038, China.
These authors contributed equally to this work: Yang Xu, Quanying Liu.
Received 2024-12-6; Accepted 2025-1-13; Published 2025-1-27
Abstract
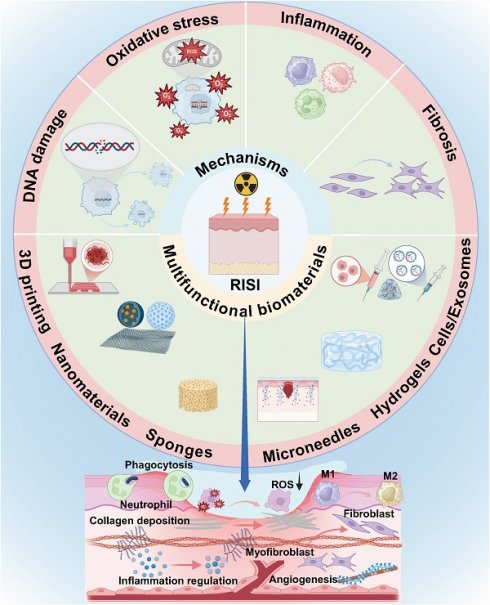
Radiation-induced skin injury (RISI) is a prevalent complication following nuclear accidents and radiotherapy for tumors. The associated side effects may include erythema, desquamation, ulceration, and in severe cases, necrosis of certain skin tissues. These adverse reactions significantly impact the quality of life for patients and contribute to both psychological distress and economic burdens. However, there is currently no standardized protocol for the treatment and management of RISI. In comparison to traditional pharmaceuticals, the utilization of biomaterials in addressing radiation-induced diseases has garnered increasing attention due to their superior biocompatibility and outstanding functionality. Nevertheless, comprehensive reviews on this topic remain scarce. In this context, this paper systematically elucidates the pathogenesis of RISI, subsequently introducing the clinical manifestations and advancements in treatment for RISI. It emphasizes a comprehensive discussion on the design and innovation of novel biomaterials aimed at treating and protecting against RISI, while also illustrating the mechanisms by which multifunctional biomaterials enhance both treatment efficacy and protective measures for radiation-induced skin conditions. Finally, it addresses the challenges encountered by multifunctional biomaterials in managing radiation-related diseases and outlines potential directions for future research efforts. The objective of this review is to investigate the therapeutic and protective effects of multifunctional biomaterials in relation to radiation-induced skin injury, thereby providing significant reference value for the design and clinical application of innovative materials.
Keywords: Multifunctional, Biomaterials, Radiation-induced skin injury, Wound healing
1. Introduction
The widespread utilization of nuclear technology across various sectors, including industry, medicine, science and technology, and the military, heightens the potential for nuclear accidents and radiation-related harm [1,2]. The skin is the largest organ in the human body, serving multiple functions including the regulation of immunity and metabolism. It acts as the primary barrier against external threats, making it particularly vulnerable to damage [3]. Radiation-induced skin injury (RISI) is a common complication associated with nuclear accidents and tumor radiotherapy [4]. For instance, in the context of tumor radiotherapy, despite significant advancements in the precision and standardization of radiation therapy, the incidence of RISI is gradually on the rise. Approximately 95% of patients undergoing radiotherapy for tumors experience varying degrees of skin damage. Adverse reactions may present as erythema, peeling skin, and in severe cases, ulcers or even necrosis of skin tissue [5]. These complications not only severely impact patients' quality of life but also impose substantial psychological stress and economic burdens. Furthermore, they can interfere with ongoing treatment regimens and disrupt the overall process of radiation therapy [6]. Therefore, it is imperative to identify effective strategies for the treatment or prevention of skin damage resulting from ionizing radiation [7].
The normal process of skin wound healing typically progresses through four distinct stages: hemostasis, inflammation, proliferation, and remodeling [8]. In contrast to standard wounds, radioactive skin lesions induce DNA damage and result in elevated levels of reactive oxygen species (ROS) production [9]. With the accumulation of irradiation time and dose, the clinical and pathological manifestations of RISI may include, but are not limited to, dry and wet desquamation, erythema, edema, abnormal pigmentation. In severe cases, skin ulcers and fibrosis can occur, potentially leading to secondary infections that adversely affect the patient's overall health [10]. Currently, there is no standardized gold standard treatment for RISI in clinical practice, and the existing research lacks universality. To date, several commercially available topical ointments have emerged, primarily composed of small molecular drugs that chemically neutralize toxic free radicals within skin cells. However, their effectiveness is significantly limited by unsatisfactory solubility, poor chemical stability, susceptibility to inactivation during long-term storage, restricted capacity to eliminate free radicals, and notable side effects. Consequently, the availability and practicality of these treatments are severely compromised [11]. Addressing these limitations is crucial for enhancing the therapeutic efficacy of RISI. In light of these challenges, there exists an urgent necessity to develop a novel strategy aimed at improving both radiation protection and treatment effectiveness for the skin.
In recent years, multifunctional biomaterials have increasingly emerged as a significant focus of research in the field of regenerative medicine. Compared to traditional pharmaceuticals, these materials are extensively utilized for tissue repair and regeneration due to their advantages in biocompatibility, bioactivity, chemical stability, and degradability [12]. Functional biomaterials not only exhibit structural and compositional similarities to the extracellular matrix, thereby promoting cell proliferation and migration while regulating the local microenvironment, but they also function as effective carriers for cells, drugs, or bioactive substances. This capability enables targeted delivery to specific sites, ultimately enhancing therapeutic outcomes [13]. The researchers have discovered that these multifunctional biomaterials have significantly advanced in the protection and treatment of radiation-induced skin disorders. Therefore, it is crucial to integrate and interpret current research developments in this field to provide clinical guidance for the treatment and prevention of radiation-related illnesses.
Considering the rapid growth of interest in this field and the pressing need for effective treatments, this paper systematically elucidates the pathogenesis of RISI, subsequently introducing the clinical manifestations and advancements in treatment for RISI. It emphasizes a comprehensive discussion on the design and innovation of novel biomaterials aimed at treating and protecting against RISI, while also illustrating the mechanisms by which multifunctional biomaterials enhance both treatment efficacy and protective measures for radiation-induced skin conditions. Finally, it addresses the challenges encountered by multifunctional biomaterials in managing radiation-related diseases and outlines potential directions for future research efforts. This work aims to encourage and inspire a greater number of researchers to advance the design of innovative biomaterials for the treatment and prevention of radiation-related diseases. It seeks to foster the development of new radiation protection agents and aspires to facilitate the translation of relevant research into clinical applications, thereby realizing their potential value in practice.
2. Mechanisms of radiation-induced skin injury
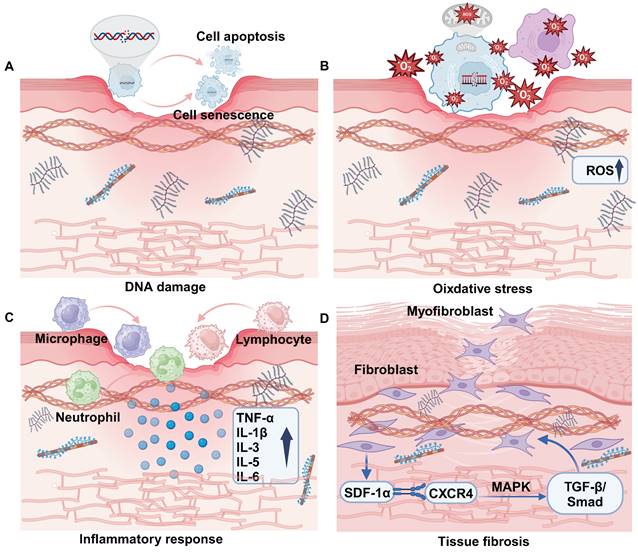
The mechanism of RISI is intricate. Currently, it is widely accepted that ionizing radiation can induce both reversible and irreversible damage to the DNA of biological tissues and cells. This damage may lead to DNA mutations and structural abnormalities, affecting gene transcription and protein levels, potentially resulting in cell death. Additionally, ionizing radiation triggers the production of highly reactive molecules such as hydroxyl free radicals, which attack proteins, lipids, and DNA. This process activates various cellular signaling pathways that result in the release of inflammatory factors, contributing to cellular and tissue damage (Figure 1) [14]. Simultaneously, blood vessel impairment caused by ionizing radiation disrupts the normal supply of nutrients, exacerbates tissue hypoxia, and promotes fibrosis process [15]. As a result, the prolonged inflammatory response coupled with ongoing cellular damage and repair processes leads to excessive activation of fibroblasts. This results in abnormal deposition of collagen and extracellular matrix proteins, rendering the skin difficult to heal and causing it to appear erythematic, swollen, and ulcerated.
Pathogenesis of radiation-induced skin injury. (A) DNA damage. (B) Oxidative stress. (C) Inflammatory response. (D) Tissue fibrosis.
2.1 DNA damage
DNA is an organic molecule that serves as a crucial repository of genetic information essential for the development and maintenance of life. Its chemical properties and structure can be readily altered under the influence of ionizing radiation (IR). DNA damage encompasses both physical and chemical alterations that directly or indirectly compromise DNA strands, including single-strand breaks, double-strand breaks, base deletions, and cross-linking damage (Figure 1A) [16]. These lesions can be identified using a range of techniques, including single-cell gel electrophoresis and pulsed-field gel electrophoresis. DNA damage occurs in vivo and is repaired through various mechanisms such as base excision repair, nucleotide excision repair, mismatch repair, homologous recombination repair, and non-homologous end joining. These processes work together to mitigate the potentially harmful effects of DNA damage [17]. When repair mechanisms are compromised, a significant accumulation of DNA damage occurs within the organism, resulting in cellular senescence, apoptosis, or mutations that adversely affect skin integrity [18].
Notably, radiation-induced senescent cells, despite their inability to undergo cell division and being permanently arrested in proliferation, consistently demonstrate apoptotic resistance, metabolic activity, and the secretion of pro-inflammatory and pro-fibrotic molecules. Additionally, they induce changes in the adjacent microenvironment. Among these effects, the bystander effects associated with ionizing radiation-induced cellular senescence are primarily mediated by exosomes and microRNAs (cells that are not directly exposed to ionizing radiation also exhibit senility-like features when they receive signaling molecules released by irradiated cells) [19]. For example, exosomes derived from senescent cells are enriched with a variety of inflammatory mediators, such as IL-6, IL-8, TNF-α, etc. These factors have the potential to activate inflammatory signaling pathways in surrounding healthy cells, inducing them to enter a chronic low-grade inflammatory environment similar to the state of aging. Additionally, they may disrupt normal cellular metabolism by impairing mitochondrial function or elevating levels of ROS [20]. At the same time, a significant number of microRNAs (miRNAs) implicated in the aging process have been identified, and their alterations can lead to DNA damage, γH2AX lesion formation, increased ROS, and oxidative stress. For instance, miR-34a promoted cellular senescence by targeting SIRT1 and downregulating its expression, which subsequently activated p53 [21]. The overexpression of miR-21 has been shown to diminish the replicative lifespan of human umbilical vein endothelial cells (HUVECs) [22]. Additionally, miR-22 facilitated cellular senescence in human fibroblasts and epithelial cells by targeting CDK6, Sp1, and SIRT1 [23]. Currently, the p16-pRB and p53-p21 pathways are widely recognized as significant mechanisms underlying radiation-induced aging [24]. It has been reported that mitochondrial dysfunction, ferritin autophagy, and the inflammation cascade induced by cyclic guanosine monophosphate (cGMP)-adenosine monophosphate (AMP) synthase (cGAS) may also serve as potential mechanisms contributing to radiation-induced aging [25]. Our research team has identified human positive cofactor 4 (PC4) as a potential target in the context of cellular aging. PC4 accumulated and became activated during the aging process, contributing to its acceleration by disrupting mTOR-regulated protein homeostasis [26]. The overall process by which ionizing radiation induces DNA damage remains complex and is not yet fully elucidated. Nevertheless, research indicated that emerging techniques in DNA nanotechnology and atomic force microscopy (AFM) hold promise for uncovering the fundamental mechanisms underlying DNA damage [9].
2.2 Oxidative stress
Ionizing radiation can inflict damage on DNA either directly or indirectly, primarily through the radiolysis of water, which generates free radicals that subsequently attack cellular membrane lipids, proteins, and DNA. This process leads to oxidative stress and ultimately results in cellular damage. Mitochondria are organelles tasked with energy production within cells and play a crucial role in the regulation of oxidative stress [27]. Free radicals generated by ionizing radiation enhance the expression of cyclooxygenases (COXs), nitric oxide synthases (NOS), lipoxygenases (LOX), and NADPH oxidase, thereby impacting mitochondrial function [28]. Increased levels of ROS lead to mitochondrial dysfunction, which is typically characterized by impaired respiratory chain activity, structural abnormalities, depletion of the cellular ATP pool, disruption of cell signaling pathways, and an elevated production of mitochondria-derived ROS (mtROS) (Figure 1B) [29]. These mtROS may undermine the integrity of mitochondrial membranes, leading to the release of mitochondrial ligands or damage-associated molecular patterns (DAMPs), which can further exacerbate mitochondrial dysfunction [28]. At the tissue level, the accumulation of ROS and oxidative stress can disrupt endothelial cell connections, resulting in damage to vascular endothelial cells. This process increases permeability and further compromises vascular epithelial function, ultimately leading to tissue hypoxia and necrosis [30]. In radiation-induced skin injury, TGF-β binds to its receptor to up-regulate the expression of miRNA-21, thereby inhibiting the production of superoxide dismutase 2 (SOD2), which is an important factor in the elimination of ROS [31].
2.3 Inflammatory response
Ionizing radiation causes damaged cells to release inflammatory mediators that attract immune cells (including lymphocytes, macrophages, and neutrophils) to the damaged skin area. These cells release various cytokines and chemokines that further amplify the inflammatory response [32]. The early inflammatory response is primarily characterized by an increase in pro-inflammatory cytokines (such as IL-1β, IL-3, IL-5, IL-6, and TNF-α) and chemokines. These mediators contribute to skin tissue injury and compromise barrier integrity by activating the local inflammatory responses of eosinophils and neutrophils (Figure 1C) [33]. In addition, skin exposure to ionizing radiation induces cellular senescence of keratinocytes. These aging keratinocytes will not only over proliferate, leading to the destruction of the structural function of the epidermis, but also further secrete inflammatory mediators and cause radiation dermatitis [34]. Cellular iron death induced by lipid peroxidation and the formation of ferritin in keratinocytes is also a contributing factor to necrotizing skin inflammation [35,36].
Further intensification of the inflammatory response is implicated in the activation and intranuclear migration of nuclear factor-κB (NF-κB). Initially, ionizing radiation induces an increase in intracellular ROS, which subsequently activates the IKK (IκB kinase) complex. This activation leads to the phosphorylation and degradation of IκB (inhibitory factor κB). Following the degradation of IκB, NF-κB is liberated from the cytoplasm and transferred into the nucleus. Within the nucleus, NF-κB binds to the promoter region of target genes, thereby promoting the expression of various inflammatory mediators (such as IL-1β, IL-6, TNF-α, etc.) and chemokines. The activation of ROS further extends to the NLRP3 pathway, facilitating the release of Caspase-1 and the extracellular secretion of pro-inflammatory cytokines such as IL-1β and IL-8. This cascade triggers inflammation and promotes apoptosis [37]. Based on this, NLRP3 and NF-κB may be regarded as promising targets for mitigating radiation-induced skin inflammatory damage. This can be achieved by directly or indirectly inhibiting their upstream signaling pathways, thereby alleviating skin damage through the reduction of the inflammatory response. Due to the intricate nature of the skin immune response, further investigation into the interactions occurring among epithelial cells, stromal cells, and various immune cell populations is essential. This research will enhance our understanding of the mechanisms that regulate skin immune homeostasis and inflammation in the context of ionizing radiation [24].
2.4 Tissue fibrosis
Radiation-induced fibrosis is a pathological condition characterized by the persistent over-activation of fibroblasts, leading to the destruction of normal tissue components. This process results in an excessive accumulation of extracellular matrix, which can ultimately cause irreversible damage to the affected tissues [38,39]. Skin fibrosis is a complex process that involves the intricate interactions among various cells and cytokines [40]. Ionizing radiation facilitates the activation of myofibroblasts by engaging various signaling pathways, including TGF-β and Wnt/β-catenin. Additionally, it can stimulate fibroblasts, endothelial cells, and vascular smooth muscle cells, prompting their transformation into myofibroblasts [41]. For instance, the interaction between stromal cell-derived factor-1α (SDF-1α) and its receptor CXCR4 activates the TGF-β/Smad signaling pathway through downstream MAPK signaling. This cascade ultimately contributes to the promotion of fibrosis (Figure 1D) [42]. When activated myofibroblasts secrete alpha-smooth muscle actin (α-SMA) and types I, III, and IV collagen, integrins link the alpha-SMA stress fibers to collagen, creating high contractility and hardening the skin tissue microenvironment, which can eventually lead to its sclerosis and dysfunction [43]. In addition, microvascular injury may lead to skin fibrosis. The exposure of subcutaneous extracellular matrix (ECM) components to platelets promotes the excessive secretion of von Willebrand factor (vWF) and platelet-activating factor (PAF), while simultaneously diminishing the production of nitric oxide (NO), prostacyclin, and the transmembrane glycoprotein thrombomodulin (TM), along with their fibrinolytic activity [44]. These alterations ultimately trigger an anti-fibrinolytic response and initiate a clotting cascade, resulting in coagulation and vascular occlusion, which accelerates the process of fibrosis [45].
3. Clinical manifestation and treatment status of RISI
Radiation skin injuries can be categorized into acute and chronic types. Furthermore, the severity of these injuries can be classified to facilitate the development and implementation of appropriate treatment plans and measures [46]. Acute radiation-induced skin injury refers to acute radiation dermatitis and radiation skin ulcer caused by multiple large doses of external radiation in one or a short period of time (a few days) [47]. The pathophysiological changes of acute RISI mainly involve erythema, dry and wet desquamation, skin necrosis, ulceration, and bleeding, which usually occur within 90 days [48]. According to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0, acute radiation-induced skin injury can be classified into 5 grades from grade 0 to grade IV according to the degree of injury. The specific skin injury and manifestations are shown in Table 1 [49]. Different from acute RISI, chronic RISI is a delayed adverse reaction with long latency, obvious potential, progression, persistence and recurrence. Pathophysiological changes in chronic RISI involve symptoms such as pigmentation, chronic skin atrophy, ulcers, telangiectasia, fibrosis, and skin cancer [50]. The grading standards for chronic radiation dermatitis are primarily based on the criteria established by the American Cancer Radiation Therapy Association Group. These standards typically categorize the condition into 6 grades ranging from 0 to V degrees (Table 2) [51].
Classification and manifestation of acute radiation-induced skin injury
| Damage grade | Changes in the skin |
|---|---|
| Grade 0 | Unchanged |
| Grade I | Erythema or dry desquamation |
| Grade Ⅱ | Moderate erythema or flaky wet desquamation |
| Grade Ⅲ | Fused wet desquamation or pitted edema with a diameter ≥1.5 cm, not limited to the fold |
| Grade Ⅳ | Full dermal skin necrosis or ulceration |
Classification and manifestation of chronic radiation-induced skin injury
| Damage grade | Changes in the skin |
|---|---|
| Grade 0 | Unchanged |
| Grade I | Mild atrophy, hyperpigmentation, slight hair loss |
| Grade Ⅱ | Lamellar atrophy, moderate capillaries dilation, complete hair exfoliation |
| Grade Ⅲ | Marked atrophy, marked telangiectasia |
| Grade Ⅳ | Ulceration |
| Grade Ⅴ | Died as a direct result of a late radioactive reaction |
RISI can significantly impact patients' quality of life and the overall effectiveness of treatment following radiation therapy. Consequently, the management of RISI has been a focal point for both clinicians and researchers. While numerous clinical approaches exist for the prevention and treatment of RISI, both domestically and internationally, many of these methods lack targeted and effective protocols.
3.1 Topical care
The management of RISI is an integral part of the entire radiation therapy process; however, there is currently no standardized approach to care. Traditional skin care encompasses the cleaning and maintenance of the patient's skin, which can effectively prevent wound infections, alleviate physical discomfort, and facilitate subsequent treatment for patients. The irradiated area of the patient's skin should be maintained in a clean and dry condition. Initially, it is essential to use a mild non-irritating cleanser to gently cleanse the affected skin; water temperature should not be excessively high, and alkaline soaps should be avoided [52]. Following cleansing, a moisturizing cream containing vitamin E, hyaluronic acid, and other beneficial ingredients should be applied to keep the skin hydrated. This helps reduce external irritation while ensuring that wounds remain in a dry and well-ventilated state. It is also important to avoid using irritating dressings that could exacerbate the condition or lead to secondary damage from friction with clothing. It is crucial to recognize that excessive or improper washing can disrupt the biophysical properties of the skin barrier and may exacerbate symptoms of inflammation or injury. Therefore, it is essential to pay attention to both the duration and frequency of cleansing. It is recommended that patients clean their wounds once or twice daily, avoiding prolonged immersion during radiation therapy.
3.2 Drug therapy
The medications employed in the treatment of radiation injuries primarily consist of hormone therapies for anti-inflammatory purposes, vascular agents for antioxidant therapy, and superoxide dismutase [53]. The inflammatory response resulting from acute RISI is typically managed with local corticosteroids. These agents exhibit therapeutic effects owing to their anti-inflammatory, immunosuppressive, anti-proliferative, and vasoconstrictive properties. For instance, they effectively inhibit cytokine surges induced by radiation, diminish the occurrence of wet desquamation, and postpone the onset of dermatitis [54]. Statins possess anti-inflammatory, immunomodulatory, antioxidant, metabolic, and antimicrobial properties that can enhance the management of skin diseases and facilitate the healing of ulcerative wounds. Notably, they have been demonstrated to significantly alleviate radiation-induced swelling, itching, and pain [55]. Melatonin is a potent free radical scavenger with notable antioxidant and anti-inflammatory properties, capable of maintaining mitochondrial homeostasis under various experimental conditions and exhibits significant anti-aging effects [56]. Amifostine, recognized for its antioxidant capabilities, was the first clinical radioprotective agent approved by the United States Food and Drug Administration (FDA). However, its clinical application has been limited due to pronounced adverse reactions such as nausea, vomiting, and fatigue, as well as restricted routes of administration [57]. Topical emulsions of triethanolamine have demonstrated efficacy in the treatment of radiation dermatitis. These formulations facilitate the removal of necrotic tissue, promote fibroblast proliferation, mitigate in vitro vascular alterations, restore CD34 expression, enhance epithelial cell proliferation, and decrease IL-1 secretion. Sucralfate is a widely used anti-ulcer medication when administered orally. In topical formulations, sucralfate has demonstrated significant barrier protection, antibacterial activity, anti-inflammatory effects, and the ability to promote angiogenesis. However, clinical outcomes regarding the efficacy of sucralfate in radioactive skin therapy have yielded mixed results. The largest and most rigorously conducted studies reported no significant impact of sucralfate on alleviating dermatitis severity or relieving symptoms in patients. Similar findings were presented by Wells et al. Conversely, Maiche et al. assessed that a 7% sugar sulfate cream could substantially mitigate acute radiation reactions affecting the skin [58]. In addition, our research group has discovered that, compared to rapamycin, cordycepin effectively can inhibit cellular senescence through the activation of NRF2 and AMPK pathways. This finding suggested that cordycepin may offer a more effective treatment for alleviating radiation-induced skin ulcers and held promise as a potential palliative option for managing such conditions [59,60]. Similarly, dasatinib plus quercetin was shown to alleviate radioactive skin ulcers by removing senescent cells [61]. It is essential to recognize that the pharmacological management of RISI constitutes a common and significant intervention, capable of alleviating symptoms, promoting healing, and mitigating complications to varying extents (Table 3). However, it is important to acknowledge that drug treatments may also lead to substantial side effects, and individual patients can exhibit considerable variability in their responses to the same medication. Therefore, we must consider diverse prevention and treatment strategies based on the underlying pathogenesis and select the most appropriate medication tailored to each specific case in order to achieve optimal outcomes.
3.3 Cytokines
Epidermal growth factor (EGF) plays a crucial role in promoting the proliferation of human epidermal stem cells, fibroblasts, and keratinocytes [62]. Experimental studies have demonstrated that EGF, which is released by platelets, macrophages, and fibroblasts, plays a significant role in promoting wound healing through the process of skin re-epithelialization [63]. There are clinical studies using recombinant human epidermal growth factor (rh-EGF) to treat radiation-induced chronic wounds for 16 weeks. In comparison to flap surgery and conventional treatments, rh-EGF has demonstrated significant efficacy in alleviating symptoms and promoting the healing process of chronic ulcer wounds [64]. Fibroblast growth factor (FGF) can promote capillary regeneration and improve local blood circulation, and the healing time of RISI patients treated with FGF was shorter than that of conventional therapy [65]. Recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) can be effectively utilized in the management of refractory wounds following chemoradiotherapy. It demonstrates both local anti-infective properties and promotes wound healing [66]. Chen et al. have discovered that senescent fibroblasts accumulate at the site of RISI and can modulate the local immune microenvironment through the secretion of IL-33. This process accelerated epithelialization and collagen deposition, thereby contributing to wound healing. These findings offered valuable insights for developing intervention strategies targeting senescent cells in RISI (Table 3) [67]. Although the topical application of growth factors offers a relatively gentle and operationally simple therapeutic approach, the use of a single growth factor may have limited efficacy and is prone to inactivation in vitro, which could necessitate the combination of multiple growth factors and precise control of dose and timing of administration. However, there is still a lack of sufficient large-scale randomized controlled clinical trial evidence to comprehensively evaluate the effectiveness and reliability of this approach.
3.4 Surgical treatment
RISI can result in severe complications such as skin ulcers, necrosis, and infections. In cases where patients do not respond to conservative treatment or present with advanced conditions, surgical intervention becomes a crucial option. Some researchers have employed Doppler ultrasound to design axial skin flaps for the repair of radioactive ulcers on the chest wall in patients following radical breast cancer surgery.
Studies on the drug therapy and cytokines to the treatment of radiation-induced skin injury
| Treatment methods | Composition | Functions | Limitations | Ref |
|---|---|---|---|---|
| Drug therapy | Superoxide dismutase | Scavenging free radicals | Limited routes of administration, poor stability | [53] |
| Corticosteroids | Anti-inflammatory, antioxidant, immunosuppressive | Long-term use is easy to cause skin atrophy | [54] | |
| Statins | Antibacterial, anti-inflammatory, antioxidant | Lack of substantial clinical evidence | [55] | |
| Melatonin | Scavenging free radicals, inhibiting apoptosis | Lack of large-scale, randomized controlled trials | [56] | |
| Amifostine | Antioxidant | Obvious side effects (nausea, vomiting, fatigue, etc.) | [57] | |
| Triethanolamine | Recruit macrophages, stimulate granulation tissue formation | Potential irritation, allergic reactions | [54] | |
| Sucralfate | Antibacterial, anti-inflammatory, promote angiogenesis | The clinical efficacy is controversial | [58] | |
| Cordycepin | Anti-DNA damage, anti-cell aging | Low bioavailability, high cost | [59] | |
| Quercetin | Anti-DNA damage, removal of senescent cells, antioxidation | Difficult to extract and purify | [61] | |
| Cytokines | EGF | Promote re-epithelialization | High cost, easy inactivation | [63] |
| FGF | Enhance angiogenesis | [65] | ||
| rhGM-CSF | Anti-infection, promote wound healing | [66] |
Their findings indicated that these patients experienced successful flap survival, adequate blood circulation, and no recurrence of ulcers during postoperative follow-up. Pulsed dye laser therapy is primarily used to address abnormally dilated capillaries, thereby reducing erythema and inflammatory responses [68]. Hyperbaric oxygen therapy primarily alleviates pain and improves wound healing by increasing local oxygen tension and enhancing angiogenesis [69]. While surgical treatment can remove diseased tissue to prevent further deterioration of the condition, it is time-consuming, carries higher surgical risks, has a long recovery period, and requires a higher level of professional expertise from the surgeon.
The studies mentioned above all exhibit limitations in the treatment of RISI, particularly in their failure to classify patients based on their skin's sensitivity to radiation. Therefore, it is essential to determine whether the patient suffers from any conditions that may increase radiation sensitivity, such as diabetes and vascular diseases. Additionally, an initial assessment of the patient's radiation sensitivity can be made through direct observation of skin condition and minimal erythema dose testing, with findings documented [70]. Further evaluation can be conducted by measuring specific biomarkers, such as DNA damage repair capacity and levels of cellular apoptosis, to quantify individual radiation sensitivity [71]. Although we have identified genes that are altered in skin tissue following radiation exposure through high-throughput sequencing, defining truly "radiation-specific" genes requires additional research to confirm which genes are uniquely or predominantly activated or inhibited under radiation conditions while remaining relatively quiescent in other environments [72]. This would provide a theoretical foundation and technical support for developing new strategies for radiation protection. Furthermore, current clinical approaches for managing RISI predominantly rely on pharmacological interventions. While the aforementioned drugs possess varying degrees of antioxidant and anti-inflammatory properties, they suffer from poor chemical stability and limited free radical scavenging capabilities. Consequently, satisfactory therapeutic outcomes have yet to be achieved. Most widely used traditional medicines primarily focus on alleviating symptoms rather than addressing underlying issues. Consequently, it is imperative to conduct comprehensive investigations aimed at identifying more effective prevention and control strategies.
4. Research progress of biomaterials in radiation-induced skin injury
In recent years, the utilization of biomaterials in the treatment of radiation-related diseases and in radiation protection has garnered increasing attention. Unlike traditional pharmaceutical compounds, biomaterials offer a range of advantages, including exceptional biological activity, chemical stability across various physiological conditions, superior biocompatibility, and targeted delivery capabilities (Table 4) [73]. It is crucial to emphasize that the strategic integration of specific biomaterials with particular therapeutic agents can yield synergistic therapeutic effects, thereby paving new avenues for the treatment of radiation-induced ailments (Figure 2).
4.1 Stem cells and exosomes
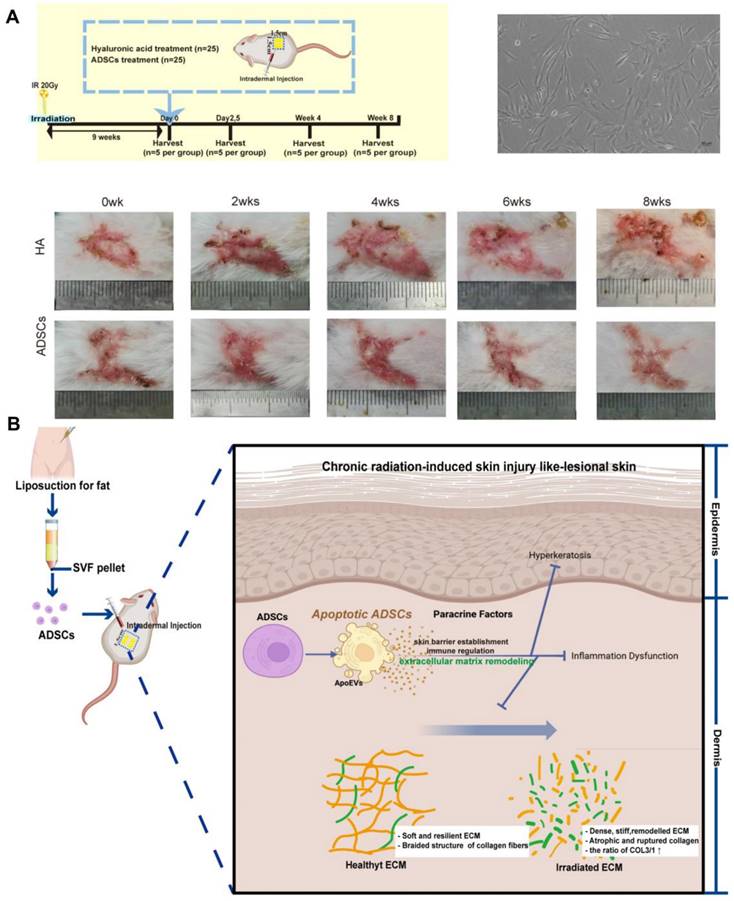
Therapies utilizing stem cells have been documented to enhance wound healing following exposure to radiation [101]. Zhu et al. discovered that adipose-derived stem cells (ADSCs) injected into the skin of patients suffering from chronic radiation dermatitis can significantly alleviate symptoms associated with this condition, including fibrosis and irregular collagen deposition. Furthermore, these cells promote the regeneration of skin affected by severe radiation damage. The proposed therapeutic mechanism may involve ADSCs undergoing apoptosis, which in turn facilitates the remodeling and reorganization of the dermal collagen matrix, thereby enhancing the vitality of radiation-damaged skin in murine models (Figure 3) [74]. The researchers discovered that bone marrow mesenchymal stem cells (BMSCs) were also effective in alleviating skin inflammation and fibrosis in mice suffering from acute RISI [75]. Human-induced pluripotent stem cells (iPSCs) demonstrate a positive impact on the healing of irradiated skin wounds by facilitating re-epithelialization and enhancing angiogenesis when compared to the PBS group [76]. Although mesenchymal stem cells are known to effectively promote tissue repair following injury, there is a growing body of evidence suggesting that their therapeutic effects are primarily mediated through paracrine mechanisms. Consequently, exosomes derived from stem cells have recently emerged as a promising cell-free strategy for enhancing skin regeneration, thereby addressing the challenges associated with the immunogenicity of stem cells [102]. Studies have demonstrated that microRNAs (miRNAs) derived from mesenchymal stem cell (MSC)-derived exosomes play a significant role in mitigating both acute and chronic radiation damage. For instance, miRNA-210 had been shown to facilitate DNA repair by modulating hypoxia-inducible factor 1 (HIF-1), promote the proliferation and migration of epithelial cells, and participate in immune responses. These findings suggested that irradiated cells may leverage MSC-derived exosomes miRNAs to enhance their resistance to ionizing radiation [103]. And miRNA-21 inhibited apoptosis in vascular endothelial cells and mitigated ionizing radiation-induced vascular damage in vitro by targeting the PTEN gene which regulating the PI3K/AKT signaling pathway [104]. Engineered exosomes have garnered significant attention due to their distinctive biological properties and versatility [77]. These modified exosomes can be enhanced through the incorporation of drugs, growth factors, gene editing techniques, and various other modification methods to facilitate targeted therapeutic interventions [105,106].
Overall, both stem cells and exosomes play a pivotal role in tissue repair; however, their retention and stability in vivo present significant challenges [107]. In light of these obstacles, hydrogels have emerged as reliable carriers for the delivery of bioactive substances. As a highly hydrophilic gel characterized by a unique three-dimensional network structure, hydrogels not only exhibit biocompatibility but also possess the ability to rapidly absorb and retain substantial amounts of water without dissolving. Furthermore, they are anticipated to offer specialized functions such as promoting blood coagulation, enhancing tissue adhesion, exhibiting antibacterial activity, providing anti-inflammatory properties, and demonstrating antioxidant effects. These functionalities are essential for facilitating the collaboration between stem cells and exosomes in promoting skin tissue regeneration and repair.
Multifunctional biomaterials for radiation-induced skin injury and its therapeutic mechanism.
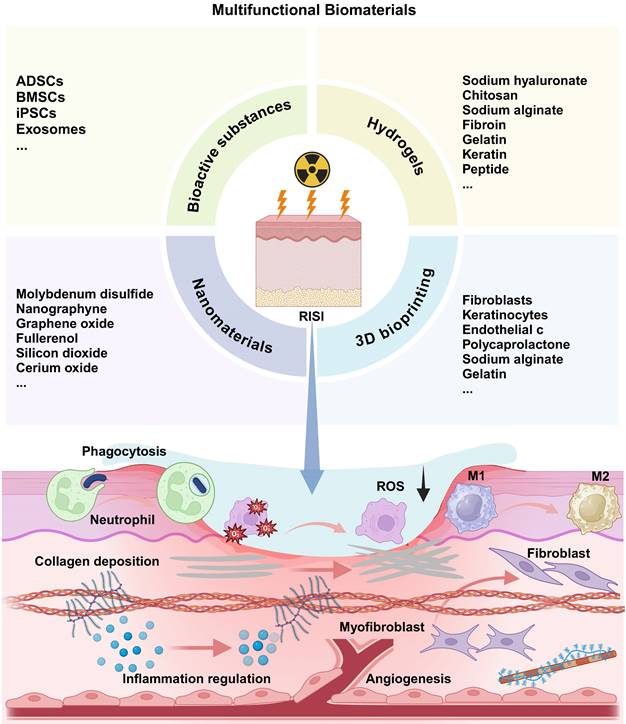
Biomaterial-based treatment strategies for radiation-induced skin injury
| Strategy | Composition | Functions | Limitations | Ref |
|---|---|---|---|---|
| Stem Cells and exosomes | ADSCs | Anti-inflammatory, inhibit skin fibrosis, prevent irregular collagen deposition | Immunogenicity, tumorigenic risk | [74] |
| BMSCs | Reduce inflammation and fibrosis | [75] | ||
| iPSCs | Promote re-epithelialization, enhance angiogenesis | [76] | ||
| Natural Exosomes | Promote the proliferation and migration of epithelial cells, inhibit the apoptosis of vascular endothelial cells | Undefined composition, difficult to extract and purify | [74] | |
| Engineering Exosomes | Targeted therapy | [77] | ||
| Hydrogels | Gallic acid | Antioxidant | Poor stability, limited skin permeability | [78] |
| EGCG | Antioxidant, promote angiogenesis | [79] | ||
| Tannic acid | Tissue adhesion, free radical scavenging, immune regulation | [80] | ||
| Curcumin | Antioxidant, anti-inflammatory | Poor solubility | [80] | |
| Deferoxamine | Reduce inflammation, promote angiogenesis | Potential cytotoxicity | [36] | |
| Retinoic acid | Anti-inflammatory, accelerate fibrocyte proliferation | Long-term use can damage the skin barrier | [36] | |
| Hyaluronic acid | Reduce inflammation, maintain cell activity, relieve pain | Poor mechanical property | [81] | |
| Gelatin Methacryloyl | Physical absorption of low energy X-rays | [82] | ||
| Sodium alginate | Antibacterial, promote angiogenesis | [83] | ||
| Chitosan | Antibacterial, enhance cell adhesion | [84] | ||
| Keratin | Promote keratinocyte and fibroblast migration, reduce inflammation | Extraction complexity | [85] | |
| Polypeptide | Antioxidant, promote epidermal tissue regeneration and angiogenesis | High cost | [86,87] | |
| Glucomannan | Regulate the polarization of macrophages towards M2 phenotype | Poor water solubility | [88] | |
| Sildenafil citrate | Promote re-epithelialization, vascularization and granulation tissue formation | Insufficient mechanical strength | [88] | |
| Ferulic acid | Antioxidant, inhibition of NLRP3 inflammasome activation to reduce inflammation | The optimal therapeutic concentration is unclear | [89] | |
| Acellular Dermal Matrix | Regulate macrophage polarization, enhance angiogenesis, prevent excessive fibrosis, promote lipogenesis | High cost, limited mechanical properties, potential immunogenicity | [90,91] | |
| Nanomaterials | Molybdenum disulfide | Scavenging free radicals | Poor dispersity, long-term safety to be verified | [92] |
| Graphdiyne | Good chemical stability, broad-spectrum free radical scavenging ability | [93] | ||
| Graphene oxide | Antibacterial, reduce the radiation sensitivity of HaCaT cells | [7] | ||
| Fullerenols | Antioxidant | [94] | ||
| Silicon dioxide | Antioxidant | [95] | ||
| Cerium oxide | Antioxidant | [95] | ||
| VEGF-chitosan nanoparticles | Protect vascular endothelial cells, promote angiogenesis | High cost, relatively complex process | [96] | |
| 3D printing | Fibroblasts | Reduce inflammation, assess DNA damage | Time-consuming, complex to operate | [97,98] |
| Keratinocytes | Anti-inflammatory, promote wound healing | [97] | ||
| Endothelial cells | Vascular remodeling | [99] | ||
| Polycaprolactone encapsulated amoxicillin | Antibacterial | Uncontrollable release rate and timing | [100] | |
| Sodium alginate-gelatin encapsulated rhEGF | Enhance cell proliferation and adhesion | [100] |
4.2 Intelligent responsive hydrogels
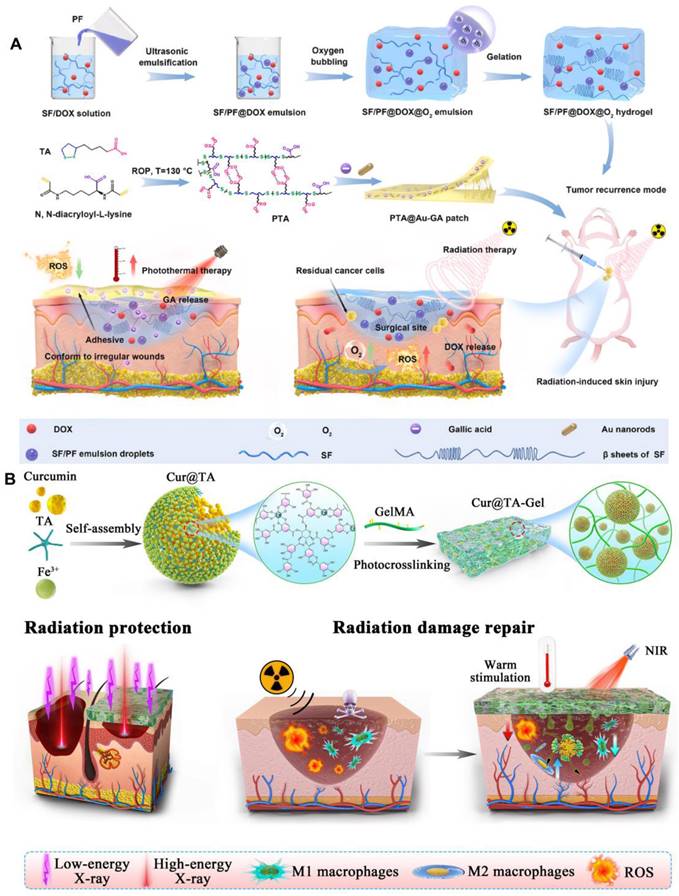
The environmental responsiveness of hydrogels represents an advanced drug delivery strategy that adapts to variations in external conditions, including temperature [108], photothermal effects [78], pH [109], hydrogen peroxide (H2O2) [110], enzymes [111], and glucose [112] etc. This capability enables the precise release of drugs at optimal times and targeted locations (Table 5) [113]. Zhang et al. developed a biodegradable fibroin/perfluorocarbon (SF/PF) hydrogel loaded with doxorubicin (DOX), as well as an antioxidant bioadhesive containing gold nanorods (AuNRs) and gallic acid (GA). These innovative formulations are designed to effectively inhibit tumor recurrence and mitigate skin radiation damage by providing mild photothermal therapy alongside antioxidant properties (Figure 4A) [78,114]. Wang et al. grafted the photosensitive group methacrylate anhydride (MA) onto chitosan (CHI) and gelatin (GEL), subsequently incorporating epigallocatechin gallate (EGCG) through physical means. The resulting composite photosensitive hydrogel exhibited excellent swelling properties, favorable rheological characteristics, and biocompatibility. Furthermore, it had been shown to promote angiogenesis and demonstrated a significant therapeutic effect on radiation skin injuries [79]. An immunomodulatory sticky hydrogel patch, encapsulated with curcumin and tannic acid, exhibited tissue adhesion and photothermal properties. This innovative patch demonstrated a robust ability to clear ROS and facilitated the polarization of macrophages towards the M2 phenotype. It was effectively utilized for the repair of radiation dermatitis and provided radiological protection (Figure 4B) [80]. By hybridizing the luminescent 3D terbium (III) metal-organic framework (Tb-MOF) with sodium alginate (SA) gel, utilizing terbium (III) as a crosslinking agent, researches successfully prepared Tb-MOF/Tb-AG hydrogels. These hydrogels exhibited multiple luminescence centers and an ample number of uranium binding sites, enabling rapid response to uranium detection. This innovative approach held promise for applications in human health [115]. It had been reported that a novel composite hydrogel, developed from physically cross-linked hyaluronic acid, can effectively enhance the healing of skin ulcers induced by radiation. This was achieved through the application of freeze-thaw technology, which incorporated small molecule drugs such as deferoxamine and retinoic acid [32].
(A) Therapeutic effects of ADSCs on chronic radiation dermatitis skin regeneration. (B) Illustration of the therapeutic mechanisms of apoptotic ADSCs on chronic radiation dermatitis. Adapted with permission from [74], copyright 2023 Springer Nature.
(A) Schematic illustration of the fabrication of SF/PF@DOX hydrogel and PTA@Au-GA patch and their application in treatment of breast cancer recurrence and radiation skin injury. Adapted with permission from [114], copyright 2024 Elsevier. (B) Schematic diagram illustrating of the preparation process of the nanohybrid hydrogel patch and the principle of its application in radiation protection and radiation dermatitis. Adapted with permission from [80], copyright 2023 Elsevier.
In addition, functional hydrogels demonstrate significant potential in the treatment of RISI. Keratin-based hydrogels can enhance wound healing by promoting cell proliferation and collagen synthesis following radiation exposure [85]. Multifunctional peptide hydrogels that mimic the extracellular matrix effectively repair RISI by eliminating harmful ROS and facilitating angiogenesis [86,87]. Glycopeptide hydrogels derived from glucomannan can mediate macrophage polarization towards the M2 phenotype, thereby improving the persistent inflammatory microenvironment associated with RISI. This approach significantly reduced epithelial hyperplasia and accelerated wound healing [88]. The treatment of radiation-induced skin wounds in SD rats using sildenafil citrate hydrogel not only facilitated skin re-epithelialization and collagen deposition but also mitigated inflammatory infiltration [116]. The ferulic acid hydrogel developed by Huang et al. has demonstrated a significant ability to reduce inflammation and oxidative stress through the inhibition of radiation-induced NLRP3 inflammasome activation, thereby promoting the reconstruction of skin tissue [89]. Porcine acellular dermal matrix hydrogels have been reported to effectively enhance the healing of RISI in rat models by modulating the inflammatory microenvironment, promoting angiogenesis, while simultaneously preventing excessive fibrosis and encouraging lipogenesis [90,91].
4.3 Nanomaterials
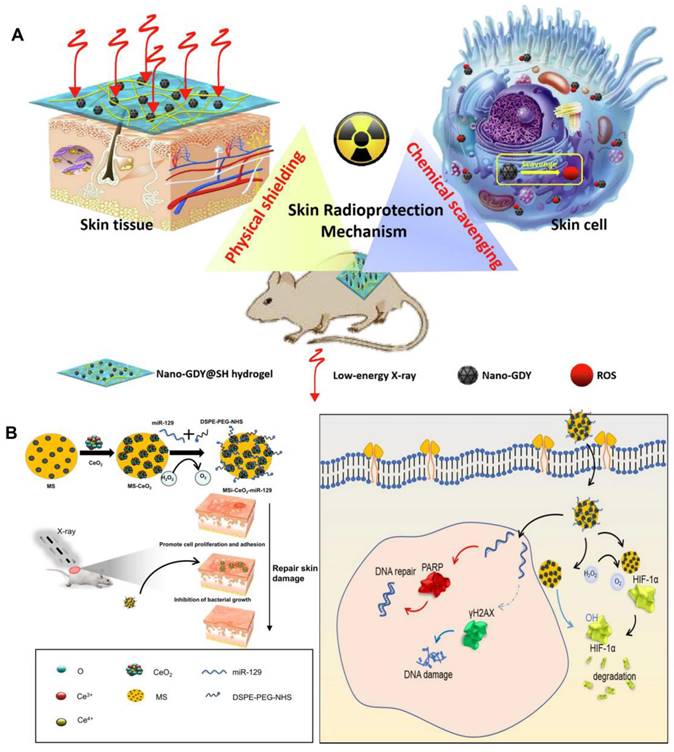
With the rapid advancement of nanotechnology in the field of biology, the integration of multifunctional nanomaterials with hydrogels presents a promising new strategy for radiological protection dressings. On one hand, nanomaterials have demonstrated numerous advantages in radiation protection, including efficient and broad-spectrum free radical scavenging capabilities [122]. On the other hand, due to the three-dimensional network structure inherent to hydrogels, they not only serve as carriers for nanoparticles but also possess significant potential for absorbing low-energy ionizing radiation owing to their high moisture retention properties [123]. For instance, molybdenum disulfide (MoS2) nanosheets were extensively utilized in the biomedical field due to their large specific surface area and excellent biocompatibility, which enabled them to effectively eliminate various free radicals. Incorporating these nanosheets into hydrogels not only addressed the issue of poor mechanical properties of hydrogels but also imparted remarkable antioxidant capabilities, thereby enhancing the radiation protection effect on the skin [92]. Similarly, due to the excellent chemical stability and broad-spectrum free radical scavenging ability of graphdiyne nanoparticles [93], sodium hyaluronate hydrogel loaded with it can effectively mitigate low-energy X-ray-induced skin edema and ulcers in mice, while also promoting wound healing (Figure 5A) [82]. Graphene oxide had garnered attention as a wound repair material, particularly when combined with interferon-α-induced protein 6 (IFI6) hydrogel. This combination not only exhibited significant antibacterial properties but also reduced the radiation sensitivity of HaCaT cells, offering a promising solution for the repair of radioactive skin wounds [7]. Fullerenols were recognized as "free radical sponges." The fullerenol-sodium hyaluronate hydrogel developed by Zhao et al. effectively mitigated ROS-induced damage and improved the viability of irradiated human keratinocytes, thereby alleviating radiation dermatitis [94]. Zhou et al. utilized mesoporous silica to effectively anchor and disperse cerium oxide (CeO2) nanoparticles containing miR129 serum, resulting in the formation of nanocapsules with a distinctive core-shell structure. Notably, these nanocapsules demonstrate anti-reactive oxygen species (anti-ROS), anti-HIF-1α, and anti-radiation properties. This study underscores the clinical potential of miR129-loaded nanoparticles in modulating PARP and γH2AX, thereby reducing radiosensitivity and enhancing resistance to radiation-induced damage (Figure 5B) [95]. Vascularization plays a critical role in tissue regeneration following RISI. Numerous studies have investigated the potential of nanoparticles loaded with vascular endothelial growth factor (VEGF) to promote angiogenesis during wound healing. To advance this strategy, Yu et al. developed a system utilizing chitosan (CS) nanoparticles encapsulated with VEGF-165. By modifying these nanoparticles with sodium tripolyphosphate (TPP), the system effectively packaged VEGF-165 and facilitated its sustained local release over an extended period. This approach significantly increased the concentration of VEGF-165, thereby enhancing microvascular remodeling and mitigating the progression of radioactive skin damage [96].
The response elements, mechanisms and treatment effects of intelligent response hydrogels
| Response element | Example | Assembly mechanism | Treatment effect | Ref |
|---|---|---|---|---|
| Temperature responsive | Pluronic F127 | Composed of hydrophilic poly(ethylene oxide) (PEO) blocks at both ends and a hydrophobic poly(propylene oxide) (PPO) block in the middle | Stimulate the expression of vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1) to promote wound healing | [108] |
| Photothermal-responsive | Polydopamine | A lot of phenolic hydroxyl groups | Antioxidant, anti-inflammatory | [117] |
| pH-responsive | Alginate grafted with dopamine | Amidation | Antibacterial, regulate ROS, promote cell proliferation | [118] |
| H2O2-responsive | L-arginine- coupled chitosan | Amidation | Promote angiogenesis and collagen deposition | [119] |
| Enzyme responsive | Multi-arm PEG | The function of matrix metalloproteinases | Reduce inflammation | [111,120] |
| Glucose-responsive | phenylboronic acid | Boronic ester bonds | Anti-inflammatory, promote the secretion of VEGF | [121] |
Nanomaterials in the therapeutic management of RISI: (A) The scheme of skin radioprotection by nano-GDY@SH hydrogel via both physically shielding of low-energy X-ray and chemically scavenging of broad-spectrum free radicals. Adapted with permission from [82], copyright 2022 Elsevier. (B) Schematic illustration showing the fabrication of MS-CeO2-miR129 and its application for RISI healing. Adapted with permission from [95], copyright 2022 Springer Nature.
4.4 3D bioprinting
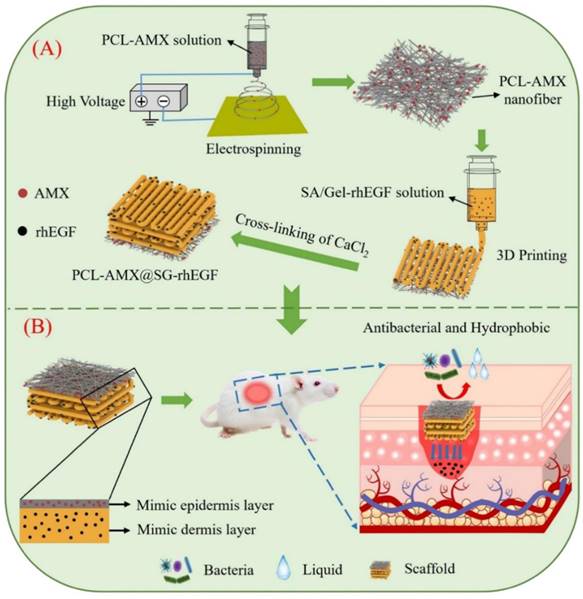
In recent years, bioprinting has demonstrated significant potential in the regeneration and repair of tissues and organs. In cases of irregular defects resulting from ionizing radiation, 3D printing technology can be tailored to meet the specific needs of individual patients. Furthermore, the customizable nature of printing inks combined with a variety of printing methods enhanced the effectiveness of treatments for RISI [124]. Using bio-inks derived from the patient's own fibroblasts and keratinocytes, researchers developed personalized skin repair materials through 3D printing technology. These innovative materials were applied to patients suffering from radiation skin injuries. The results demonstrated that the bioprinted skin repair material significantly accelerated wound healing and effectively reduced inflammatory responses [97]. The development of multi-layer composite materials designed to replicate the epidermis and dermis of the skin had yielded promising results. Song et al. incorporated amoxicillin into polycaprolactone nanofibers to create an antibacterial layer, while external human epidermal growth factor (rhEGF) was integrated with sodium alginate-gelatin for 3D printing as the inner layer of the scaffold. This innovative approach demonstrated remarkable drug release and antibacterial properties, significantly enhanced cell adhesion and proliferation, and effectively promoted wound healing (Figure 6) [100]. Some researchers have suggested that isolating and culturing normal skin fibroblasts, followed by reprogramming them into induced pluripotent stem cells (iPSCs), represents a promising strategy for differentiating iPSCs into keratinocytes and endothelial cells. These differentiated cells can serve as seed cells for 3D bioprinted skin grafts. However, it is important to emphasize that the utilization of iPSCs necessitates the establishment of robust and standardized operating procedures [99]. Chai et al. evaluated low-dose radiation-induced DNA damage and repair by 3D printing human foreskin fibroblast constructs [98]. Although 3D bioprinting has found extensive application in the domains of tissue engineering and regenerative medicine, it continues to encounter numerous challenges. These challenges encompass aspects such as printing technology, bio-inks, and the selection and compatibility of cells. Furthermore, various factors influence the progression of skin radiation injuries, making early outcomes difficult to predict. It is essential to accurately assess the symptoms associated with skin damage in order to facilitate personalized treatment approaches. This may involve in-situ printing of cells and materials directly onto patients' wounds; however, this process is inherently complex. Additionally, ensuring sufficient vascularization of the engineered structures is crucial for maintaining the long-term viability of any bioprinted tissue construct. Without adequate vascularization, essential nutrients and gas exchange cannot be effectively achieved.
Schematic diagram depicting two aspects: (A) The preparation process of the scaffold; (B) The bioactive therapeutic mechanism of the scaffold in the wound area. Adapted with permission from [100], copyright 2024 Elsevier.
5. Summary and prospects
In this paper, we provide a comprehensive review of the mechanisms, clinical manifestations, treatment status, and research advancements concerning multifunctional biomaterials in the context of radiation-induced skin injury diseases. Despite significant progress made in recent years regarding the application of biomaterials for treating radiation skin injuries, several challenges persist that must be addressed before these innovations can be effectively translated into clinical practice:
(1) Complexity of the damage mechanism: The pathogenesis and injury mechanisms associated with various diseases represent critical guidelines for material design and warrant further investigation. The degree of radiation-induced reaction is not entirely dependent on the irradiation dose, but also closely related to the irradiation area and volume, which makes it difficult to judge the results in the early stage. Skin damage resulting from ionizing radiation occurs through two primary pathways: direct attack on DNA leading to its damage, and indirect harm caused by ROS generated when ionizing radiation interacts with water, which can also inflict damage on DNA and other cellular components. However, this description serves only as a preliminary overview. The pathogenesis of RISI is highly complex, resembling a chain reaction that encompasses multiple biological processes including injury signaling pathways, oxidative stress responses, inflammation, immune activation, and microvascular impairment. Consequently, researchers must transcend traditional notions of "target cells" to identify potential damaging factors. It is essential to delve deeper into molecular structures, cellular frameworks, and related signaling pathways in order to develop targeted therapeutic strategies [125]. Therefore, a more comprehensive understanding of the mechanisms underlying radiation-induced tissue dysfunction is essential. This knowledge holds significant implications for the medical management of radiation injuries and informs the design and development of innovative, effective mechanism-based biomaterials.
(2) Safety of biomaterials: The safety of biomaterials in vivo is of paramount importance. Despite the vigorous advancement in biomaterial-mediated therapies and radiation protection research, substantiating their advantages for clinical applications remains in its early stages. Current efforts are primarily focused on the synthesis of multifunctional biomaterials, conducting in vitro experiments, and validating findings through animal models. Ongoing research must prioritize human specificity and safety assessments, as these are critical factors for enabling biomaterials to progress toward clinical applications. Consequently, when exploring novel biomaterials for the treatment of radiation skin injuries, implementing the following strategies may enhance their biosafety and efficacy: a) For the mechanisms of RISI, a comprehensive understanding of the interactions between biomaterials and cellular signaling pathways, as well as metabolic networks, is essential for designing biomaterials with targeted therapeutic activity [126]. b) By employing interdisciplinary approaches and high-throughput screening techniques, novel categories of effective natural or synthetic materials are identified to enhance the therapeutic index [73]. c) The specific nature of skin damage resulting from radiation must be thoroughly considered. For instance, the application of localized drug administration, along with percutaneous absorption and skin penetration, may enhance treatment efficacy. Additionally, it is important to take into account the antibacterial properties and adhesion characteristics of the materials used. d) The design of drug delivery systems for biomaterials must not only achieve high efficiency in drug loading but also ensure that the drug release rate is controllable. This is essential to prevent initial burst release or prolonged inefficient release. Furthermore, it is crucial for these systems to maintain stability during storage and transportation to avoid degradation or inactivation.
(3) Multifunctional design of biomaterials: While biomaterials have demonstrated significant advantages in the management of radioactive skin diseases, the clinical evidence supporting their superiority remains in its early stages. It is understood that the pathogenesis and clinical manifestations of RISI vary with increasing radiation dose and duration of irradiation, underscoring the importance of developing biological materials with targeted therapeutic applications. In the early acute phase of radiation damage, utilizing nanomaterials that possess anti-cellular senescence and antioxidant properties may represent a more effective approach [127]. 3D-printed biomaterials enriched with highly active ingredients not only facilitate personalized customization but also aid in regulating inflammatory responses and promoting tissue repair during the intermediate stages of injury [128]. The moist environment provided by hydrogels enhances subsequent wound healing and can help mitigate scar formation [129]. However, it is important to recognize that the functionality of individual biomaterials is inherently limited; thus, optimizing composite designs for these materials is essential. Factors such as DNA damage repair, cellular aging delay, varying effects across different stages of radiation-induced skin injury, simultaneous drug loading and controlled release functionalities, as well as microenvironment regulation within irradiated skin wound tissues must all be considered in the design process for biomaterials [130]. Consequently, extensive research and clinical trials are essential to investigate various biomaterials and ascertain their critical role in RISI therapy.
(4) Efficacy of clinical treatment: Although biomaterials have been extensively evaluated for safety in animal models, clinical studies remain preliminary. Currently, several urgent questions persist regarding the toxicity and systemic immune responses induced by these biomaterials, their mechanisms of action in radiation-induced diseases, and the efficacy of protective measures-issues that require further investigation by researchers. Given the unique characteristics of various organs and tissues, the severity of radiation-induced diseases exhibits significant heterogeneity. Furthermore, most studies on biomaterials have predominantly focused on tissues and organs such as the skin, gastrointestinal tract, and lungs [131-133]. It is crucial to broaden research efforts to encompass other clinically relevant sites affected by radiation damage, including the eyes, liver, bladder, and hematopoietic system. It is crucial to emphasize the necessity of conducting a comprehensive evaluation of biological materials, and this process must be standardized. First, by leveraging bionic principles, we can design materials with enhanced biocompatibility, thereby improving therapeutic outcomes. Second, the integration of artificial intelligence with bioprinting techniques holds promise for producing biomaterial structures with greater specificity, thus paving the way for personalized therapeutic interventions. Finally, employing organoid models may serve as an effective preclinical tool for assessing the performance of biomaterials, thereby facilitating advancements in the management of radiation-induced diseases.
In summary, the rapid advancements in materials science and regenerative medicine have led to the discovery of new biomaterials that exhibit remarkable capabilities in antioxidant activity, cell proliferation, immune regulation, and angiogenesis. These biomaterials hold significant potential for the treatment and protection of diseases associated with radiation damage. Consequently, the future clinical application and commercialization of smart biomaterials remain highly anticipated. However, achieving success necessitates further verification and evaluation of these systems. It is imperative to strive towards providing more effective and safer solutions for managing radiation-induced diseases and enhancing radiation protection at the earliest opportunity.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (82030056) and Postdoctoral Innovation Talents Support Program of China (No. BX20230489).
Author contributions
Yang Xu: Writing-original draft, Supervision, Funding acquisition. Quanying Liu: Conceptualization, Writing-original draft. Wenfeng Li: Writing-original draft. Zhihe Hu: Writing-original draft. Chunmeng Shi: Writing-review & editing, Supervision, Funding acquisition.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zheng K, Zhu X, Guo S, Zhang X. Gamma-ray-responsive drug delivery systems for radiation protection. Chem Eng J. 2023;463:142522
2. Yan T, Yang P, Bai H, Song B, Liu Y, Wang J. et al. Single-cell RNA-Seq analysis of molecular changes during radiation-induced skin injury: the involvement of Nur77. Theranostics. 2024;14:5809-25
3. Peña OA, Paul Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25(8):599-616
4. Aleksandra P, Anastazja SS, Andrzej T, Tomasz R, Krzysztof S, Grażyna KW. Dermoscopy of acute radiation-induced dermatitis in patients with head and neck cancers treated with radiotherapy. Sci Rep. 2023;13(1):15711
5. Singh M, Alavi A, Wong R, Akita S. Radiodermatitis: A review of our current understanding. Am J Clin Dermatol. 2016;17(3):277-92
6. Wang YR, Yang L, Liu B, Liao S, Fu X, Zhou Y. et al. Radiation skin injury care in radiotherapy for oncology: mechanisms, drug therapy and novel biomaterial application strategies. Adv Ther. 2023;6(11):2300024
7. Hao J, Sun M, Li D, Zhang T, Li JJ, Zhou DJ. An IFI6-based hydrogel promotes the healing of radiation-induced skin injury through regulation of the HSF1 activity. J Nanobiotechnology. 2022;20(1):288
8. Guo BL, Dong RN, Liang YP, Li M. Haemostatic materials for wound healing applications. Nat Rev Chem. 2021;5(11):773-91
9. Ameixa J, Bald I. Unraveling the complexity of DNA radiation damage using DNA nanotechnology. Acc Chem Res. 2024;57(11):1608-19
10. Singh VK, Seed TM. Pharmacological management of ionizing radiation injuries: current and prospective agents and targeted organ systems. Expert Opin Pharmacother. 2020;21(3):317-37
11. Liu L, Liang Z, Ma S, Li L, Liu X. Radioprotective countermeasures for radiation injury (Review). Mol Med Rep. 2023;27:1-14
12. Liang YP, He JH, Guo BL. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15(8):12687-722
13. Xu Y, Ma L, Wang Y, Shi C. Engineering strategies of biomaterial-assisted exosomes for skin wound repair: Latest advances and challenges. Chin Chem Lett. 2025;36:109766
14. Elena O, Rosario SP, Juan IV, Eduardo G, Blanca P, José ME. et al. Nuclear and radiological emergencies: biological effects, countermeasures and biodosimetry. Antioxidants. 2022;11(6):1098
15. Yu ZX, Xu CY, Song B, Zhang SH, Chen C, Li CL. et al. Tissue fibrosis induced by radiotherapy: current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J Transl Med. 2023;21(1):708
16. Zhang CC, Liu JB, Wu J, Ranjan KK, Cui XP, Wang XD. et al. Key molecular DNA damage responses of human cells to radiation. Front Cell Dev Biol. 2024;12:1422520
17. Monfared YK, Heidari P, Klempner SJ, Mahmood U, Parikh AR, Hong TS. et al. DNA damage by radiopharmaceuticals and mechanisms of cellular repair. Pharmaceutics. 2023;15(12):2761
18. Yan Y, Fu J, Kowalchuk RO, Wright CM, Zhang R, Li X. et al. Exploration of radiation-induced lung injury, from mechanism to treatment: A narrative review. Transl Lung Cancer Res. 2022;11(2):307-22
19. Ibragimova M, Kussainova A, Aripova A, Bersimbaev R, Bulgakova O. The molecular mechanisms in senescent cells induced by natural aging and ionizing radiation. Cells. 2024;13:550
20. Bogdanowicz P, Roullet N, Bensadoun P, Bessou-Touya S, Lemaitre J-M, Duplan H. Reduction of senescence-associated secretory phenotype and exosome-shuttled miRNAs by Haritaki fruit extract in senescent dermal fibroblasts. Int J Cosmet Sci. 2023;45:488-99
21. Munk R, Panda AC, Grammatikakis I, Gorospe M, Abdelmohsen K. Chapter Four- Senescence-Associated MicroRNAs. Int Rev Cell Mol Biol. 2017;334:177-205
22. Hanna D, Barbara P-K, Lucia T-Z, Carina S, Klaus F, Martina W.-F. Chang, et al. High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging Cell. 2013;12:446-458
23. Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A. et al. miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol. 2011;193:409-24
24. Chen WC, Wang Y, Zheng J, Chen Y, Zhang C, Yang W. et al. Characterization of cellular senescence in radiation ulcers and therapeutic effects of mesenchymal stem cell-derived conditioned medium. Burns & Trauma. 2023;11:tkad001
25. Chen Z, Cao K, Xia Y, Li Y, Hou Y, Wang L. et al. Cellular senescence in ionizing radiation (Review). Oncology Reports. 2019;42:883-94
26. Chen L, Liao FY, Wu J, Wang ZW, Jiang ZY, Zhang C. et al. Acceleration of ageing via disturbing mTOR-regulated proteostasis by a new ageing-associated gene PC4. Aging Cell. 2021;20:e13370
27. Shimura T, Sasatani M, Kawai H, Kamiya KJ, Kobayashi JY, Komatsu KS. et al. ATM-mediated mitochondrial damage response triggered by nuclear DNA damage in normal human lung fibroblasts. Cell Cycle. 2017;16(24):2345-54
28. Marchi S, Guilbaud E, Tait S-WG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23(3):159-73
29. Liu CC, Wei JL, Wang XZ, Zhao Q, Lv JC, Tan ZN. et al. Radiation-induced skin reactions: oxidative damage mechanism and antioxidant protection. Front Cell Dev Biol. 2024;12:1480571
30. Wei J, Wang B, Wang H, Meng L, Zhao Q, Li X. et al. Radiation-induced normal tissue damage: Oxidative stress and epigenetic mechanisms. Oxid Med Cell Longev. 2019;2019:3010342
31. Farrugia C-JE, Burke ES, Haley ME, Bedi KT, Gandhi MA. The use of aloe vera in cancer radiation: An updated comprehensive review. Complement Ther Clin Pract. 2019;35:126-30
32. Eming SA, Murray PJ, Pearce EJ. Metabolic orchestration of the wound healing response. Cell Metab. 2021;33:1726-43
33. Wei JL, Meng LB, Hou X, Qu C, Wang B, Xin Y. et al. Radiation-induced skin reactions: mechanism and treatment. Cancer Manag Res. 2018;11:167-77
34. Rübe CE, Freyter BM, Tewary G, Roemer K, Hecht M, Rübe C. Radiation dermatitis: radiation-induced effects on the structural and immunological barrier function of the epidermis. Int J Mol Sci. 2024;25:3320
35. Vats K, Kruglov O, Mizes A, Samovich SN, Amoscato AA, Tyurin VA. et al. Keratinocyte death by ferroptosis initiates skin inflammation after UVB exposure. Redox Biol. 2021;47:102143
36. Lei G, Mao C, Yan Y, Zhuang L, Gan B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell. 2021;12:836-57
37. Kameni LE, Januszyk M, Berry CE, Downer J-MA, Parker JB, Morgan AG. A review of radiation-induced vascular injury and clinical impact. Ann Plast Surg. 2024;92:181-85
38. Yang XJ, Ren HR, Guo XM, Hu CS, Fu J. Radiation-induced skin injury: pathogenesis, treatment, and management. Aging. 2020;12:23379-93
39. Liao FY, Chen L, Luo P, Jiang ZY, Chen ZL, Wang ZW. et al. PC4 serves as a negative regulator of skin wound healing in mice. Burns & Trauma. 2020;8:tkaa010
40. Chen X, Wu Y, Jia S, Zhao M. Fibroblast: A Novel Target for Autoimmune and Inflammatory Skin Diseases Therapeutics. Clinic Rev Allerg Immunol. 2024;66:274-93
41. Griffin MF, Huber J, Evan FJ, Quarto N, Longaker MT. The role of Wnt signaling in skin fibrosis. Med Res Rev. 2022;42:615-28
42. Horton JA, Li F, Chung EJ, Hudak K, White A, Krausz K. Quercetin inhibits radiation-induced skin fibrosis. Radiat Res. 2013;180:205-15
43. Li DJ, Berry CE, Wan DC, Longaker MT. Clinical, mechanistic, and therapeutic landscape of cutaneous fibrosis. Sci Transl Med. 2024;16:eadn7871
44. Hwang JH, Heo W, Park JI, Kim KM, Oh HT, Yoo GD. Endothelial TAZ inhibits capillarization of liver sinusoidal endothelium and damage-induced liver fibrosis via nitric oxide production. Theranostics. 2023;13(12):4182-96
45. Boraldi F, Lofaro FD, Bonacorsi S, Mazzilli A, Garcia-Fernandez M, Quaglino D. The role of fibroblasts in skin homeostasis and repair. Biomedicines. 2024;12:1586
46. Guo J, Zhang X, Mao R, Li H, Hao Y, Zhang J. et al. Multifunctional glycopeptide-based hydrogel via dual-modulation for the prevention and repair of radiation-induced skin injury. ACS Biomater Sci Eng. 2024;10:5168-80
47. Soriano JL, Calpena AC, Souto EB, Clares B. Therapy for prevention and treatment of skin ionizing radiation damage: A review. Int J Radiat Biol. 2019;95:537-53
48. Huang B, An L, Su W, Yan T, Zhang H, Yu D-J. Exploring the alterations and function of skin microbiome mediated by ionizing radiation injury. Front Cell Infect Microbiol. 2022;12:1029592
49. Hymes SR, Strom EA, Fife C. Radiation dermatitis: Clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46
50. Wilson BN, Shah R, Menzer C, Aleisa A, Sun MD, Kwong BY. et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-6
51. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-6
52. Roy I, Fortin A, Larochelle M. The impact of skin washing with water and soap during breast irradiation: a randomized study. Radiother Oncol. 2001;58:333-9
53. Ma Z, Chen Y, Tang K, Yang H, Tian M, Xi X. et al. Highly efficient prevention of radiation dermatitis using a PEGylated superoxide dismutase dissolving microneedle patch. Eur J Pharm Biopharm. 2024;201:114347
54. Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: A review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-67
55. Ghasemi A, Ghashghai Z, Akbari J, Yazdani CJ, Salehifar E, Hosseinimehr SJ. Topical atorvastatin 1% for prevention of skin toxicity in patients receiving radiation therapy for breast cancer: a randomized, double-blind, placebo-controlled trial. Eur J Clin Pharmacol. 2019;75:171-78
56. Rusanova I, Martínez-Ruiz L, Florido J, Rodríguez-Santana C, Guerra-Librero A, Acuña-Castroviejo D. et al. Protective Effects of Melatonin on the Skin: Future Perspectives. Int J Mol Sci. 2019;20:4948
57. Maurice K, Sanjay J, Ashley A, Toms VT, Mary RN, William CW. et al. Use of amifostine for cytoprotection during radiation therapy: A review. Oncology. 2020;98:61-80
58. Abtahi NB, Saffaei A, Sabzghabaee AM, Amiri R, Hosseini NS, Niknami E. Topical sucralfate for treatment of mucocutaneous conditions: A systematic review on clinical evidences. Dermatol Ther. 2022;35:e15334
59. Wang ZW, Chen ZL, Jiang ZY, Luo P, Liu L, Huang Y. et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat Commun. 2019;10:2538
60. Li C, Zhu L, Liu JX, Guo J, Xie J, Shi CM. et al. Cordycepin delays postovulatory aging of oocytes through inhibition of maternal mRNAs degradation via DCP1A polyadenylation suppression. Cell Mol Life Sci. 2023;80:372
61. Wang H, Wang Z, Huang Y, Zhou Y, Sheng X, Jiang Q. et al. Senolytics (DQ) mitigates radiation ulcers by removing senescent cells. Front oncol. 2020;9:1576
62. Fan RY, Zhang CW, Li F, Li B, McCarthy A, Zhang Y. Hierarchically assembled nanofiber scaffolds with dual growth factor gradients promote skin wound healing through rapid cell recruitment. Adv Sci. 2024;11:e2309993
63. Ryu SH, Kim YH, Lee S, Hong JP. The preventive effect of recombinant human growth factor (rhEGF) on the recurrence of radiodermatitis. J Radiat Res. 2010;51:511-7
64. Lee S, Moon SY, Kim YH, Hong JP. The use of recombinant human epidermal growth factor to promote healing for chronic radiation ulcer. Int Wound J. 2007;4:216-20
65. Song YH, Zhu YT, Ding J, Zhou FY, Xue JX, Jung JH. Distribution of fibroblast growth factors and their roles in skin fibroblast cell migration. Mol Med Rep. 2016;14:3336-42
66. Hu X, Sun H, Han C, Wang X, Yu W. Topically applied rhGM-CSF for the wound healing: A systematic review. Burns. 2011;37:729-41
67. Chen Y, Ma L, Cheng Z, Hu Z, Xu Y, Wu J. et al. Senescent fibroblast facilitates re-epithelization and collagen deposition in radiation-induced skin injury through IL-33-mediated macrophage polarization. J Transl Med. 2024;22:176
68. Rossi AM, Blank NR, Nehal K, Dusza S, Lee EH. Effect of laser therapy on quality of life in patients with radiation-induced breast telangiectasias. Lasers Surg Med. 2018;50:284-90
69. Borab Z, Mirmanesh MD, Gantz M, Cusano A, Pu LLQ. Systematic review of hyperbaric oxygen therapy for the treatment of radiation-induced skin necrosis. J Plast Reconstr Aesthet Surg. 2017;70:529-38
70. Ervin MD, Goans R, Diffenderfer-Stewart K, Aloisi B, Iddins CJ. Cutaneous Radiation Injuries: REAC/TS Clinical Experience. Disaster Med Public Health Prep. 2024;18:e33
71. Park S-Y, Park JM, Kim J, Choi CH, Chun M, Chang JH. et al. Quantitative radiomics approach to assess acute radiation dermatitis in breast cancer patients. PLOS One. 2023;18:e0293071
72. Scatena C, Murtas D, Tomei S. Cutaneous melanoma classification: The importance of high-throughput genomic technologies. Front Oncol. 2021;11:635488
73. Man JP, Shen YH, Song YJ, Yang K, Pei P, Hu L. Biomaterials-mediated radiation-induced diseases treatment and radiation protection. J Control Release. 2024;370:318-38
74. Zhu Y, Liu X, Chen X, Liao Y. Adipose-derived stem cells apoptosis rejuvenate radiation-impaired skin in mice via remodeling and rearranging dermal collagens matrix. Stem Cell Res Ther. 2024;15:1-15
75. Zheng K, Wu WZ, Yang SL, Huang LH, Chen J, Gong CG. Bone marrow mesenchymal stem cell implantation for the treatment of radioactivity-induced acute skin damage in rats. Mol Med Rep. 2015;12:7065-71
76. Flamant S, Busson E, Ribault A, L'homme BB, Massouridès E, Pinset C. et al. Human induced pluripotent stem cells as a source of mesenchymal stromal cells for the treatment of cutaneous radiation injuries. Cytotherapy. 2020;22:S44
77. Li Z, Liu J, Song J, Yin Z, Zhou F, Shen H. et al. Multifunctional hydrogel-based engineered extracellular vesicles delivery for complicated wound healing. Theranostics. 2024;14:4198-217
78. Jin L, Guo X, Gao D, Liu Y, Ni J, Zhang Z. et al. An NIR photothermal-responsive hybrid hydrogel for enhanced wound healing. Bioact Mater. 2022;16:162-72
79. Wang J, Gao L, Song J, Li S. Study of EGCG composite hydrogel for the treatment of radiation-induced skin injuries. J Appl Biomater Funct Mater. 2023;21:22808000231218996
80. Huang R, Sun W, Li W, Hu R, Meng R, Peng Z. et al. Immunomodulatory hydrogel patches loaded with curcumin and tannic acid assembled nanoparticles for radiation dermatitis repair and radioprotection. Chem Eng J. 2024;500:156869
81. Kirova YM, Fromantin I, De Rycke Y, Fourquet A, Morvan E, Padiglione S. et al. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy? Results of phase III randomised trial. Radiother Oncol. 2011;100:205-9
82. Xie J, Zhao M, Wang C, Zhu S, Niu W, Yong Y. et al. External use of Nano-graphdiyne hydrogel for skin radioprotection via both physically shielding of Low-energy X-ray and chemically scavenging of Broad-spectrum free radicals. Chem Eng J. 2022;430:132866
83. Andreea B, Bogdan N, Marius Z, Gabriela MI, Ciprian B, Vioara M. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J Pers Med. 2021;11:890
84. Park SJ, Cho W, Kim MS, Gu BK, Kang CM, Khang G. et al. Substance-P and transforming growth factor-β in chitosan microparticle-pluronic hydrogel accelerates regenerative wound repair of skin injury by local ionizing radiation. J Tissue Eng Regen Med. 2018;12:890-6
85. Chen XL, Zhai DL, Wang BC, Hao SL, Song J, Peng ZP. Hair keratin promotes wound healing in rats with combined radiation-wound injury. J Mater Sci Mater Med. 2020;31:28
86. Hao Y, Li H, Guo J. et al. Bio-Inspired Antioxidant Heparin-Mimetic Peptide Hydrogel for Radiation-Induced Skin Injury Repair. Adv Healthc Mater. 2023;12:e2203387
87. Guo J, Zhang X, Mao R, Li H, Hao Y, Zhang J. et al. ultifunctional glycopeptide-based hydrogel via dual-modulation for the prevention and repair of radiation-induced skin injury. ACS Biomater Sci Eng. 2024;10:5168-80
88. Feng ZJ, Zhang YM, Yang CF, Liu X, Huangfu YN, Zhang CG. et al. Bioinspired and Inflammation-Modulatory Glycopeptide Hydrogels for Radiation-Induced Chronic Skin Injury Repair. Adv Healthc Mater. 2023;12:e2201671
89. Huang CS, Huangfu CJ, Bai ZJ, Zhu L, Shen P, Wang NN. et al. Ningning Wang Multifunctional carbomer based ferulic acid hydrogel promotes wound healing in radiation-induced skin injury by inactivating NLRP3 inflammasome. J Nanobiotechnology. 2024;22:576
90. Liu X, Guo T, Huang Z, Chen S, Chen L, Li C. et al. Acellular dermal matrix hydrogels promote healing of radiation-induced skin injury in a rat model. J Mater Chem B. 2024;12:11218-29
91. DeCostanza L, Grogan GM, Bruce AC, Peachey CM, Clark EA, Atkins K. et al. Decellularized porcine dermal hydrogel enhances implant-based wound healing in the setting of irradiation. Acta Biomater. 2025;191:260-75
92. Luo X, Liu H, Wen J, Hu JX, Li YZ, Li GJ. Composite hydrogels with antioxidant and robust adhesive properties for the prevention of radiation-induced dermatitis. J Mater Chem B. 2024;12:6927-39
93. Xie J, Wang N, Dong X, Wang C, Du Z, Mei L. et al. Graphdiyne nanoparticles with high free radical scavenging activity for radiation protection. ACS Appl Mater Interfaces. 2019;11:2579-90
94. Zhao MR, Wang CY, Xie JN, Ji C, Gu ZJ. Eco-friendly and scalable synthesis of fullerenols with high free radical scavenging ability for skin radioprotection. Small. 2021;17:e2102035
95. Zhou DJ, Du M, Luo H, Ran FW, Zhao X, Dong Y. Multifunctional mesoporous silica-cerium oxide nanozymes facilitate miR129 delivery for high-quality healing of radiation-induced skin injury. J Nanobiotechnology. 2022;20:409
96. Yu DJ, Li S, Wang S, Li XJ, Zhu MS, Huang S. Development and characterization of VEGF165-chitosan nanoparticles for the treatment of radiation-induced skin injury in rats. Mar Drugs. 2016;14:182
97. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773-85
98. Chai WX, Kong YF, Escalona MB, Hu CS, Balajee AS, Huang Y. Evaluation of Low-dose Radiation-induced DNA Damage and Repair in 3D Printed Human Cellular Constructs. Health Phys. 2023;125(3):175-85
99. Lv XF, Zhao N, Long S, Wang GJ, Ran XZ, Gao JN. 3D skin bioprinting as promising therapeutic strategy for radiation-associated skin injuries. Wound Repair Regen. 2024;32(3):217-28
100. Song Y, Hu Q, Liu S, Wang Y, Zhang H, Chen J. et al. Electrospinning/3D printing drug-loaded antibacterial polycaprolactone nanofiber/sodium alginate-gelatin hydrogel bilayer scaffold for skin wound repair. Int J Biol Macromol. 2024;275:129705
101. Yang P, Zhang SJ, Yan T, Li FS, Zhang SY. The therapeutic application of stem cells and their derived exosomes in the treatment of radiation-induced skin injury. Radiat Res. 2023;199:182-201
102. Tan F, Li XR, Wang Z, Li JJ, Shahzad K, Zheng JL. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9:17
103. Wang HK, Zhang NN, Wang X, Tian J, Yi J, Li Yao. et al. Emerging role of mesenchymal stem cell-derived exosome microRNA in radiation injury. Int J Radiat Biol. 2024;100(7):996-1008
104. Zhang Y, Chen Z, Feng L, Jiang P, Li X, Wang X. Ionizing radiation-inducible microRNA-21 induces angiogenesis by directly targeting PTEN. Asian Pac J Cancer Prev. 2019;20:1587-93
105. Shetty AK, Upadhya R. Extracellular Vesicles in Health and Disease. Aging Dis. 2021;12:1358-62
106. Nguyen DD, Vu DM, Vo N, Tran NH, Ho DT, Nguyen T. et al. Skin rejuvenation and photoaging protection using adipose-derived stem cell extracellular vesicles loaded with exogenous cargos. Skin Res Technol. 2024;30:e13599
107. Hu W, Wang W, Chen Z, Chen Y, Wang Z. Engineered exosomes and composite biomaterials for tissue regeneration. Theranostics. 2024;14:2099-126
108. Li SS, Yang C, Li JQ, Zhang C, Zhu LL, Song Y. et al. Progress in pluronic F127 derivatives for application in wound healing and repair. Int J Nanomedicine. 2023;18:4485-505
109. Juthi AZ, Li FF, Wang B, Alam MM, Talukder ME, Qiu BS. pH-responsive super-porous hybrid hydrogels for gastroretentive controlled-release drug delivery. Pharmaceutics. 2023;15:816
110. Li JJ, Ke WD, Wang L, Huang MM, Yin W, Zhang P. et al. Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2 production for synergistic oxidation. J Control Release. 2016;225:64-74
111. Sobczak M. Enzyme-responsive hydrogels as potential drug delivery systems-state of knowledge and future prospects. Int J Mol Sci. 2022;23:4421
112. Wang JQ, Wang ZJ, Yu JC, Kahkoska AR, Buse JB, Gu Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv Mater. 2020;32:e1902004
113. Fan MH, Pi JK, Zou CY, Jiang YL, Li QJ, Zhang XZ. et al. Hydrogel-exosome system in tissue engineering: A promising therapeutic strategy. Bioact Mater. 2024;38:1-30
114. Zhang Z, Cao Q, Xia Y, Cui C, Qi Y, Zhang Q. et al. Combination of biodegradable hydrogel and antioxidant bioadhesive for treatment of breast cancer recurrence and radiation skin injury. Bioact Mater. 2024;31:408-21
115. Cui AQ, Wu XY, Ye JB, Song G, Chen DY, Xu J. et al. “Two-in-one” dual-function luminescent MOF hydrogel for onsite ultra-sensitive detection and efficient enrichment of radioactive uranium in water. J Hazard Mater. 2023;448:130864
116. Kulshrestha S, Chawla R, Singh S, Yadav P, Sharma N, Goel R. et al. Protection of sildenafil citrate hydrogel against radiation-induced skin wounds. Burns. 2020;46:1157-69
117. Fang Z, Lv Y, Zhang H, He Y, Gao H, Chen C. et al. A multifunctional hydrogel loaded with two nanoagents improves the pathological microenvironment associated with radiation combined with skin wounds. Acta Biomater. 2023;159:111-27
118. Li P, Li Y, Fu R, Duan Z, Zhu C, Fan D. NIR- and pH-responsive injectable nanocomposite alginate-graft-dopamine hydrogel for melanoma suppression and wound repair. Carbohydr Polym. 2023;314:120899
119. Zhou X, Zhao B, Wang L, Yang L, Chen H, Chen W. et al. A glucose-responsive nitric oxide release hydrogel for infected diabetic wounds treatment. J Control Release. 2023;359:147-60
120. Noddeland HK, Lind M, Jensen LB, Petersson K, Skak-Nielsen T, Larsen FH. et al. Design and characterization of matrix metalloproteinase-responsive hydrogels for the treatment of inflammatory skin diseases. Acta Biomater. 2023;157:149-61
121. Zhou W, Duan Z, Zhao J, Fu R, Zhu C, Fan D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact Mater. 2022;17:1-17
122. Ren XY, Huo MF, Wang MM, Lin H, Zhang XX, Yin J. Highly catalytic niobium carbide (mxene) promotes hematopoietic recovery after radiation by free radical scavenging. ACS Nano. 2019;13:6438-54
123. Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J. 2015;65:252-67
124. Lv X, Zhao N, Long S, Wang G, Ran X, Gao J. et al. 3D skin bioprinting as promising therapeutic strategy for radiation-associated skin injuries. Wound Repair Regen. 2024;32:217-28
125. Wijerathne H, Langston JC, Yang Q, Sun S, Miyamoto C, Kilpatrick LE. et al. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother Oncol. 2021;158:21-32
126. Kamran MZ, Ranjan A, Kaur N, Sur S, Tandon V. Radioprotective agents: Strategies and translational advances. Med Res Rev. 2016;36(3):461-93
127. Shi YR, Zhang YJ, Zhang YX, Yao JL, Guo JP, Xu XL. et al. Advances in Nanotherapy for Targeting Senescent Cells. Int J Nanomedicine. 2024;19:8797-813
128. Daikuara LY, Chen XF, Yue ZL, Skropeta D, Wood FM, Fear MW. 3D bioprinting constructs to facilitate skin regeneration. Adv Funct Mater. 2022;32:2105080
129. Chen Q, Li SY, Li K, Zhao WF, Zhao CS. A skin stress shielding platform based on body temperature-induced shrinking of hydrogel for promoting scar. Adv Sci. 2024;11:e2306018
130. Wang Y, Sun SK, Liu Y, Zhang Z. Advanced hitchhiking nanomaterials for biomedical applications. Theranostics. 2023;13:4781-801
131. Cui HQ, Zhang XY, Zhang ZZ, Zhang MY, Zhang TL, Wu LL. et al. Killing three birds with one stone: Tumor-membrane-decorated Prussian blue nanovaccines for synergistic management of skin tumors, radiation dermatitis and wounds. Compos B Eng. 2023;264:110900
132. Dasgupta Q, Jiang A, Wen AM, Mannix RJ, Man YC, Hall S. A human lung alveolus-on-a-chip model of acute radiation-induced lung injury. Nat Commun. 2023;14:6506
133. Li H, Zhao SY, Jiang M, Zhu T, Liu JJ, Feng GX. Biomodified extracellular vesicles remodel the intestinal microenvironment to overcome radiation enteritis. ACS Nano. 2023;17:14079-98
Author contact
![]() Corresponding author: Email: shicmedu.cn.
Corresponding author: Email: shicmedu.cn.
 Global reach, higher impact
Global reach, higher impact