13.3
Impact Factor
Theranostics 2025; 15(7):2672-2679. doi:10.7150/thno.108963 This issue Cite
Research Paper
Cancer-control outcomes with [177Lu]Lu-PSMA Radioligand Therapy in elderly, frail or comorbid mCRPC patients
1. Department of Urology, University Hospital Frankfurt, Goethe University Frankfurt am Main, Frankfurt, Germany.
2. Department of Nuclear Medicine, University Hospital Frankfurt, Goethe University Frankfurt am Main, Frankfurt, Germany.
3. Urologische Klinik München Planegg, Planegg, Germany.
4. Department of Urology and Urosurgery, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
5. Martini-Klinik Prostate Cancer Center, University Hospital Hamburg-Eppendorf, Hamburg, Germany.
6. Department of Urology, University Hospital Hamburg-Eppendorf, Hamburg, Germany.
Received 2024-12-17; Accepted 2025-1-7; Published 2025-1-27
Abstract
Rationale: [177Lu]Lutetium prostate-specific membrane antigen radioligand therapy ([177Lu]Lu-PSMA) is EMA-approved for certain indications in metastatic castration resistant prostate cancer (mCRPC). However, cancer-control outcomes in specific and trial-underrepresented subgroups are scant.
Methods: We relied on the FRAMCAP database to elaborate progression-free (PFS) and overall (OS) survival in elderly (≥75 yrs), frail (ECOG status ≥1) mCRPC patients or those with cardiovascular disease (CVD) treated with [177Lu]Lu-PSMA.
Results: Of 312 [177Lu]Lu-PSMA mCRPC patients, 76% were ≤75 vs. 24% >75 years. Patients >75 years received [177Lu]Lu-PSMA more frequently within the first three mCRPC lines (85% vs. 62%) and harbored more frequently ECOG status ≥2 (13% vs. 4.3%, both p < 0.01). In PFS and OS analyses, no significant difference between patients aged ≤75 vs. >75 years was observed (hazard ratios [HR] 0.97 & 0.85, both p≥0.4) with median PFS of 12.7 vs. 11.7 and OS of 15.1 vs. 19.8 months. In ECOG-stratified analyses, no PFS difference was observed, with significantly better OS for ECOG 0 vs. ≥1 (HR 1.69, p < 0.01), but not after further multivariable adjustment. In CVD-stratified analyses, PFS failed to provide significant differences between CVD vs. no CVD (HR: 1.44, p = 0.051). However, in OS analyses, significant worse OS for CVD mCRPC [177Lu]Lu-PSMA patients was observed (HR: 1.93, p < 0.01). After multivariable adjustment, CVD was an independent predictor for worse PFS and OS (both p < 0.01).
Conclusions: Real-world evidence suggests equally effective cancer-control outcomes in elderly and frail mCRPC patients treated with [177Lu]Lu-PSMA. However, patients with CVD are of higher risk for shorter PFS and OS.
Keywords: mCRPC, PFS, OS, Survival, Lu-177
Introduction
Patients with metastatic castration-resistant prostate cancer (mCRPC) face a high risk of cancer-specific mortality and significant disease-related complications due to tumor burden [1]. In recent years, the landscape of mCRPC treatment significantly changed and several new systemic treatment options and combinations have been approved, by demonstrating improvements in progression-free survival (PFS) and overall survival (OS) [2-10]. Moreover, recently the European Medicines Agency (EMA) approved [177Lu]Lutetium-vipivotidtetraxetan prostate-specific membrane antigen radioligand therapy ([177Lu]Lu-PSMA) for mCRPC patients previously treated with androgen receptor pathway inhibitors (ARPI) and taxane-based chemotherapy, based on the results of the VISION trial [11]. [177Lu]Lu-PSMA therapy, which targets prostate cancer cells with beta radiation via a molecular approach distinct from ARPI or taxane-based chemotherapy, has emerged as a pivotal component in the sequential treatment strategies for mCRPC due to its cancer-control efficacy. These effects have been further elaborated in additional randomized phase III trials in earlier treatment lines and stages of mCRPC or also in combination with ARPI administration [12-15].
Currently, elderly mCRPC patients or those with severe comorbidities or advanced frailty index such as Eastern Cooperative Oncology Group (ECOG) status are often underrepresented within phase III trials. Nonetheless, several post-hoc analyses of phase III trials in metastatic prostate cancer (hormone-sensitive [mHSPC] or mCRPC) suggested different and mostly worse cancer-control outcomes such as PFS and OS in specific patient subgroups, such as elderly or frail patients [11,16,17]. However, no data on these specific mCRPC patient subgroups treated with [177Lu]Lu-PSMA are currently available.
We addressed this knowledge gap and relied on the Frankfurt Metastatic Cancer Database of the Prostate (FRAMCAP) to elaborate cancer-control outcomes such as PFS and OS in [177Lu]Lu-PSMA-treated mCRPC patients. We hypothesized important cancer-control differences may exist in mCRPC patients treated with [177Lu]Lu-PSMA above 75 years, ECOG performance status ≥1 or with severe comorbidities such as history or active cardiovascular disease (CVD).
Materials and Methods
Study population
After receiving approval from the local ethics committee (reference number: SUG-5-2024) and adhering to the principles of the Declaration of Helsinki, we performed a retrospective analysis of all mCRPC patients documented in the prospectively maintained FRAMCAP database. A total of 1,182 patients treated at the Department of Urology, University Hospital Frankfurt, Germany, were screened. For the analysis, mCRPC patients were included if they had received at least one cycle of [177Lu]Lu-PSMA. This selection criteria resulted in 312 eligible mCRPC patients.
[177Lu]Lu-PSMA radioligand therapy
Treatment of [177Lu]Lu-PSMA was administered at the nuclear medicine department every 6-8 weeks, as previously described [18-20]. [177Lu]Lu-PSMA could be administered in accordance with EMA approval or as an individual compassionate use after previous multidisciplinary team discussion (MTD). For [177Lu]Lu-PSMA therapy, PSMA-PET/CT scan confirming PSMA-avid metastatic disease was required before initiating treatment.
Statistical analysis
Descriptive statistics included the computation of frequencies and proportions for categorical variables used in the analysis. Median values and interquartile ranges (IQR) were reported for continuous variables. Statistical significance for differences in proportions was assessed using the Chi-square test, while the t-test and Kruskal-Wallis test were employed to evaluate differences in distributions.
For PFS and OS analyses, Kaplan-Meier curve estimates with a log-rank test were used. PFS was defined as the time from beginning of [177Lu]Lu-PSMA until beginning of next sequential treatment administration for mCRPC. OS was calculated from the start of [177Lu]Lu-PSMA radioligand therapy. We conducted three different sets of PFS and OS analyses: First, patients aged >75 years at metastatic disease vs. ≤75 years, second ECOG status 0 at metastatic disease vs. ≥1 and finally patients with CVD at metastatic disease vs. without CVD.
For all cancer-control outcome estimates, univariable, as well as multivariable Cox regression models were applied. Adjustment in multivariable Cox regression models were performed for Gleason Score (6-7 vs. 8-10), synchronous vs. metachronous mHSPC and high volume mHSPC (vs. low volume). Moreover, depending on the outcome variable of interest, adjustment for age at metastatic disease (<75 vs. ≥75 yrs), ECOG status at metastatic disease (0 vs. 1-2) and CVD (vs. no CVD) was made. For PFS analyses further adjustment for the number of treatment line (1st to 7th) and for OS analyses for the number of received CRPC treatment lines was made. All tests were two sided with a level of significance set at p < 0.05. R software environment for statistical computing and graphics (version 3.4.3) was used for all analyses.
Results
Among the 312 mCRPC patients included in the study (Table 1), the median age at metastatic diagnosis was 70 years (IQR: 62-74 years), with a median PSA level at mCRPC of 16 ng/ml (IQR: 5-60 ng/ml). Overall, 47% of patients were classified as having an ECOG performance status of ≥1, and 35% had active or previously treated cardiovascular disease. At initial diagnosis, 59% presented with de novo metastatic disease, and 43% received some form of local treatment to the prostate. The median number of systemic treatments received for mCRPC was three (IQR: 2-5), while the median number of [177Lu]Lu-PSMA therapy cycles was four (IQR: 2-6). The median PSA reduction observed during [177Lu]Lu-PSMA therapy was 17% (IQR: 0-62%). At the time of mHSPC diagnosis, 65% of patients had high-volume disease, and 59% were de novo metastatic. At mCRPC stage, the majority (82%) presented with bone-only metastases, while 8.7% and 9.4% had lymph node or visceral metastases (with or without bone involvement), respectively.
Baseline characteristics: Age group ≤75 vs. >75 years
Of 312 [177Lu]Lu-PSMA treated mCRPC patients, 76% (n = 238) were ≤75 years at time of metastatic diagnosis (Table 1), while 24% (n = 74) were aged >75 years. In comparison between both age groups, the median number of received systemic treatment lines (4 vs. 3, p < 0.001) was higher for patients ≤75 years. Patients aged >75 years received [177Lu]Lu-PSMA more frequently within the first three systemic lines of mCRPC, compared to patients aged ≤75 years (85% vs. 62%, p < 0.001). Moreover, patients with ECOG status ≥2 were significantly more prevalent in patients aged >75 years (13% vs. 4.3%, p < 0.01). Conversely, no difference was observed regarding median PSA response under [177Lu]Lu-PSMA radioligand therapy (-20% vs. -14%) and rate of PSA50 (31% vs. 33%) between patients aged ≤75 vs. >75 years (both p>0.9).
[177Lu]Lu-PSMA PFS and OS analyses stratified according to age
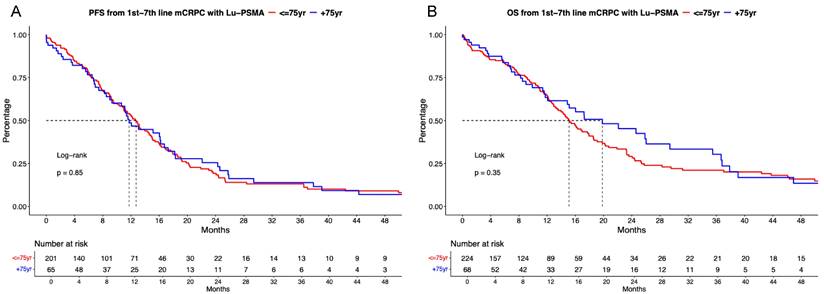
In PFS analyses between [177Lu]Lu-PSMA treated patients, no significant difference between patients aged ≤75 vs. >75 years was observed (Figure 1A, hazard ratio [HR]: 0.97, p = 0.85) with median PFS of 12.7 vs. 11.7 months. Corresponding 12-months PFS rates were 53.2 vs. 48.7%.
In OS analyses, also no differences between both age categories were observed (Figure 1B, HR: 0.85, p = 0.4) with median OS of 15.1 vs. 19.8 months. Corresponding 12-months OS rates were 64.7 vs. 63.4%. In multivariable Cox regression models, also no significant difference for PFS (Table 2A) and OS (Table 2B) were observed regarding age ≤75 vs. >75-year-old mCRPC patients receiving [177Lu]Lu-PSMA (both p≥0.4).
Characteristics of 312 metastatic castration resistant prostate cancer (mCRPC) patients receiving [177Lu] Lutetium prostate-specific membrane antigen ([177Lu]Lu-PSMA) radioligand therapy stratified according to age at metastatic disease.
| Characteristic | N | OverallN = 3121 | Age ≤75, N = 238 (76%)1 | Age >75, N = 74 (24%)1 | p-value2 |
|---|---|---|---|---|---|
| Age at metastatic disease, yrs | 312 | 70 (62, 74) | 67 (61, 71) | 79 (77, 82) | <0.001 |
| PSA at mCRPC, ng/ml | 150 | 16 (5, 60) | 16 (4, 46) | 21 (7, 91) | 0.2 |
| PSA second line mCRPC, ng/ml | 181 | 49 (13, 139) | 52 (12, 148) | 44 (16, 132) | >0.9 |
| Received systemic treatment lines for mCRPC | 312 | 3 (2, 5) | 4 (3, 5) | 3 (2, 4) | <0.001 |
| Cycles 177Lu-PSMA | 267 | 4 (2, 6) | 3 (2, 6) | 4 (2, 6) | 0.6 |
| PSA response under 177Lu-PSMA, % | 57 | 17 (0, 62) | 20 (0, 62) | 14 (0, 68) | >0.9 |
| PSA response 50% | 57 | 18 (32%) | 14 (31%) | 4 (33%) | >0.9 |
| PSA response 90% | 57 | 6 (11%) | 4 (8.9%) | 2 (17%) | 0.6 |
| Treatment 177Lu-PSMA | 308 | <0.001 | |||
| 1st line mCRPC | 28 (9.1%) | 15 (6.4%) | 13 (18%) | ||
| 2nd line mCRPC | 72 (23%) | 47 (20%) | 25 (34%) | ||
| 3rd line mCRPC | 108 (35%) | 84 (36%) | 24 (33%) | ||
| 4th line mCRPC | 38 (12%) | 35 (15%) | 3 (4.1%) | ||
| 5th line mCRPC | 40 (13%) | 34 (14%) | 6 (8.2%) | ||
| 6th line mCRPC | 16 (5.2%) | 15 (6.4%) | 1 (1.4%) | ||
| 7th line mCRPC | 6 (1.9%) | 5 (2.1%) | 1 (1.4%) | ||
| ECOG status | 186 | 0.002 | |||
| 0 | 100 (54%) | 85 (61%) | 15 (33%) | ||
| 1 | 74 (40%) | 49 (35%) | 25 (54%) | ||
| ≥2 | 12 (6.5%) | 6 (4.3%) | 6 (13%) | ||
| CVD | 189 | 66 (35%) | 47 (32%) | 19 (46%) | 0.083 |
| Gleason score 8-10 | 267 | 197 (74%) | 146 (73%) | 51 (77%) | 0.5 |
| Local therapy prostate | 312 | 133 (43%) | 104 (44%) | 29 (39%) | 0.5 |
| High volume mHSPC | 122 | 79 (65%) | 62 (64%) | 17 (68%) | 0.7 |
| De Novo mHSPC | 307 | 180 (59%) | 146 (62%) | 34 (47%) | 0.017 |
| Metastatic sites at mCRPC | 138 | 0.14 | |||
| M1a | 12 (8.7%) | 7 (6.9%) | 5 (14%) | ||
| M1b | 113 (82%) | 83 (81%) | 30 (83%) | ||
| M1c | 13 (9.4%) | 12 (12%) | 1 (2.8%) | ||
| Treatment first-line mCRPC | 312 | <0.001 | |||
| ADT mono | 26 (8.3%) | 25 (11%) | 1 (1.4%) | ||
| Chemotherapy | 56 (18%) | 52 (22%) | 4 (5.4%) | ||
| 177Lu-PSMA | 28 (9.0%) | 15 (6.3%) | 13 (18%) | ||
| ARPI | 176 (56%) | 125 (53%) | 51 (69%) | ||
| PARPi | 1 (0.3%) | 1 (0.4%) | 0 (0%) | ||
| Radium | 24 (7.7%) | 19 (8.0%) | 5 (6.8%) | ||
| None/Other/NA | 1 (0.3%) | 1 (0.4%) | 0 (0%) | ||
| 1 Median (Q1, Q3); n (%) | |||||
| 2 Kruskal-Wallis rank sum test; Fisher's exact test; Pearson's Chi-square test | |||||
Abbreviations: PSA: Prostate-specific antigen, ECOG: Eastern Cooperative Oncology group, CVD: Cardiovascular disease, mHSPC: metastatic hormone-sensitive prostate cancer, ADT: Androgen deprivation therapy, ARPI: Androgen receptor pathway inhibitor, nmHSPC: non-metastatic hormone-sensitive prostate cancer, m0CRPC: non-metastatic CRPC, PARPi: Poly adenosine diphosphate ribose polymerase inhibitor
Kaplan Meier curves depicting progression-free survival (PFS, A) and overall survival (B) in first to seventh-line metastatic castration-resistant prostate cancer (mCRPC) patients receiving [177Lu] Lutetium prostate-specific membrane antigen (Lu-PSMA) radioligand therapy and stratified according to age at metastatic disease.
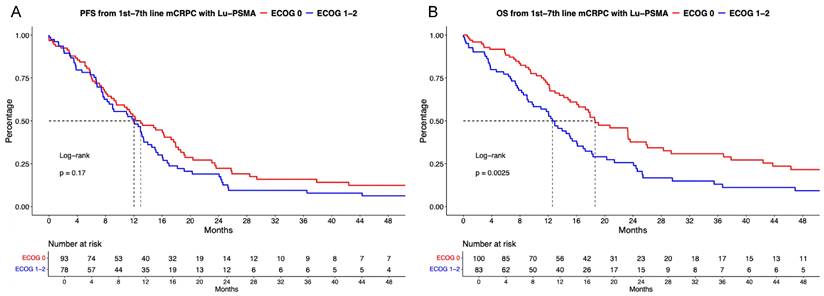
Kaplan Meier curves depicting progression-free survival (PFS, A) and overall survival (B) in first to seventh-line metastatic castration-resistant prostate cancer (mCRPC) patients receiving [177Lu] Lutetium prostate-specific membrane antigen (Lu-PSMA) radioligand therapy and stratified according to Easter Cooperative Oncology Group (ECOG) status.
[177Lu]Lu-PSMA PFS and OS analyses stratified according to ECOG status
Of [177Lu]Lu-PSMA patients with known ECOG status, 54% (n = 105) were categorized as ECOG status 0 vs. 46% (n = 95) ECOG status ≥1. In PFS analyses (Figure 2B), no significant difference between ECOG 0 vs. ≥1 was observed with a HR of 1.26 and median PFS of 13.0 vs. 12.0 months (p = 0.17). Corresponding 12-months PFS rats were 52.7% vs. 51.1% for ECOG 0 vs. ≥1. After multivariable adjustment in Cox regression models (Table 2A), no significant difference was observed (p = 0.4).
In OS analyses (Figure 2B), patients with ECOG 0 harbored significant better OS rates than patients with ECOG ≥1 undergoing [177Lu]Lu-PSMA radioligand therapy with a HR of 1.69 (p < 0.01) and median OS of 18.6 vs. 12.6 months. Corresponding 12-months OS rates were 71.2% vs. 55.5% for ECOG 0 vs. ≥1. However, after further multivariable adjustment in Cox regression models, no further differences between ECOG status groups were observed (p = 0.8, Table 2B).
[177Lu]Lu-PSMA PFS and OS analyses stratified according to CVD
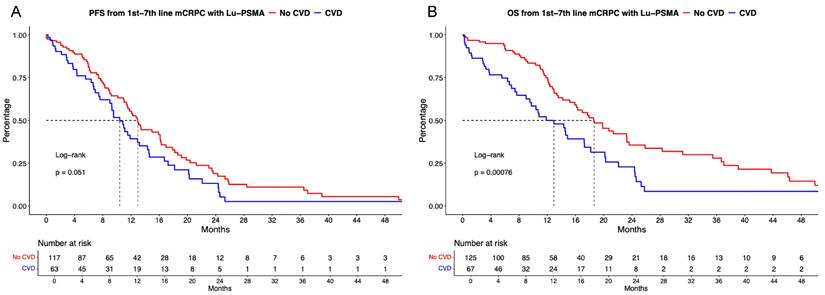
Of [177Lu]Lu-PSMA patients with available CVD status, 66% (n = 133) harbored no CVD vs. 34% (n = 70) with active or previous CVD. PFS analyses failed to provide significant differences between CVD vs. no CVD (Figure 3A), with median PFS of 13.0 vs. 10.4 months (HR: 1.44, p = 0.051). Corresponding 12-months PFS rates were 55.4% vs. 39.3% for no CVD vs. CVD groups. After multivariable adjustment, CVD was a significant predictor of shorter PFS (HR: 2.79, p < 0.01, Table 2A).
Kaplan Meier curves depicting progression-free survival (PFS, A) and overall survival (B) in first to seventh-line metastatic castration-resistant prostate cancer (mCRPC) patients receiving [177Lu] Lutetium prostate-specific membrane antigen (Lu-PSMA) radioligand therapy and stratified according active or history of cardiovascular disease (CVD).
Univariable und multivariable Cox regression models predicting progression-free survival (PFS; A) and overall survival (OS; B) in metastatic castration resistant prostate cancer (mCRPC) patients receiving [177Lu] Lutetium prostate-specific membrane antigen ([177Lu]Lu-PSMA) radioligand therapy. Abbreviation: HR: Hazard Ratio, CI: Confidence interval, ECOG: Eastern Cooperative Oncology Group, CVD: Cardiovascular disease
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| PFS | HR | CI | p value | HR | CI | p value |
| Age ≤75 yrs | Ref. | - | - | Ref. | - | - |
| Age >75 yrs | 0.97 | 0.70-1.34 | 0.9 | 0.62 | 0.21-1.88 | 0.4 |
| ECOG 0 | Ref. | - | - | Ref. | - | - |
| ECOG 1-2 | 1.26 | 0.91-1.77 | 0.17 | 0.68 | 0.29-1.64 | 0.4 |
| No CVD | Ref. | - | - | Ref. | - | - |
| CVD | 1.44 | 1.00-2.07 | 0.051 | 2.79 | 1.29-6.00 | <0.01 |
| OS | HR | CI | p value | HR | CI | p value |
| Age ≤75 yrs | Ref. | - | - | Ref. | - | - |
| Age >75 yrs | 0.85 | 0.60-1.20 | 0.4 | 0.98 | 0.25-3.91 | >0.9 |
| ECOG 0 | Ref. | - | - | Ref. | - | - |
| ECOG 1-2 | 1.69 | 1.20-2.39 | <0.01 | 0.89 | 0.34-2.36 | 0.8 |
| No CVD | Ref. | - | - | Ref. | - | - |
| CVD | 1.93 | 1.31-2.84 | <0.01 | 5.14 | 2.10-12.60 | <0.01 |
Adjustment in multivariable Cox regression models for:
Gleason Score (6-7 vs. 8-10), synchronous vs. metachronous mHSPC, high volume mHSPC (vs. low volume).
Depending on variable of interest, further adjustment for age (<75 vs. ≥75 yrs), ECOG status (0 vs. 1-2) and CVD (vs. no CVD) was made.
For PFS further adjustment for number of treatment line (1st to 7th) and for OS number of received CRPC treatment lines was made.
In OS analyses (Figure 3B), significant OS differences for [177Lu]Lu-PSMA patients without CVD vs. CVD was observed (HR: 1.93, p < 0.01) with median OS of 18.6 vs 12.9 months. Corresponding 12-months OS rates were 74.9% vs. 50.0% for no CVD vs. CVD groups. After further multivariable adjustment in Cox regression models, CVD was also an independent predictor for worse OS (HR: 5.14, p < 0.01, Table 2B).
Discussion
We initially hypothesized that important cancer-control differences may exist in mCRPC patients treated with [177Lu]Lu-PSMA and aged >75 years relative to mCRPC patients aged ≤75 years. Moreover, we also hypothesized that additional cancer-control outcome differences may exist in patients classified as ECOG ≥1 status or with history or active CVD. We tested these hypotheses within the FRAMCAP database and made several important observations.
First, we observed that in real-world setting, approximately every fourth patients (24%) receiving [177Lu]Lu-PSMA treated is aged >75 years at time of metastatic diagnosis. Moreover, we observed that patients aged above 75 years and receiving [177Lu]Lu-PSMA receive in median three systemic treatment lines for mCRPC, which were significantly less than in patients ≤75 years (four systemic treatment lines). Additionally, patients aged >75 years, received [177Lu]Lu-PSMA significantly more frequently within the first three treatment lines for mCRPC (85% vs. 62%), which might also contribute to the favorable OS outcomes. All of the above observations are of note, since they reflect a deep adaption of [177Lu]Lu-PSMA mCRPC treatment in clinical practice and treatment algorithms, as well as an early administration in elderly patients (even outside current EMA approval). Moreover, these findings are significant, since patients aged >75 years are frequently underrepresented within phase III randomized trials and the focus does not primarily lie on elderly subgroups. For example, in the VISION trial, the basis for approval of [177Lu]Lu-PSMA after previous taxan-based chemotherapy and ARPI treatment, enrolled patients with a median age of 70 years, with the youngest patient included aged 48 years [11]. Similarly, in the TheraP trial, patients were enrolled at a median age of 72 years, showing a different focus within these studies [15]. Compared to previous real-world studies, our cohort of elderly mCRPC patients indicate that [177Lu]Lu-PSMA may provide clinicians with a feasibly additional mCRPC treatment option, since median number of received treatment therapies for mCRPC was higher within our study than for example in a report by Fernando et al. in which mCRPC patients aged >75 years. In this report, 66% of patients received only one line of treatment [21]. Similar observations were also made within other publications, addressing elderly mCRPC patients [22,23].
Second, when cancer-control outcomes of [177Lu]Lu-PSMA mCRPC patients aged >75 years at time of metastases were compared, comparable median PFS and OS outcomes were observed and no difference were found after further additional adjustment in Cox regression models. This clearly shows the high effectivity of [177Lu]Lu-PSMA also in elderly patients. Moreover, PSA responses were similar between both age groups. These observations are of importance, since no previous published report focused on cancer-control outcomes of elderly [177Lu]Lu-PSMA patients. Subgroup analyses of currently available phase III trials such as the VISION or SPLASH trial provide only analyses of patients aged ≥65 years [11,14]. A previously presented abstract at ESMO 2024 comparing [177Lu]Lu-PSMA in French mCRPC patients pre-treated with at least one taxane-based and at least one APRI-based treatment in patients ≥75 years found also no difference between PFS and OS [24]. However, PFS was shorter within these cohort with 7 vs. 8 months for patients aged ≥75 vs. <75 years. These differences may be explained by the heavily pre-treatment of mCRPC patients, while patients within our study also received [177Lu]Lu-PSMA in more previous systemic treatment lines of mCRPC and median PFS usually decreased with every additional treatment line [25]. Moreover, PFS definitions may differed. However, our data on reported OS rates are similar to previously published real-world reports about [177Lu]Lu-PSMA treatment, irrespectively of stratification regarding age [19,26-28]. Moreover, in further sensitivity analyses for OS defined from beginning of mCRPC, also no difference in OS was observed for comparison in both age categories (data not shown).
Finally, we further explored our above findings within [177Lu]Lu-PSMA mCRPC patients stratified according to ECOG performance status and CVD. Here, we observed that patients with worse ECOG performance status are of higher risk of death under [177Lu]Lu-PSMA. However, these OS difference vanished after adjustment for differences in baseline patient and tumor characteristics in multivariable Cox regression models. In CVD analyses, CVD showed to be associated with shorter non-significant PFS (p = 0.051) and significant shorter OS. Moreover, CVD was independently associated with worse PFS and OS even in multivariable adjusted Cox regression models. These observations are of note since previously mCRPC studies reported worse cancer-control outcomes with worse ECOG performance status, but these findings have never been investigated within specific [177Lu]Lu-PSMA mCRPC patients [29]. Similarly, stratification according to CVD in [177Lu]Lu-PSMA has never been addressed. However, a previously published small-sized report (n = 11) of mCRPC patients undergoing [177Lu]Lu-PSMA showed no severe cardiotoxicity profile or adverse events [30]. Although our study could not differentiate between deaths due to cardiotoxic events and those related to cancer progression, this represents an important area for further investigation. Notably, prospective trials such as VISION and TheraP, as well as real-world data, have not identified significant cardiotoxicity associated with [177Lu]Lu-PSMA that would necessitate routine pretreatment cardiac assessments [11,15]. The observed worse PFS in patients with CVD might instead be explained by factors such as higher PSA levels in advanced lines of mCRPC and fewer median cycles of [177Lu]Lu-PSMA received in this cohort (data not shown). These findings underline the need for future studies to validate the observed associations in prospective settings and to better understand the interplay between preexisting CVD and outcomes in mCRPC patients undergoing [177Lu]Lu-PSMA treatment.
In addition to the above-mentioned limitation, our study should be interpreted in its light of the retrospective and single-center design. Moreover, some missing data, limitations in their further distinction (such as different CVD conditions), as well as other not reported variables may have influenced cancer-control outcomes. Further, no information regarding adverse events or blood values other than PSA were available, similar to possible dose reductions or adjustments in treatment schedules. Moreover, in comparison to other trails one has to keep in mind that used [177Lu]Lu-PSMA was seen as equivalent to commercially available one. Finally, some of the reported subgroup analyses may lack of sample size and therefore may limit the findings.
Taken together, the current real-world cohort of [177Lu]Lu-PSMA mCRPC patients suggest feasible cancer-control outcomes such as PFS and OS for patients aged >75 years. Therefore, [177Lu]Lu-PSMA should be considered as a safe and important cornerstone in the treatment of elderly mCRPC patients. Moreover, the study provides important observations of [177Lu]Lu-PSMA mCRPC with advanced ECOG performance status and CVD. Especially, patients with CVD should be treated with caution to prevent cardiotoxic events.
Acknowledgements
This study was part of the EPIC-REAP project (Enhancing Prostate cancer care In Germany Combining Real-world data And AI for Enhanced Analysis and Precision) supported by the Mildred-Scheel Nachwuchszentrum Frankfurt.
Data availability declaration
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Hospital Frankfurt, number: SUG-5-2024.
Competing Interests
Mike Wenzel receives speaker honoraria or is consultant for Accord, Johnsen&Johnsen, Pfizer, MSD, Astra Zeneca, Ipsen, Markus Graefen receives speaker honoraria or is consultant for Intuitive Surgical, Metronic, Ipsen, Astellas, Johnson & Johnson und Takeda, Tobias Maurer reports speaker fees from ABX, Astellas, Bayer, Sanofi-Aventis and Phillips, consultant fees from ABX, Advanced Accelerator Applications International S.A., Ascenian, Astellas, Axiom, Blue Earth Diagnostics, GEMoAb, Novartis, ROTOP Pharma and Telix, and research funding from ABX, Brainlab, Intuitive Surgical and Telix, Felix Chun receives speaker honoraria or is consultant for Astellas, AstraZeneca , Bayer, Johnson & Johnson, Lumenis, Molecular Health, Olympus, Pfizer, Philipp Mandel receives speaker honoraria or is consultant for AMGEN, Astellas, AstraZeneca, Bayer, IPSEN, Johnson & Johnson, MSD, Novartis, Orion, Pfizer.
References
1. Cornford P, Tilki D, van den Bergh RCN, Bries E, Eberli D, De Meerleer G. et al. EAU Guidelines on prostate cancer. Edn. presented at the EAU Annual Congress Paris. 2024 ISBN 978-94-92671-23-3
2. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, De Souza P. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-48 doi:10.1056/NEJMoa1209096
3. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512 doi:10.1056/NEJMoa040720
4. de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154 doi:10.1016/S0140-6736(10)61389-X
5. Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S. et al. Survival with olaparib in metastatic castration-resistant prostate xancer. N Engl J Med. 2020;383(24):2345-2357 doi:10.1056/NEJMoa2022485
6. Parker C, Nilsson S, Heinrich D, O'Sullivan JM, Fossa SD, Chodacki A. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223 doi:10.1056/NEJMoa1213755
7. Agarwal N, Azad AA, Carles J, Fay AP, Matsubara N, Heinrich D. et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402(10398):291-303 doi:10.1016/S0140-6736(23)01055-3
8. Chi KN, Sandhu S, Smith MR, Attard G, Saad M, Olmos D. et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol. 2023;34(9):772-782 doi:10.1016/j.annonc.2023.06.009
9. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg C, Miller K. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197 doi:10.1056/NEJMoa1207506
10. Wenzel M, Borkowetz A, Lieb V, Hoffmann MA, Borgmann H, Hoefner T. et al. Efficacy of cabazitaxel in fourth or later line of therapy in metastatic castration-resistant prostate cancer: multi-institutional real-world experience in germany. Urol Oncol. 2022;40(12):538.e7-538.e14 doi:10.1016/j.urolonc.2022.09.011
11. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K. et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091-1103 doi:10.1056/NEJMoa2107322
12. Morris MJ, Castellano D, Herrmann K, de Bono JS, Shore ND, Chi KN. et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): a phase 3, randomised, controlled trial. Lancet. 2024;404(10459):1227-1239 doi:10.1016/S0140-6736(24)01653-2
13. Emmett L, Subramaniam S, Crumbaker M, Nguyen A, Joshua AM, Weickhardt A. et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024;25(5):563-571 doi:10.1016/S1470-2045(24)00135-9
14. Sartor O, Jiang DM, Smoragiewicz M, Zibelman M, Flechon A, El-Haddad G. et al. Efficacy of 177Lu-PNT2002 in PSMA-positive mCRPC following progression on an androgen-receptor pathway inhibitor (ARPI) (SPLASH). Annals of Oncology (2024) (Suppl 2): 1-72. 10.1016/annonc/annonc1623.
15. Hofman MS, Emmett L, Sandhu S, Iravani A, Buteau JP, Joshua AM. et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 2024;25(1):99-107 doi:10.1016/S1470-2045(23)00529-6
16. Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R. et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24 doi:10.1056/NEJMoa1903307
17. Mourey L, Boyle HJ, Roubaud G, McDermott RS, Supiot S, Tombal BF. et al. Efficacy and safety of abiraterone acetate plus prednisone and androgen deprivation therapy +/- docetaxel in older patients (≥70 years), with de novo metastatic-castration sensitive prostate cancer, compared to younger patients (<70 years): The PEACE-1 trial. J Clin Oncol. 2023;41(Suppl 6):20-20 doi:10.1200/JCO.2023.41.6_suppl.20
18. Mader N, Nguyen Ngoc C, Kirkgöze B, Baumgarten J, Groener D, Klimek K. et al. Extended therapy with [177Lu]Lu-PSMA-617 in responding patients with high-volume metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2023;50(6):1811-1821 doi:10.1007/s00259-023-06119-1
19. Wenzel M, Koll F, Hoeh B, Humke C, Siech C, Mader N. et al. Real-world comparison of cabazitaxel versus 177Lu-PSMA radiopharmaceutical therapy in metastatic castration-Resistant prostate cancer. J Nucl Med. 2025;66:61-66 doi:10.2967/jnumed.124.268807
20. Wenzel M, Koll F, Hoeh B, Humke C, Reis H, Wild P. et al. Cancer-control outcomes of patients with metastatic castration-resistant prostate cancer with BRCA gene or tumor suppressor mutations undergoing 177-Lutetium prostate-specific membrane antigen radioligand therapy. JCO Precis Oncol. 2024Dec;8:e2400645 doi: 10.1200/PO-24-00645. PMID: 39642328
21. Fernando M, Anton A, Weickhardt A, Aza AA, Uccellini A, Brown S. et al. Treatment patterns and outcomes in older adults with castration-resistant prostate cancer: Analysis of an Australian real-world cohort. J Geriatr Oncol. 2023;14(8):101621 doi:10.1016/j.jgo.2023.101621
22. Freedland SJ, Davis M, Epstein AJ, Arondekar B, Ivanova JI. Real-world treatment patterns and overall survival among men with metastatic castration-resistant prostate cancer (mCRPC) in the US medicare population. Prostate Cancer Prostatic Dis. 2024;27:327-333 doi:10.1038/s41391-023-00725-8
23. Wenzel M, Hoeh B, Wagner N, Koll F, Siech C, Humke C. et al. Treatment patterns and oncological outcomes of older adults with metastatic prostate cancer in real-world setting. J Am Geriatr Soc. 2024 doi:10.1111/jgs.19045
24. Tonnelet D, Farce J, Agrigoroaie L, Bros M, Mourey L, Lacombe M. et al. Characteristics, tolerance and effectiveness of patients aged more or less than 75 years treated with [177Lu]Lu-PSMA-617 as part of France's early access program. Annals of Oncology. 2024;35:S995
25. Wenzel M, Siech C, Hoeh B, Koll F, Humke C, Tilki D. et al. Contemporary treatment patterns and oncological outcomes of metastatic hormone-sensitive prostate cancer and first- to sixth- line metastatic castration-resistant prostate cancer patients. Eur Urol Open Sci. 2024;66:46-54 doi:10.1016/j.euros.2024.06.010
26. Satapathy S, Yadav MP, Ballal S, Sahoo RK, Bal C. [177Lu]Lu-PSMA-617 as first-line systemic therapy in patients with metastatic castration-resistant prostate cancer: a real-world study. Eur J Nucl Med Mol Imaging. 2024;51(8):2495-2503 doi:10.1007/s00259-024-06677-y
27. Khreish F, Ghazal Z, Marlowe RJ, Rosar F, Sabet A, Maus S. et al. 177 Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: Initial 254-patient results from a prospective registry (REALITY Study). Eur J Nucl Med Mol Imaging. 2022;49(3):1075-1085 doi:10.1007/s00259-021-05525-7
28. Almuradova E, Seyyar M, Arak H, Tamer F, Kefeli U, Koca S. et al. The real-world outcomes of Lutetium-177 PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer: Turkish Oncology Group multicenter study. Int J Cancer. 2024;154(4):692-700 doi:10.1002/ijc.34749
29. Assayag J, Kim C, Chu H, Webster J. The prognostic value of eastern cooperative oncology group performance status on overall survival among patients with metastatic prostate cancer: a systematic review and meta-analysis. Front Oncol. 2023;13:1194718 doi:10.3389/fonc.2023.1194718
30. Jafari E, Amini AL, Ahmadzadehfar H, Bagheri D, Assadi M. Cardiotoxicity and cardiac monitoring following the use of radiotheranostics agents including 177Lu-PSMA for prostate cancer and 177Lu-DOTATATE for neuroendocrine tumors Nuklearmedizin. 2021 Apr;60(2):99-105. doi: 10.1055/a-1332-8230. PMID: 33461224.
Author contact
![]() Corresponding author: Mike Wenzel, M.D., Department of Urology, Goethe University Hospital Frankfurt, Frankfurt/Main, Germany. Phone: +49 69 6301 83147; Fax: +49 69 6301 80069; E-Mail: Mike.Wenzelde.
Corresponding author: Mike Wenzel, M.D., Department of Urology, Goethe University Hospital Frankfurt, Frankfurt/Main, Germany. Phone: +49 69 6301 83147; Fax: +49 69 6301 80069; E-Mail: Mike.Wenzelde.
 Global reach, higher impact
Global reach, higher impact