13.3
Impact Factor
Theranostics 2024; 14(18):7054-7071. doi:10.7150/thno.102037 This issue Cite
Review
Unlocking the dual role of LSD1 in tumor immunity: innate and adaptive pathways
1. State Key Laboratory of Esophageal Cancer Prevention & Treatment; Key Laboratory of Advanced Drug Preparation Technologies, Ministry of Education of China; Key Laboratory of Henan Province for Drug Quality and Evaluation, Henan Province; School of Pharmaceutical Sciences; Academy of Medical Sciences; Tianjian Laboratory of Advanced Biomedical Sciences; Zhengzhou University, Zhengzhou, Henan 450001, China.
2. Department of Breast Surgery, Henan Provincial People's Hospital, People's Hospital of Zhengzhou University, People's Hospital of Henan University; Henan Provincial Engineering Research Center of Breast Cancer Precise Prevention and Treatment, Zhengzhou, Henan 450003, China.
3. XNA platform, School of Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, Henan 450001, China.
4. School of Medicine, Taizhou University, Taizhou, Zhejiang 318000, China.
Received 2024-8-7; Accepted 2024-10-10; Published 2024-10-21
Abstract
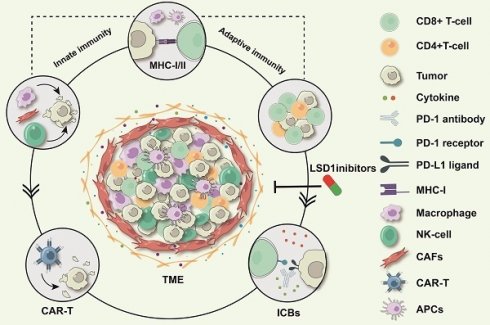
The roles of innate and adaptive immunity are crucial in both the development of cancer and its response to treatment. Numerous studies have demonstrated that histone lysine-specific demethylase 1 (LSD1) is overexpressed in various cancers. Elevated levels of LSD1 intricately modulate immune checkpoints, the function of immune cells, and the expression of immunomodulators, impacting both innate and adaptive immunity. Moreover, compelling evidence suggests that inhibiting LSD1 enhances tumor immunity, suppresses tumor growth, and improves the effectiveness of immunotherapy. However, a comprehensive classification of LSD1's role in both innate and adaptive immunity is lacking. In this review, we outline the role of LSD1 in tumor immunity in terms of both innate and adaptive immunity, summarizing the mechanisms associated with LSD1-mediated tumor immunity and its potential regulatory capacity in tumor immune escape. Finally, we summarize the research status of LSD1 inhibitors in tumor immunotherapy, which be valuable for promoting the development of effective LSD1-targeted agents used as combination immunotherapy drugs.
Keywords: LSD1, Tumor microenvironment, Immune checkpoint blockade, Immune cell, Cancer immunotherapy
1. Introduction
The human body maintains health through innate and adaptive immunity, which work to recognize and eliminate damaged and tumor cells. In cancer, both systems play crucial roles [1]. Adaptive immunity, primarily mediated by B and T cells, is central to the fight against cancer and involves several key steps: antigen recognition, clonal expansion, the effector phase, and immune memory [2, 3]. On the other hand, the innate immune system is activated by sensing tissue damage and recruiting cells to the affected areas. For example, macrophages capture and digest diseased cells through phagocytosis, while natural killer (NK) cells can recognize and kill infected or cancerous cells, suppressing tumor development without the need for antigen presentation [2-4]. The tumor microenvironment (TME) is a complex ecosystem with both pro-tumor and anti-tumor tendencies. It comprises innate immune cells, adaptive immune cells, immunosuppressive cells, interstitial tissues, microvessels, and various cytokines and chemokines [5-10]. Tumor cells typically create an immunosuppressive environment by recruiting immunosuppressive cells [11, 12], secreting factors that suppress the immune response [13-16], overexpressing antigens like PD-L1 [17, 18], exhibiting low major histocompatibility complex (MHC) [19, 20], or releasing tumor exosomes to sustain their survival and progression [21, 22].
LSD1, also known as KDM1A, is a crucial epigenetic target involved in various physiological and pathological processes through its demethylase activity on both histone and non-histone substrates [23-25]. Numerous studies have shown that LSD1 is highly expressed in many cancers, including gastric cancer [26], squamous cell carcinoma [27], hepatocellular carcinoma [28], prostate cancer [29], and breast cancer [30]. High LSD1 expression is generally associated with poor prognosis. Currently, several LSD1 inhibitors are in clinical trials and show considerable potential in cancer treatment [31, 32]. Recent research has highlighted the important role of LSD1 in tumor immunity. Abnormally high expression of LSD1 influences tumor immunity in multiple cancers by regulating immune checkpoints [33-35], antigen presentation [36, 37], and other pathways [38]. Consequently, LSD1 is a potential target for modulating the TME, offering significant research value and application potential in tumor immunotherapy. Inhibiting LSD1 expression or activity can alter the immunosuppressive characteristics of the TME and enhance the antitumor immune response, ultimately improving the efficacy of tumor immunotherapy.
In this review, the biological function of LSD1 and its implications in immunotherapy are elucidated from the perspectives of innate and adaptive immunity.
2. LSD1 and cancer
LSD1, a member of the flavin adenine dinucleotide-dependent amine oxidase superfamily, was the first histone demethylase to be identified [39]. It interacts with the nuclear REST corepressor 1 (CoREST) transcriptional blocking complex [40]and histone deacetylases 1 and 2 (HDAC1/2) [41]. In complex with CoREST and the nucleosome remodeling and deacetylase, LSD1 removes methyl groups from monomethyl and dimethyl histone H3 lysine 4 (H3K4me1/me2), leading to transcriptional repression [39, 42-45]. Beyond histones, LSD1 targets several non-histone proteins, including DNA methyltransferase 1 (DNMT1), E2F transcription factor 1 (E2F1), tumor suppressor p53, transcription signal transducer and activator of transcription 3 (STAT3), and hypoxia-inducible factor-1 (HIF-1α) [24]. Through the demethylation of these substrates, LSD1 is involved in various cellular processes, such as cell proliferation and differentiation [46], senescence [47], epithelial-mesenchymal transition [48], chromatin regulation [42], angiogenesis [49], cancer stem cell regulation [50], glycolysis and mitochondrial metabolism [28].
There is growing evidence that abnormal expression of LSD1 in tumors contributes to poor immunotherapy outcomes [51-56]. Inhibiting LSD1 expression and transcriptional activity presents a promising new avenue for the immunotherapy of malignant tumors [57, 58]. LSD1 mediates tumor immunity through various mechanisms, such as regulating autophagy-related gene expression to mediate tumor-associated autophagy [59, 60], affecting immune checkpoint protein expression [34, 61], upregulating immunosuppressive molecules [62, 63], reducing tumor antigen presentation [36, 37], and interfering with hypoxia-mediated tumor physiology and pathogenesis [64, 65]. The elucidation of LSD1's molecular mechanisms in tumor immune escape underscores its potential as a target for tumor immunotherapy. The following sections will explore the intricate roles and molecular mechanisms of LSD1 in both innate and adaptive immunity.
3. LSD1 regulates innate immunity
Innate immunity is the first line of defense against invading pathogens and disturbances in homeostasis. Key players in innate immunity include cells derived from bone marrow precursors, such as NK cells, macrophages, and dendritic cells (DCs). These cells are essential for resisting and eliminating pathogenic microorganisms [66]. In the early stages of cancer, the innate immune system can recognize and eliminate tumor cells. However, as the tumor develops, the TME becomes immunosuppressive, allowing tumor cells to escape immune surveillance. In this context, innate immune cells can become significant factors promoting tumor growth and metastasis [67]. Studies have shown that LSD1 can affect the anti-tumor activity of innate immune cells, either directly or indirectly. The following sections will discuss the role of LSD1 in innate immunity, focusing on its effects on different cell types.
3.1 Regulation of NK cells
NK cells are crucial innate lymphocytes involved in anti-tumor activity, antiviral defense, and immune regulation. They also participate in hypersensitivity reactions and autoimmune diseases. NK cells recognize and kill target cells, and activation receptors on their surface, such as NKG2D, NKP46, NKP30, and NKP44, play a key role in promoting their anti-tumor activity [68-70]. Studies have found that LSD1 inhibitors can enhance the anti-tumor activity of NK cells by affecting their metabolism or the expression of NK cell ligands on tumor cells. This section will explore these mechanisms in detail (Figure 1A).
LSD1's regulation on innate immunity. A. LSD1 regulates the anti-tumor activity of NK cells through two pathways: LSD1 inhibition up-regulated the expression of NK cell ligand on AML; Scaffolding inhibitors reduce oxidative phosphorylation and glycolysis of NK cells and induce mitochondrial depletion of reactive oxygen species and antioxidant glutathione. B. LSD1 inhibition promotes the differentiation of TAM into M1 macrophages, promotes the infiltration of M1 macrophages, and inhibits the progression of TBNC. C. LSD1 inhibition decreased the proportion of CAFs in TME and inhibited the progression of TNBC. Inhibition of LSD1 can restore the expression of IRF8 in MDS progenitor cells, promote CD141Hi cDCs differentiation.
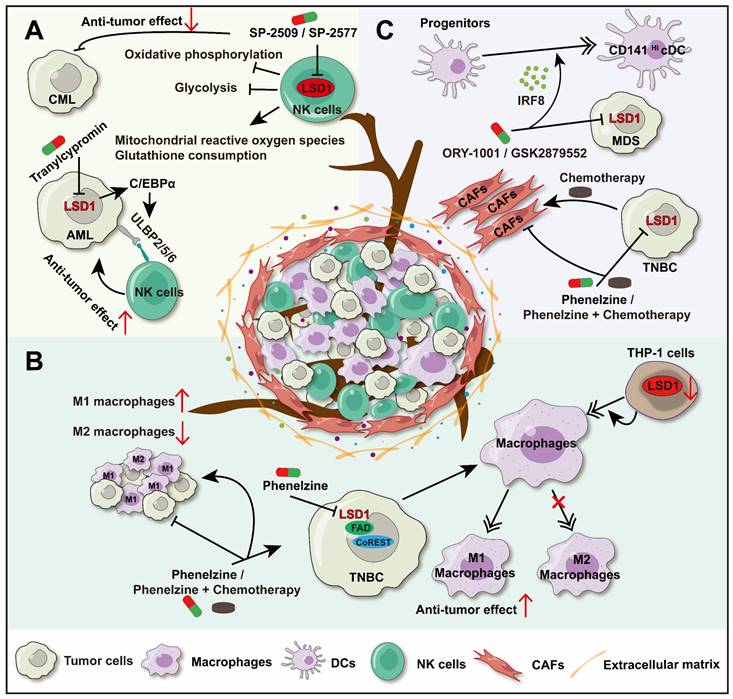
UL16-binding proteins (ULBPs), ligands of NKG2D, are expressed on the surface of tumor cells and can sensitize NK cells to kill these cells. The LSD1 inhibitor (Tranylcypromine) can upregulate the expression of ULBP2/5/6 by promoting CCAAT/enhancer-binding protein α (C/EBPα) expression in acute myeloid leukemia (AML) cells, thereby increasing NK cells ability to lyse AML cells [71]. Similarly, the LSD1 inhibitor (GSK-LSD1) also enhanced the cytotoxicity and tumor infiltration of NK cells in diffuse intrinsic pontine glioma (DIPG) cells [72]. However, there are variations in the effects of LSD1 inhibitors on NK cells activity. Studies have shown that scaffolding inhibitors (SP-2509 and SP-2577) can impair the ability of NK cells to lyse chronic myelogenous leukemia (CML). SP-2509 and SP-2577 reduce the oxidative phosphorylation and glycolysis of NK cells, induce the consumption of mitochondrial reactive oxygen species and the antioxidant glutathione, which lead to dissolution function of NK cells weakened. Moreover, scaffold inhibitors have certain toxicity to NK cells [72, 73].
These current study of LSD1's regulation on NK cells were based on LSD1 inhibitor, while the regulations of different inhibitors are not consistent. On one hand, inhibiting LSD1 (Tranylcypromine) promotes the infiltration of NK cells and enhances their cytotoxicity. On the other hand, scaffold LSD1 inhibitors can weaken the anti-tumor ability of NK cells and produce a certain toxicity to NK cells, leading to the development of resistance to immunotherapy in tumors. Further research is needed to explain the differences among LSD1 inhibitors. There is still much research space for the deep molecular mechanism of LSD1 on NK immunity.
3.2 Regulation of macrophages
Tumor-associated macrophages (TAM) are the most abundant infiltrating inflammatory cells in TME and play a key role in regulating the TME [74]. TAM can differentiate into two phenotypes: M1 macrophages (tumor suppressor subtype) and M2 macrophages (tumor promoting subtype). While, the former exerting antitumor effects through cytotoxic activity and antigen presentation, and the latter can promote tissue repair, tumor cell proliferation, and inhibit anti-tumor immunity [75]. Immunosuppressive cells in the TME are key contributors to tumor resistance against immune checkpoint blockade (ICB) therapy. They inhibit CD8+ T cell function and promote tumor growth [76]. Targeting the highly infiltrated M2 macrophages could help overcome this resistance and enhance the anti-tumor effects in coordination with adaptive immunity.
LSD1 inhibitor (phenelzine), which targets both the flavin adenine dinucleotide (FAD) and CoREST binding domains of LSD1. It facilitates macrophages differentiate into M1 phenotype with antitumor effects in triple-negative breast cancer (TNBC) [77]. In addition, phenelzine alone or combined with chemotherapy can increase the infiltration of M1 macrophages in the TME, while reduce M2 macrophages infiltration, and inhibit tumor growth consequently [78]. In another study, LSD1 inhibition in THP-1 cells produced effects similar to those seen with the inhibitor. Downregulation of LSD1 induced the differentiation of THP-1 monocytes into macrophages by upregulating the methylation of the interleukin-6 (IL-6) promoter at H3K4. Further research on the impact of LSD1 on macrophage polarization is highly anticipated [79]. Besides, interleukin-4 (IL-4) is a key cytokine creating an inflammatory inhibitory microenvironment in TAMs, inducing polarization of M2 macrophages [9]. A study reveals that LSD1 was a co-activator of IL-4, and LSD1 inhibition reduces the expression of IL-4-induced genes in macrophages, but further research is needed to explore this phenomenon in TAMs [80] (Figure 1B).
LSD1 has been shown to play a key role in macrophage maturation [81], morphology maintenance [82], and inflammatory behavior across various disease conditions [83]. While most current studies focus on LSD1's regulation of macrophage polarization, this opens new avenues for exploring how LSD1 influences macrophages in cancer. Given the high infiltration of macrophages in solid tumors, targeting LSD1 to enhance their anti-tumor activity presents a promising strategy for cancer immunotherapy.
3.3 LSD1 regulates innate immunity via DCs and cancer-associated fibroblasts (CAFs)
In solid tumors, CD141Hi conventional dendritic cells (CD141Hi cDCs) play a significant role in anti-tumor immune surveillance and immunotherapy response. In myelodysplastic syndromes (MDS), the level of CD141Hi cDCs was positively correlated with the longer overall survival rate of MDS patients. Further studies found that there were fewer progenitors dedicated to DCs differentiation in MDS bone marrow, and these progenitors expressed lower levels of interferon regulatory factor-8 (IRF8), which is the primary regulator of CD141Hi cDCs differentiation. However, inhibition of LSD1 (ORY-1001 or GSK2879552) can restore the expression of IRF8 in MDS progenitor cells and promote the differentiation of CD141Hi cDCs [84] (Figure 1C). These data suggest that LSD1 has a significant role in modulating DCs.
Except for immune cells, LSD1 regulates the function of non-immune components in the TME. CAFs, a key part of the tumor microenvironment, are involved in angiogenesis, extracellular matrix remodeling, and immunosuppression, creating a supportive environment for tumor cells [85]. Thus, targeting CAFs presents a novel approach to tumor therapy. Since CAFs are involved throughout the tumor immune process, they are tentatively grouped under the innate immunity segment in this review. Studies have shown that in breast cancer in situ, paclitaxel monotherapy increases the proportion of CAFs in the TME, while inhibition of LSD1 (Phenelzine) alone or combined chemotherapy reduces CAFs infiltration significantly [78]. Meanwhile, abnormally elevated LSD1 expression in CAFs has been linked to poor patient survival [86], and direct inhibition of CAFs has been shown to reduce their activity and diminish their immunosuppressive function [87] (Figure 1C). All indications are that LSD1 may be an important cause of high infiltration of CAFs in the TME, resulting in immune escape.
Currently, the investigation into LSD1 within DCs and CAFs remains in its nascent stages. Limited studies have proposed that the inhibition of LSD1 could potentially facilitate the differentiation of DCs towards a more immunoreactive state and attenuate the tumor-supporting function of CAFs consequently exerting a pivotal role in innate immunity. Furthermore, this substantiates the notion that LSD1 serves as a promising target within the realm of innate immunity.
Based on the previous discussion, it is evident that LSD1 intersects with various aspects of the innate immune response. Thus, directing therapeutic interventions towards LSD1 may potentiate the innate immune system attributes of promptness, and affect inflammation within the context of tumor immunity, which vigilantly monitoring and eradicating the migration of tumor cells. What's more, the innate immune system can cascade towards activating, regulating, and imprinting memory responses in adaptive immunity through pathogen recognition and antigen representation and presentation. Hence, the potential utility of LSD1 inhibitors has come to the fore, as they hold promise in rendering tumor cells more readily discernible to the immune system, consequently augmenting the efficacy of immunotherapeutic agents such as immune checkpoint inhibitors.
4. LSD1 regulates adaptive immune processes
In a previous section, the role of LSD1 in regulating innate immune cells within the TME was emphasized. Moreover, it is widely acknowledged that effective anti-tumor defenses hinge on the activation of adaptive immune responses, which primarily orchestrated by T and B cells. T-cell-mediated immune response is a stepwise process involving multiple stages and coordinated mechanisms [88]. (a) Antigen recognition and presentation: the antigen was released by cancer cell, and then recognized and presented to T cells by DCs or antigen-presenting cells (APCs). (b) T cell activation, proliferation, differentiation, and depletion: T cells begin to proliferate and differentiate into different T cell subtypes once receive signals from APCs. (c) T cell transport, invasion, effect stage: T cells transport and infiltrate into the tumor and stroma to recognize and kill cancer cells. However, any anomaly in any stage of this process can become a bottleneck in oncotherapy. The immunomodulatory role of LSD1 in these pathways has attracted much attention. Due to the limited reports on LSD1's regulation of B-cell-mediated immune responses, most studies have focused on its role in B-cell malignancies. Therefore, only a brief overview is provided in this paper. This chapter emphasizes the regulation of T-cell-mediated immune responses by LSD1 in cancer cells.
4.1 Regulation of T cells
4.1.1 LSD1 affects antigen presentation and T cell activation
Antigen presenting process is the key to T cell activation. The antigens were presented by the MHC, and recognized by T cells through specific T cell receptor (TCR), causing T cell activation. MHC-I mainly presents antigens to CD8+ T cells, while MHC-II mainly presents antigens to CD4+ T cells [89, 90]. In many cancer types, tumor cells can diminish or completely abolish the expression of MHC-I molecules through diverse mechanisms. Consequently, this renders tumor cells incapable of being effectively identified and targeted by CD8+ T cells, leading to immune evasion, namely, escaping immune surveillance and attack by the host. Enhancing the antigen presentation process was a crucial step in the anti-tumor immunotherapy [91, 92]. Studies have shown that LSD1 can affect the antigen presentation process by regulating the expression of MHC-I and MHC-II molecules, and this will be discussed in the following sections (Figure 2).
In melanoma cells, LSD1 deletion enhances T cell activation and infiltration by upregulating the expression of MHC-I encoding genes. Mechanistically, LSD1 deficiency reduces the activity of RNA-induced silencing complex (RISC) components, leading to the accumulation of double-stranded RNA (dsRNA). This dsRNA stress activates MDA5, which subsequently upregulates MHC-I and triggers type 1 interferon (IFN) responses [34]. Small cell lung cancer (SCLC) is a highly invasive neuroendocrine tumor characterized by early acquired treatment resistance and limited benefits from ICB. The inhibition of MHC-I is a key mechanism for T cell immune therapy resistance in SCLC [93]. Inhibition of LSD1 has been shown to promote the conversion from a high neuroendocrine (NE) gene expression to a low NE phenotype in SCLC, improving NOTCH signaling activation. Additionally, LSD1 inhibition significantly restored MHC-I expression and triggered intrinsic IFN signaling, both of which enhanced tumor immunogenicity and significantly increased CD8+ T cell activation and infiltration [36, 37]. In addition, LSD1 inhibition also up-regulates MHC-I class molecule-mediated antigen presentation in mouse breast tumor cells. However, the exact mechanism is not reported in detail [94].
The regulation of MHC-I and MHC-II molecules by LSD1 is not only limited in tumor cells, but also in mesenchymal stem cells (MSCs). It has been reported that inhibition of LSD1 can result in the upregulation of dsRNA stress and its related response elements in MSCs, which include pattern recognition receptors (PRRs), Type I IFN, and IFN-stimulating genes (ISGs). Depending on these, LSD1 inhibition can enhance the ability of MSCs to present immunogenic peptides to CD8+T cells by inducing the expression of MHC-I molecules on the surface of MSCs, which induces an effective anti-tumor immune response in consequence [95]. In addition, LSD1 inhibition can upregulate macrophage CD80/86, MHC-II genes [77]. Further research on whether targeting LSD1 can enhance macrophage antigen presentation to CD4+ T cells and boost the anti-tumor immune response is highly anticipated [96].
LSD1 regulates antigen presentation and T cell activation. Inhibition of LSD1 leads to upregulation of MHC-I expression in different cell types, promoting T cell activation and infiltration. In melanoma, loss of LSD1 results in upregulation of MHC-I gene expression. LSD1 deficiency leads to accumulation of dsRNA by reducing RISC components, which activate the IFN pathway subsequently, and then upregulate MHC-I expression. In SCLC, inhibition of LSD1 promotes enhanced tumor immunogenicity and increased CD8+ T cell activation and infiltration, which is achieved by restoring MHC-I expression, triggering intrinsic IFN signaling, and other pathways. In MSCs, LSD1 inhibition also promotes MHC-I expression by inducing dsRNA stress, while upregulating the level of ISGs, IFN, and PRRs. Additionally, Inhibition of LSD1 up-regulates the expression of MHC-II in macrophages and promotes antigen presentation to CD4+T cells.
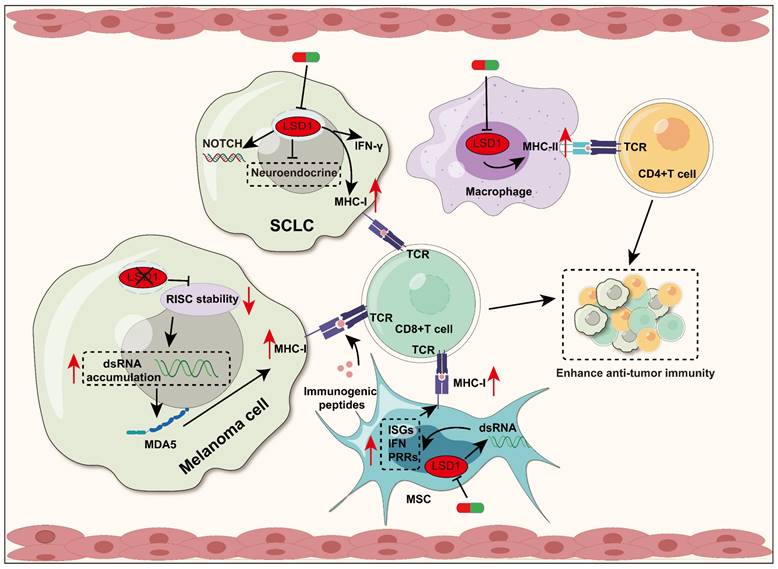
In short, elevated LSD1 expression can suppress the antigen-presenting process of tumor cells, thereby attenuating the recognition and cytotoxicity of T cells against tumor. Consequently, utilizing LSD1 inhibitors can enhance tumor immunogenicity and thereby promote anti-tumor immunity.
4.1.2 LSD1 affects the differentiation, infiltration and depletion of T cells
As previously discussed, LSD1 plays a critical role in governing the activation, differentiation, infiltration, and depletion of T cells within the context of anti-tumor immunity. There is literature suggesting that inhibiting LSD1 can induce the differentiation of CD4+ T cells into Th1 cells by upregulating Runx2, Tbx21, and Eomes in CD4+T cells. This process may have a promoting effect on tumor immunity [97]. Additionally, targeting LSD1 can enhance CD8+ T cell infiltration into tumors and boost the anti-tumor immune response through various pathways, such as increasing chemokine expression [98-100], downregulating PD-L1 [33], and accumulating dsRNA [34, 95]. Particularly excitingly, precise and transient LSD1 inhibition during T cell receptor activation and Interleukin-2 (IL-2) signaling significantly enhanced the memory phenotype of mouse CD8+ T cells. This intervention also enabled these cells to produce multiple cytokines, resist depletion, and sustain antigen-dependent and independent persistence post adoptive transfer [101]. Meanwhile, research has shown that targeting LSD1 induces extensive epigenomic remodeling in T cells by modulating their transcriptional profiles, which promotes T cell phenotypic stability and function [102]. In summary, altering the status of LSD1 can serve as a potential anti-cancer strategy. This strategy enhances T cell activation, differentiation, and infiltration through multiple mechanisms, reverses T cell depletion, and thus enhances T cell attack on tumors. To better understand the role of LSD1 in tumor immunity, the regulatory effects of LSD1 on T cells are summarized in Table 1.
4.2 Regulation of B cells
While the crucial role of T cells in tumor immunity is well understood, the contribution of B cells is often overlooked. In fact, B cells have significant prognostic value and have become an important predictor of immune checkpoint inhibitor response in a variety of cancers. B cells have a variety of functions, such as antigen presentation, antibody production, regulation of the TME, and promote immune cells to attack tumor cells [109]. LSD1 has been reported to be necessary for the proliferation and differentiation of B cells [110, 111]. Interestingly, the role of LSD1 in regulating B cell differentiation was not always coincident. On the one hand, in germinal center-derived lymphoma, conditional loss of LSD1 can inhibit the constitutive expression of BCL6 and promote the expression of genes related to proliferation of germinal center B cells, which further inhibit the differentiation of germinal center B cells and delay the development of BCL6-driven lymphoma significantly [112]. In addition, inhibition of LSD1 has been shown to improve proteasome inhibitor sensitivity and overcome drug resistance in patients with multiple myeloma (MM). Therefore, LSD1 inhibitor combined with proteasome inhibitor may have the potential to be expansibility used to other B cell malignancies treatment [113]. Meanwhile, it was also reported that LSD1 could inhibit disease progression by regulating abnormal plasma cells, which were effector B cells [114]. Currently, LSD1's regulation of B cells is mainly studied in the context of B-cell malignancies. We review this chapter and suggest that in the future more attention should be paid to the potential role of LSD1 in the regulation of tumor-killing B cells, which would be desirable or be used for reference or guidance.
5. LSD1 plays a crucial role in tumor immunotherapy
Immunotherapy has advanced rapidly in recent years, offering tremendous potential to combat tumors by modulating the human immune system. [115]. The main methods include: (a) Immunomodulators, which regulate cell-cell interactions of the immune system, tumor immunosurveillance and clearance processes, such as chemokines and cytokines [116, 117]; (b) ICB therapy, which activates the immune system to attack tumor cells by inhibiting checkpoint molecules, such as CTLA-4, PD-L1, and CD47 [118]; (c) Cell therapy, a type of cell therapy in which live cells are injected into the patients' body to enhance the immune effect to treat the disease, such as T-cells, NK-cells, and stem cells [119]; (d) Cancer vaccines, which prepare tumor-specific antigens to stimulate the immune system to produce an immune response against the tumor [120].
LSD1 affects T-cell function
| Cancers | Molecular mechanism | Biological function | References | |
|---|---|---|---|---|
| T cell activation and Infiltration | Melanoma | LSD1 deletion up-regulates MHC-Ⅰ expression | Promote the activation and infiltration of CD8+T cells | [34] |
| SCLC | LSD1 inhibition enhances the induction effect of IFN-γ on MHC-I, and then increases the expression level of MHC-I | Enhance the activation and infiltration of CD8+ T cells | [36] | |
| T cell activation | TNBC | LSD1 inhibition upregulates MHC-I expression | Inhibit breast cancer progression | [94] |
| TNBC | Inhibition of LSD1 up-regulates macrophage MHC-II | Promotes antigen delivery to CD4+ T cells, thus promoting anti-tumor immunity | [77] | |
| T cell differentiation | / | Inhibition of LSD1 increased the expression levels of Runx2, Tbx21, Eomes and H3K4 in CD4+ T cells | Promote the development of CD4+ T cells to Th1 cells secreting IFN-γ, and promote tumor immunity | [97] |
| T cell Infiltration | Melanoma and CRC | / | Simultaneous inhibition of LSD1 and TGF-β combined with anti-PD-1 therapy significantly increased the infiltration and cytotoxicity of CD8 + T cells | [61] |
| NSCLC | Inhibition of LSD1 promotes ERGIC1-mediated IFNGR1 stabilization and membrane transport, leading to upregulation of MHC-I/PD-L1 in tumors | Inhibition of LSD1 combined with anti-PD-1 therapy promoted CD8+T cell infiltration and inhibited the progression of NSCLC | [103] | |
| TNBC | Inhibition of LSD1 up-regulates CCL5, CXCL9, CXCL10 and PD-L1 | LSD1 inhibition combined with anti-PD-1 decreased Ki-67 levels in TNBC and promoted CD8+ T cell infiltration | [99] | |
| Melanoma | Inhibition of LSD1 increased the number of Slamf6+Tim-3-Texprog cells in tumors and increased the expression level of Tcf1 in ID2-deficient CD8+ T cells. | Inhibition of LSD1 can save the damage of CD8+ T cells caused by the deletion of Id2 gene, and the efficacy of anti-PD-1 therapy is low. | [104] | |
| T cell depletion | Melanoma | Inhibition of LSD1 promotes the production of effector cytokines and decreases the expression of related inhibitory receptors. | Inhibition of LSD1 reverses T cell exhaustion and enhances T cell persistence and anti-tumor efficacy. | [101] |
| T cell killing ability | GC | / | Inhibition of LSD1 enhanced the sensitivity of T cells to GC cells, improved its killing ability to GC cells, and inhibited tumor progression | [105-108] |
SCLC: Small cell lung cancer; NSCLC: Non-small cell lung cancer; TNBC: Triple negative breast cancer; GC: Gastric cancer; CRC: Colorectal cancer
It was mentioned in the previous chapter that LSD1 can regulate immune cell differentiation and function, and weaken immune response in some tumors. Inhibition of LSD1 activity can enhance the function of immune cells and improve the efficacy of immunotherapy, making LSD1 a potential immune regulatory target. This chapter will primarily review the effects of LSD1 on immunomodulators and immune checkpoint molecules, as well as the efficacy of combining LSD1 inhibitors with ICB therapy or CAR-T therapy, based on existing studies.
5.1 Regulation of immunomodulators
Tumor-associated immune factors are biologically active molecules related to tumor occurrence, development, and immune regulation. They play essential roles in processes such as tumor immune surveillance and immune escape. For instance, cytokines (such as IL-6, IL-10, transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF) and chemokines (such as CXCL9, CXCL10) are represented, and these factors can regulate the effective function of T cells, thereby changing the balance of tumor immune response [121-126].
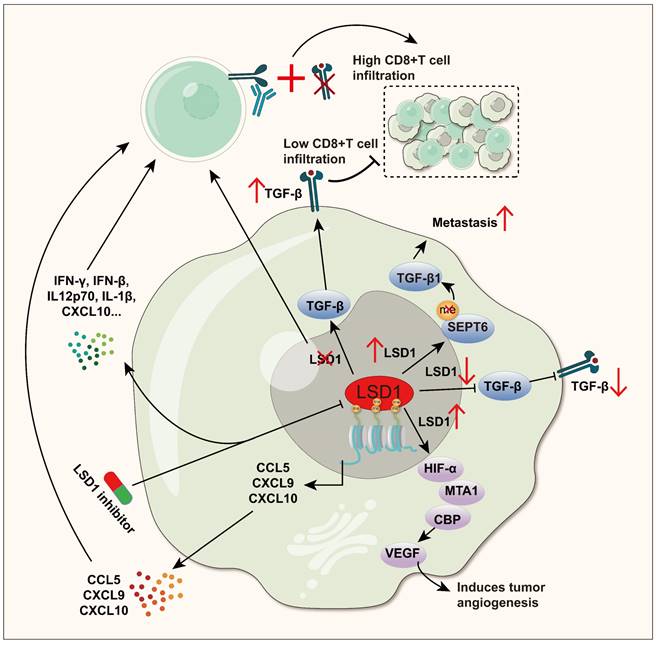
Highly expressed TGF-β has immunosuppressive effects, promoting tumor cells to evade immune surveillance [127]. Meanwhile, TGF-β can also enhance tumor vascular endothelial cell proliferation and angiogenesis, creating a favorable microenvironment by providing oxygen and nutrition, which further promote tumor development [128, 129]. Inhibiting TGF-β signaling can interrupt the epithelial-mesenchymal transition (EMT) process, enhance tumor immune response, and reduce the risk of tumor metastasis, which has broad clinical application prospects [130-132]. LSD1 has been reported to have a significant effect on TGF-β. In GC, reducing LSD1 can decrease the expression of TGF-β [63]. In NSCLC, LSD1-mediated demethylation of SEPT6 protein promotes cancer cell metastasis by activating the TGF-β1 pathway [62]. However, the positive correlation between LSD1 and TGF-β in GC and NSCLC has not been deeply studied in immune regulation. Conversely, LSD1 and TGF-β show a negative correlation in breast cancer. Similarly, it is also not clear whether LSD1 regulates immunity accordingly [133]. Meanwhile, the absence of LSD1 promotes TGF-β expression in melanoma and colorectal cancer (CRC) cells, and the up-regulated TGF-β counteracts the anti-tumor effects of LSD1 deletion-induced T cell infiltration by inhibiting the cytotoxicity of CD8+ T cells within the tumor. Simultaneous elimination of LSD1 and TGF-β, combining with PD-1 blockade, can significantly enhance CD8+ T cell infiltration and cytotoxicity [61].
VEGF is a key survival factor and mitogen for vascular endothelial cells. In the VEGF family, VEGF-A and VEGF-C play important roles in tumor immunity. There is extensive research on VEGF-A, and the term "VEGF" is often referred to VEGF-A [134]. However, VEGF-A possesses certain immunosuppressive properties, as its upregulation can promote T cell exhaustion, inhibit DCs maturation, and enhance the recruitment of regulatory T (Treg) cells and tumor-promoting M2 macrophages [135]. In contrast, VEGF-C often exhibits positive effects in tumor immunity, such as promoting the activation of CD8+T cells in brain tumors. Furthermore, it enhances the chemotherapeutic efficacy and anti-tumor immunity against brain tumors significantly [136]. LSD1 actively regulates VEGF expression in various cancers. In GC, downregulating LSD1 can inhibit the metastatic potential of GC cells and activate the VEGF-C-mediated PI3K/AKT signaling pathway [137]. In prostate cancer (PC), LSD1 upregulation is associated with PC recurrence and VEGF-A upregulation [138]. Additionally, in human breast cancer, stable HIF-1α induced by LSD1 interacts synergistically with CBP and MTA1 to promote tumor angiogenesis induced by VEGF, providing continuous oxygen and nutrition for tumor growth [139]. However, according to current research, it remains unclear whether LSD1 regulating VEGF leads to immunosuppression.
Chemokines are a class of cytokines that regulate immune cell migration and lymphoid tissue development [140, 141]. In the development of cancer, they play a core role in guiding immune cells infiltrate to tumor sites, shaping the immune characteristics of the TME, and often inhibiting tumor growth. Additionally, chemokines can also directly act on non-immune cells in the TME, such as tumor cells, stromal cells, and vascular endothelial cells [142, 143]. In TNBC, inhibiting LSD1 enhances the enrichment of H3K4me2 in the promoter regions of chemokines CCL5, CXCL9, and CXCL10, which promotes the attraction of CD8+ T cells into the TME and exerts strong killing power [99]. This result was further confirmed in a recent study [98]. In small cell carcinoma of the ovary hypercalcemic type (SCCOHT), inhibiting LSD1 enhances the release of cytokines IFN-γ, IFN-β, IL12p70, IL-1β, IP-10 (CXCL10), IL-10, IL-2, IL-6, MCP-1 (CCL2), and IL-8 (CXCL8), revealing the important role of LSD1 inhibition in promoting antitumor immunity [144].
Overall, LSD1 promotes tumor progression by directly or indirectly influencing TGFβ, VEGF, and chemokines (Figure 3). Cytokines are key mediators of cellular communication in the TME, crucial for activating and regulating innate and adaptive immune responses [145]. For example, as mentioned earlier, simultaneous inhibition of LSD1 and TGF-β, along with PD-1 blockade in melanoma and CRC, significantly enhanced CD8+ T cell infiltration and cytotoxicity. In TNBC, LSD1 inhibition also increased the expression of chemokines CCL5, CXCL9, and CXCL10, recruiting CD8+ T cells into the TME, further strengthening the adaptive immune response. Cytokines are crucial pathways through which LSD1 influences immune cells in the TME, yet they remain less studied. A promising new direction is to further explore how LSD1 modulates cytokines to influence tumor immunity. This, combined with current insights into LSD1's effects on innate and adaptive immunity, could lead to new therapeutic strategies to enhance anti-tumor immunity.
LSD1 interferes with the anti-tumor immune process by regulating the expression of TGF-β, VEGF and Chemokines. TGF-β: In GC and NSCLC, LSD1 positively regulates TGF-β and promotes cancer cell metastasis, while in melanoma and CRC, LSD1 negatively regulates TGF-β, while eliminating LSD1 and TGF-β and jointly blocking PD-1 can significantly enhance CD8+ T cell infiltration and cytotoxicity. VEGF: Stabilized by LSD1, HIF1α collaborates with CBP and MTA1 to boost VEGF-triggered angiogenesis in human breast cancer. Chemokines: In TNBC and SCCOHT, inhibition of LSD1 can enhance the secretion of chemokines such as CCL5, CXCL9, CXCL10 and CCL2, and recruit CD8+ T cells into the TME to play a powerful killing ability.
5.2 LSD1 and ICB therapy
5.2.1 Regulation of immune checkpoint molecules
Immune checkpoint molecules, including PD-1, PD-L1/PD-L2, CTLA-4, and CD47, serve as negative co-stimulatory factors regulating the duration of immune responses. Dysregulated expression of these molecules can disrupt normal immune function [146]. Current studies on LSD1's impact on immune checkpoints predominantly center around PD-1/PD-L1, with varying regulatory dynamics observed across different cancers, indicating intricate and diverse regulatory mechanisms. In GC, LSD1 exhibits positive regulation of PD-L1, whereas in melanoma, the relationship is reversed [33, 34]. Furthermore, the association between LSD1 and other immune checkpoint molecules like CTLA-4 remains unclear. Additionally, the synergistic effects of LSD1 inhibitors in combination with ICB, notably anti-PD-1/PD-L1 therapies, have been extensively documented for their significant efficacy across various cancer types. The expression of PD-1, PD-L1 and other immune checkpoint molecules can directly affect the efficacy of ICB, and numerous studies have highlighted LSD1's pivotal role in modulating the expression of these molecules. Thus, Table 2 meticulously outlines LSD1's biological impacts on tumor immunity and immunotherapy by detailing its influence on immune checkpoint molecule expression in various cancers.
5.2.2 LSD1 inhibitors combined with ICB therapy
ICB, as one of the most successful immunotherapies, has been approved for several oncology indications. However, its overall efficacy is not ideal, with only 20% of patients with solid tumors achieved complete remission after treatment [151]. The expression of immune checkpoint molecules on tumor cells is closely related to the clinical response of ICBs [152]. As previously mentioned, inhibition of LSD1 regulates the expression of immune checkpoint molecules in immune cells as well as tumor cells, suggesting that LSD1 inhibitors show promising potential as a combinatory treatment in ICB therapy.
As an illustration, in TNBC (Figure 4A), inhibition of LSD1 (HCI-2509) resulted in elevated expression of T cell chemokines (CCL5, CXCL9, CXCL10) and PD-L1. These chemokines facilitated infiltration of CD8+ T cells into the TME, where they exerted cytotoxic effects. Moreover, compared to PD-1 antibodies alone, combining HCI-2509 with PD-1 antibodies markedly increased CD8+ T cell infiltration and efficacy in overcoming PD-1 antibodies resistance [99]. Conversely, in CC (Figure 4B), inhibition of LSD1 (ORY-1001) directly reduced PD-L1 and CD47 on the surface of tumor cells. Inhibiting LSD1 in combination with either anti-CD47 or PD-L1 treatments was more effective in inhibiting tumor growth compared to using a single blockade strategy [35].
LSD1 affects the expression of immune checkpoints
| Cancers | Regulation | Immune checkpoints | Function and mechanism | References |
|---|---|---|---|---|
| Melanoma | Negative | PD-1 | 1. LSD1 loss leads to an increase in PD-1-expressing CD8 T cells in tumor infiltration | [147] |
| CRC | Positive | PD-1 | 1. LSD1 deficiency enhanced the survival rate of CD8+ tumor-infiltrating leukocytes (TILs) progenitor cells and decreased the expression of PD-1 protein on the surface of CD8+ TILs cells, without affecting the frequency of PD-1+ cells | [102] |
| Melanoma | Negative | PD-L1 | 1. Knockout of LSD1 enhances the expression of PD-L1 in tumor cells, and combined therapy with anti-PD-1 can overcome tumor resistance to PD-1 blocking, thus playing a significant tumor inhibitory effect | [34] |
| TNBC | Negative | PD-L1 | 1. Inhibition of LSD1 leads to increased enrichment of H3K4me2 on chemokines and PD-L1 promoters, thereby up-regulating the expression of PD-L1 and promoting the release of t cell chemokines (CCL5, CXCL9, CXCL10) | [99] |
| HNSCC | Negative | PD-L1 | 1. LSD1 deletion induced PD-L1 expression in tumor cells and inhibited tumor stem-like features 2. Combined treatment with LSD1 inhibitor and anti-PD-1 can reduce Ki-67 level, promote CD8+T cell infiltration, overcome tumor immune evasion, and significantly inhibit tumor growth | [100] |
| OCCC/SCCOHT | Negative | PD-L1 | 1. Inhibition of LSD1 can enhance the expression of tumor PD-L1, while combined anti-PD-L1 treatment can significantly increase the distribution of CD8+T cells in tumor infiltrating, and enhance the anti-tumor effect | [144] |
| OSCC | Negative | PD-L1 | 1. Inhibition of LSD1 can enhance the expression of PD-L1 in tumors, and combined use of YAP inhibitors can further increase the expression of PD-L1 2. The combination of LSD1 inhibition and anti-PD-1 /PD-L1 treatment can inhibit tumor growth more effectively | [148] |
| SCLC | Negative | PD-L1 | 1. Inhibition of LSD1 activates NOTCH pathway, weakens neuroendocrine features of SCLC, and enhances SCLC's response to PD-1 therapy 2. Inhibition of LSD1 increased the expression of tumor PD-L1, and combined with anti-PD-L1 treatment significantly enhanced the infiltration and anti-tumor effect of CD8+ T cells | [36, 37] |
| CC | Positive | PD-L1 | 1. Decreased H3K4me2 levels in CD47 and CD274 promoters activated by LSD1, directly decreased the expression of CD47 and PD-L1 2. The combination of LSD1 inhibitor and anti-PD-L1 can significantly enhance the effect of inhibiting tumor growth | [35] |
| HCC | Positive | PD-L1 | 1. LSD1 interacts with myocyte enhancer Factor 2D (MEF2D) to reduce its methylation, causing demethylated MEF2D to bind to the promoter of PD-L1 and activate its expression 2. miR-329-3p targets LSD1 mRNA and reduces its expression to inhibit the expression of PD-L1, thereby enhancing the lethal effect of T cells on HCC cells | [149] |
| GC | Positive | PD-L1 | 1. Both inhibition of LSD1 and decrease of LSD1 expression could decrease the level of PD-L1 in GC cells 2. Loss of LSD1 inhibits tumor growth by reducing exosome PD-L1 and increasing T cell activity | [33, 105-107] |
| LUAD | Positive | PD-L1 | 1. Inhibition of LSD1 reduces PD-L1 expression through JAK pathway, thereby inhibiting the proliferation, migration and tumor growth of LUAD cells | [150] |
| CC | Positive | CD47 | 1. The decrease of LSD1 increases the H3K4me2 level in CD47 promoter, which directly leads to the decrease of CD47 expression 2. LSD1/ wild-type p53/miR-34a signal axis targets the 3' untranslated region (3' utr) of CD47, which is involved in regulating the expression of CD47 3. The combination of LSD1 inhibitor and anti-CD47 treatment is more effective in inhibiting tumor growth than the single use strategy | [35] |
| OCCC/SCCOHT | / | CTLA-4 | 1. The combination of LSD1 inhibitor and anti-CTLA-4 significantly improved PBMC permeability in SWI/SNF mutant ovarian cancer | [144] |
SCLC: Small cell lung cancer; NSCLC: Non-small cell lung cancer; TNBC: Triple negative breast cancer; GC: Gastric cancer; CRC: Colorectal cancer; HNSCC: Head and Neck Squamous Cell Carcinoma; OCCC: Ovarian clear cell carcinoma; SCCOHT: Small cell carcinoma of the ovary hypercalcemic type; OSCC: Oral squamous cell carcinoma; HCC: Hepatocellular carcinoma; CC: Cervical cancer; LUAD: Lung adenocarcinoma
LSD1 plays a crucial role in tumor immunotherapy. A. In TNBC, inhibition of LSD1 resulted in increased expression of CCL5, CXCL9, CXCL10, and PD-L1, which attracted CD8+ T-cells into the TME and acted as killers. The combination of HCI-2509 and PD-1 antibodies further enhanced the therapeutic effect. B. Inhibition of LSD1 results in decreased expression of PD-L1 and CD47 on the surface of CC tumor cells. The therapeutic effect of LSD1 inhibitors is enhanced when used in conjunction with PD-L1 or CD47 antibodies. Lack of LSD1 results in decreased expression of PD-L1 in GC exosomes, increased T cell activity, and restoration of T cell ability to attack tumors. C. Inhibition of LSD1 in tumor cells enhanced CAR T cell cytotoxicity against neuroblastoma, whereas LSD1 inhibition in CD19-CAR T cells also promoted their proliferation rate and cytokine secretion.
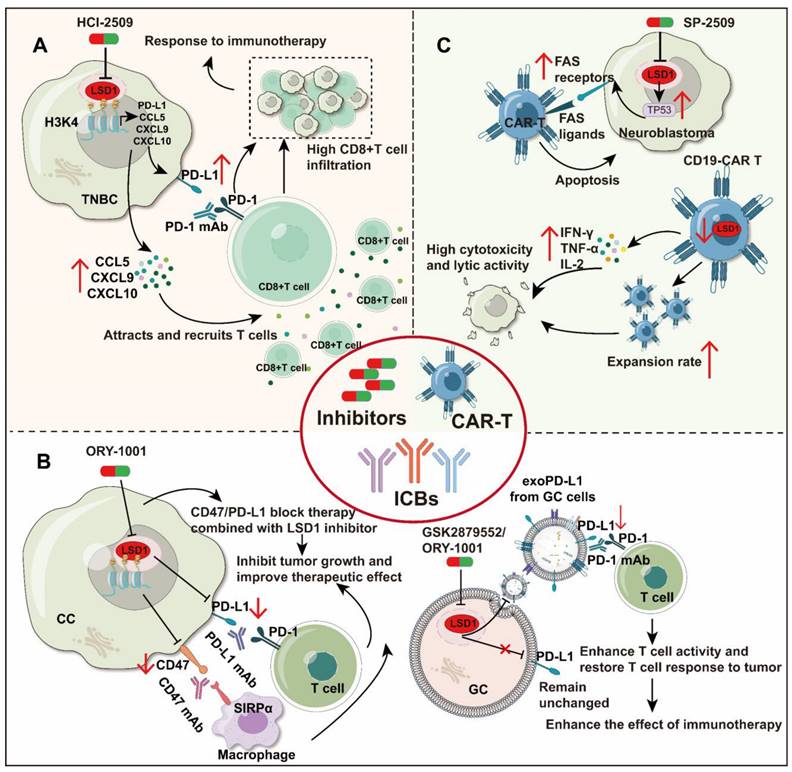
In addition, exosomal PD-L1 plays a pivotal role in ICB therapy. It inhibits cytokine production and promotes T-cell apoptosis, enabling tumors to evade immune system attacks. Therefore, reducing exosomal PD-L1 levels may enhance patient sensitivity to PD-L1/PD-1 therapy and enhance immune efficacy [153, 154]. In GC (Figure 4B), deletion of LSD1 reduces PD-L1 expression in exosomes but does not affect PD-L1 on the cell membrane of gastric cancer cells. Inhibition of LSD1 (GSK2879552 and ORY1001) prevents the translocation of exosomal PD-L1, improves T-cell activity, and restores T-cells to attack tumors, overcoming the immunosuppression [33]. Furthermore, the deletion of RNF20 was observed to render cancer cells more sensitive to PD-1 antibody in mouse breast cancer. However, ectopic expression of LSD1 was demonstrated to be capable of reversing this phenomenon [155]. This indicates that the combination of LSD1 inhibitors with PD-1 antibodies may hold therapeutic promise. Throughout the above report, an intriguing phenomenon has emerged: regardless of the regulatory relationship between LSD1 and PD-L1, the combined blockade of PD-(L)1 and inhibition of LSD1 has shown effectiveness in suppressing tumor growth across various cancer types. Table 3 provides a comprehensive overview of LSD1 inhibitors pivotal role in enhancing tumor immunotherapy. In conclusion, LSD1 inhibition has emerged as a potent adjunctive approach in immunotherapy, facilitating the conversion of "cold tumors" to "hot tumors" and mitigating immunotherapy resistance.
5.2.3 LSD1 inhibitors combined with CAR-T therapy
CAR-T cell therapy has made important breakthroughs in the field of immunotherapy, mainly applied to hematological malignancies such as lymphoma and leukemia [156]. However, scaling up CAR-T therapy to solid tumors remains a challenge. The efficacy of CAR-T therapy in solid tumors is limited by a variety of factors, such as antigen heterogeneity and loss, limited potency and persistence, poor infiltration ability, and TME inhibition [157]. Combining small molecule inhibitors with CAR-T cells treatment may be a promising new therapeutic strategy in solid tumors, and LSD1 is a good option for CAR-T cell combination therapy.
Studies have confirmed that inhibition or deletion of LSD1 can enhance the lethality of CAR-T cells to tumor cells [158, 159] (Figure 4C). In neuroblastoma, inhibiting LSD1 (SP-2509) in tumor cells can increase the expression of FAS receptors on the tumor cell surface by promoting TP53 mediated gene transcription activation. Therefore, FAS ligands on CAR-T cells can bind to FAS receptors on the tumor cell surface even without antigen expression, attacking tumor cells consequently [158]. Moreover, priming with LSD1 inhibitors (GSK-LSD1) increases the persistence and antitumor efficacy of human CD19-CAR T cells in both leukemia and melanoma models [101]. Meanwhile, in lymphoma, knocking down LSD1 in CD19-CAR T cells can also significantly improve the anti-tumor effect by maintaining the proliferation rate of CD19-CAR T cells, then promote their secretion of IFN-γ, tumor necrosis factor-α (TNF-α) and IL-2, and enhance cytotoxicity and lytic activity [159]. Thus, pharmacological inhibition of LSD1 could be exploited to improve adoptive T cell therapy (Table 3).
These studies show that the anti-tumor effect of CAR T cells can be improved by inhibiting LSD1 activity, which providing a new auxiliary strategy for improving the efficacy of CAR T cell therapy, and giving a new idea for CAR T cell design. However, the above findings need further clinical verification to determine the feasibility and safety of clinical application.
6. Conclusion and perspective
In this review, the applications of LSD1 in remodeling tumor immunity and its significant potential in tumor immunotherapy are comprehensively summarized. LSD1 has the unique ability to regulate both the innate and adaptive immune systems. An in-depth analysis of the literature indicates that LSD1 is a promising target for tumor immunoregulation and immunotherapy.
Currently, the significance of LSD1 inhibition in cancer therapy lies primarily in its multifaceted impact on the immune system. Here are the key points: (a) Innate immune effects: LSD1 regulates macrophage polarization and promotes their differentiation towards inflammatory phenotypes. Enhanced NK cell activity: Inhibiting LSD1 is anticipated to augment NK cell cytotoxicity against tumor cells, albeit the precise molecular mechanisms necessitate further investigation. (b) Adaptive immune regulation: LSD1 is pivotal in T cell-mediated immune processes. Specifically, LSD1 inhibitors can reinstate the expression of MHC-I molecules on tumor cell surfaces, thereby aiding in the activation and enhancement of T cell immune responses against tumors. (c) Immune checkpoint regulation: Inhibition of LSD1 can regulate the expression of PD-L1 on the surface of tumor cells, which is crucial for enhancing the efficacy of anti-PD-1/PD-L1 immunotherapy. Specifically, inhibiting LSD1 may mitigate tumor immune escape to T cells, making immune checkpoint inhibitor therapy more effective. In conclusion, targeted therapy against LSD1 holds significant promise in augmenting tumor immunogenicity, boosting immune cell activity, and optimizing immune checkpoint efficacy, thereby positioning it as a promising candidate for future synergistic immunotherapy strategies.
However, the research on targeting LSD1 in anti-tumor immunity still has a long way to go. For instance, various classes of LSD1 inhibitors exhibit opposite impacts on NK immunity. Scaffold LSD1 inhibitors (reversible) can induce toxicity in NK cells and impair the ability of NK cells to lysate tumor cells. Conversely, the irreversible LSD1 inhibitor can effectively enhance the lytic effect of NK cells on tumor cells, while with minimal toxicity to NK cells. This may be that reversible LSD1 inhibitors impair the metabolic function of NK cells. Hence, future studies on LSD1 regulation of NK cells should focus on the detrimental effects of reversible LSD1 inhibitors. Furthermore, in numerous human malignancies, T cell presence within tumor lesions correlates with improved patient prognosis. However, T cell infiltration is often poor in solid tumors, leading to suboptimal responses to T cell immunotherapy [161].
Application of LSD1 inhibitors in the field of tumor immunity
| Name | Structure | Type | Functions | References |
|---|---|---|---|---|
| GSK-LSD1 | 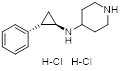 | Irreversible LSD1 inhibitor | 1. Promote CD8+ T cell infiltration and antitumor response 2. Stimulate antigen presentation and enhance T cell-mediated cytotoxicity 3. Enhanced intra-tumoral persistence of exhausted T cells 4. Enhance the persistence and antitumor efficacy of human CD19-CAR T cells. | [34, 94, 101] |
| ORY-1001 (Phase 1/2) | 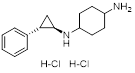 | Irreversible LSD1 inhibitor | 1. Increase the expression level of MHC-I on the surface of tumor cells, promoting the transcription of antigen presentation-related genes 2. Further stimulate the interferon signaling pathway to induce the tumor intrinsic immunogenicity 3. Combining LSD1 inhibitors with ICB therapy effectively enhances the immune response against treatment-resistant tumors | [35, 37, 103] |
| Bomedemstat (Phase 1/2) | 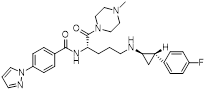 | Irreversible LSD1 inhibitor | 1. Increase the expression of MHC-I on the tumor cell surface, enhancing the induction of MHC-I by IFN-γ 2. Inhibition of LSD1 can enhance the sensitivity of tumors to PD-1 inhibitory responses, promoting CD8+ T cell infiltration and achieving strong tumor growth inhibition | [36] |
| Compound 3s | 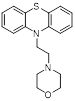 | Reversible LSD1 inhibitor | 1. Down-regulated PD-L1 on tumor cell surface 2. Promote T cell killing ability | [106] |
| SP2509 | 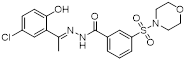 | Reversible LSD1 inhibitor | 1. Promote the increase of CD8+ T cell infiltration 2. Combined with anti-PD-(L)1 therapy, it can overcome tumor immune evasion and significantly inhibit tumor growth 3. Reduce the level of Ki-67 in tumors 4. Promote FAS ligands on CAR T cells to bind to FAS receptors of tumor cells and enhance their killing ability. | [99, 100, 148, 158] |
| SP-2577 (Phase 1/2) | 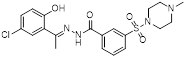 | Reversible LSD1 inhibitor | 1. Promote T cell infiltration | [144] |
| Phenelzine | 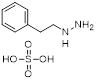 | Irreversible MAO inhibitor | 1. Combined with chemotherapy to reduce tumor volume and eliminate mesenchymal features 2. Promote the tumor-killing immune response of M1 macrophages | [78] |
| Tranylcypromine (Phase 1/2) |  | Irreversible MAO inhibitor | 1. The combination with PD-1 antibody significantly inhibited tumor growth and lung metastasis 2. Reduce the level of Ki-67 in tumors 3. Promote CD8+ T cell infiltration | [99] |
| Compound 6x | 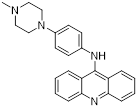 | Reversible LSD1 inhibitor | 1. Down-regulated PD-L1 on tumor cell surface 2. Promote T cell killing ability | [107] |
| Compound 5ac | 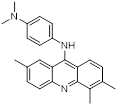 | Reversible LSD1 inhibitor | 1. Inhibit the stemness of tumor cells 2. Down-regulated PD-L1 on tumor cell surface 3. Promote T cell killing ability | [105] |
| Compound Z-1 | 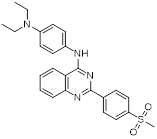 | Reversible LSD1 inhibitor | 1. Down-regulated PD-L1 on tumor cell surface 2. Promote T cell killing ability | [108] |
| GSK2879552 (Phase 1/2 terminated) | 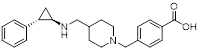 | Irreversible LSD1 inhibitor | 1. Prolong responses to PD-1 blockade 2. Promote CD8+ T cell infiltration 3. Sustain T cell invigoration | [102, 160] |
In the process of reviewing the literature, we have found that targeting LSD1 in combination with anti-PD-1/PD-L1 therapy not only enhances treatment efficacy but also promotes T cell infiltration and activation in the TME. Further studies have highlighted the controversial role of LSD1 in regulating the PD-1/PD-L1 axis across different cancer types. This may be linked to the complex role of LSD1 in the TME. PD-L1 expression is regulated by various signaling pathways, and LSD1 can influence its levels by targeting key nodes within these pathways. Additionally, different cancers have distinct TME and signaling networks, which may contribute to the variability in LSD1's effects. Thus, the potential of combining LSD1 inhibitors with ICBs in various cancer types warrants further discussion. In cancers where LSD1 and PD-L1 are positively feedback-regulated, such as GC and HCC, LSD1 inhibitors can reduce tumor immune escape by downregulating PD-L1 expression. When used in combination with ICBs, this may enhance T-cell activity and amplify the anti-tumor immune response by weakening the immunosuppressive TME. Conversely, in cancers where LSD1 and PD-L1 are negatively feedback-regulated, such as melanoma and NSCLC, high LSD1 expression inhibits PD-L1 antigen presentation, which results in a low immunotherapy response. In these cases, LSD1 inhibitors may restore the sensitivity and response to ICB therapy. This dual action could make combination therapy more effective in certain immunosuppressive TME. In addition, to this, LSD1 inhibitors may also indirectly affect the PD-1/PD-L1 signaling pathway by modulating other immune-related pathways, such as NK cell activity or macrophage polarization, but this is only one of our hypotheses. In this context, LSD1 inhibitors may synergize with ICBs to remodel the TME at multiple levels, thereby enhancing therapeutic efficacy. Moreover, macrophages play a crucial role in maintaining tissue homeostasis and the TME, exhibiting the highest infiltration rate among immune cells in solid tumors [162]. Research indicates that LSD1 inhibition promotes the inflammatory polarization of macrophages, potentially enhancing T cell infiltration and cytotoxicity. Consequently, combining LSD1 targeting with T cell immunotherapy holds promise for enhancing immunotherapy efficacy in solid tumors via highly infiltrated macrophages. Finally, despite the challenges posed by solid tumors to the expansion of CAR-T therapy applications, combining CAR-T therapy with immune checkpoint therapy has shown significant tumor inhibition in select cancer patients. However, variability in PD-1, PD-L1, and CTLA-4 expression levels limits the overall efficacy of CAR-T therapy combined with ICB therapy [163]. Meanwhile, TGF-β as a critical immunosuppressive factor for T cells, several clinical trials that TGF-β targeting combined with CAR-T therapy have been approved (NCT00889954, NCT04227275, NCT03089203, NCT03198546) [164-167]. Addressing this issue, LSD1 served as the upstream regulator of PD-L1 and TGF-β, and targeting LSD1 inhibition holds the potential to down-regulate both factors in certain cancer types. Therefore, it is anticipated and exciting to study whether the addition of LSD1 inhibitors to the dual-combination regimen that targeting TGF-β combined with CAR-T therapy will produce a better anti-tumor effect.
In general, this review systematically summarizes the role of LSD1 in tumor immunity from the perspective of innate immunity and adaptive immunity for the first time, highlighting LSD1's pivotal role as a bridge between these immune pathways. And this provides new perspectives and a theoretical foundation for exploring its potential applications in tumor immunotherapy.
Abbreviations
AML: Acute myeloid leukemia; APCs: Antigen-presenting cells; CoREST: REST corepressor 1; CAFs: Cancer-associated fibroblasts; C/EBPα: CCAAT/enhancer-binding protein α; CML: Chronic myelogenous leukemia; CD141Hi cDCs: CD141Hi conventional dendritic cells; CRC: Colorectal cancer; CC: Cervical cancer; DNMT1: DNA methyltransferase 1; DCs: Dendritic cells; DIPG: Diffuse intrinsic pontine glioma; dsRNA: Double-stranded RNA; E2F1: E2F transcription factor 1; EMT: Epithelial-mesenchymal transition; FAD: Flavin adenine dinucleotide; GC: Gastric cancer; HDAC1/2: Histone deacetylase 1 and 2; H3K4me1/me2: Monomethyl and dimethyl histone H3 lysine 4; HIF-1α: Hypoxia-inducible factor-1; HNSCC: Head and neck squamous cell carcinoma; HCC: Hepatocellular carcinoma; ICB: Immune checkpoint blocking; IL-6: Interleukin-6; IL-4: Interleukin-4; IRF8: Interferon regulatory factor-8; IFN: Interferon; ISGs: IFN-stimulating genes; LSD1: Histone lysine-specific demethylase 1; LUAD: Lung adenocarcinoma; MHC: Major histocompatibility complex; MDS: Myelodysplastic syndromes; MSCs: Mesenchymal stem cells; MM: Multiple myeloma; MEF2D: Myocyte enhancer factor 2D; NK: Natural killer cells; NSCLC: Non-small cell lung cancer; OCCC: Ovarian clear cell carcinoma; OSCC: Oral squamous cell carcinoma; PRRs: Pattern recognition receptors; PC: Prostate cancer; RISC: RNA-induced silencing complex; STAT3: Signal transducer and activator of transcription 3; SCLC: Small cell lung cancer; SCCOHT: Small cell carcinoma of the ovary hypercalcemic type; TME: Tumor microenvironment; TAM: Tumor-associated macrophages; TNBC: Triple-negative breast cancer; TCR: T cell receptor; TNBC: Triple negative breast cancer; TGF-β: Transforming growth factor-β; Treg: Regulatory T; TNF-α: Tumor necrosis factor-α; ULBPs: UL16-binding protein; VEGF: Vascular endothelial growth factor; NE: Neuroendocrine.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 22477114 to YCZ); Basic Research of the Key Project of the High Education from the Education Department of Henan Province (No. 22ZX008 to YCZ, China); National Natural Science Foundation of China (No. U21A20416 and 82020108030 to HML, China); the Youth Supporting Program from Henan Province (No. 2021HYTP060 to YCZ, China); the Young Top Talent Program from Henan Association for Science and Technology; R&D of Key Project of Henan Province (No. 241111312500 to YCZ, China); and the China Postdoctoral Science Foundation (No. 2021M702942 to LJZ, China); National Natural Science Foundation of China (No. 82102937 to PLS, China); Health Commission of Henan Province (No. YXKC2021026 to PLS, China).
Author contributions
Yu Zhang: Writing - original draft, Writing - review & editing. Ningjie Guo, Haoyi Zhu, Mengyang Liu, Jiahui Hao, Shoukai Wang, Ting Guo, M. A. A. Mamun, Jingru Pang, and Qi Liu collected the related papers and drafted the manuscript. Yichao Zheng, Hongmin Liu, Pilei Si and Lijuan Zhao - Supervision & founding acquisition. Pilei Si and Lijuan Zhao Writing - review & editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Heras-Murillo I, Adán-Barrientos I, Galán M, Wculek SK, Sancho D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat Rev Clin Oncol. 2024;21:257-77
2. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nature Immunology. 2013;14:1014-22
3. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annual Review of Immunology. 2011;29:235-71
4. Maiorino L, Daßler-Plenker J, Sun L, Egeblad M. Innate Immunity and Cancer Pathophysiology. Annu Rev Pathol. 2022;17:425-57
5. Guo S, Deng CX. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int J Biol Sci. 2018;14:2083-93
6. Laplane L, Duluc D, Larmonier N, Pradeu T, Bikfalvi A. The Multiple Layers of the Tumor Environment. Trends Cancer. 2018;4:802-9
7. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-37
8. de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374-403
9. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. Journal of Hematology & Oncology. 2019;12:76
10. Ocaña MC, Martínez-Poveda B, Quesada AR, Medina M. Metabolism within the tumor microenvironment and its implication on cancer progression: An ongoing therapeutic target. Med Res Rev. 2019;39:70-113
11. Dysthe M, Parihar R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1224:117-40
12. Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739-52
13. Batlle E, Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924-40
14. Chan LC, Li CW, Xia W, Hsu JM, Lee HH, Cha JH. et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J Clin Invest. 2019;129:3324-38
15. Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L. et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790-802
16. Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S. et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011;20:781-96
17. Wang H, Zhou Z, Zhang J, Hao T, Wang P, Wu P. et al. Pumilio1 regulates NPM3/NPM1 axis to promote PD-L1-mediated immune escape in gastric cancer. Cancer Lett. 2024;581:216498
18. Wang B, Zhou Y, Zhang J, Jin X, Wu H, Huang H. Fructose-1,6-bisphosphatase loss modulates STAT3-dependent expression of PD-L1 and cancer immunity. Theranostics. 2020;10:1033-45
19. Dhatchinamoorthy K, Colbert JD, Rock KL. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol. 2021;12:636568
20. Garrido F, Aptsiauri N. Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology. 2019;158:255-66
21. Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20:209-15
22. Jiang C, Zhang N, Hu X, Wang H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol Cancer. 2021;20:117
23. Culhane JC, Cole PA. LSD1 and the chemistry of histone demethylation. Curr Opin Chem Biol. 2007;11:561-8
24. Gu F, Lin Y, Wang Z, Wu X, Ye Z, Wang Y. et al. Biological roles of LSD1 beyond its demethylase activity. Cell Mol Life Sci. 2020;77:3341-50
25. Perillo B, Tramontano A, Pezone A, Migliaccio A. LSD1: more than demethylation of histone lysine residues. Exp Mol Med. 2020;52:1936-47
26. Zhao LJ, Fan QQ, Li YY, Ren HM, Zhang T, Liu S. et al. LSD1 deletion represses gastric cancer migration by upregulating a novel miR-142-5p target protein CD9. Pharmacol Res. 2020;159:104991
27. Yuan C, Li Z, Qi B, Zhang W, Cheng J, Wang Y. High expression of the histone demethylase LSD1 associates with cancer cell proliferation and unfavorable prognosis in tongue cancer. J Oral Pathol Med. 2015;44:159-65
28. Sakamoto A, Hino S, Nagaoka K, Anan K, Takase R, Matsumori H. et al. Lysine Demethylase LSD1 Coordinates Glycolytic and Mitochondrial Metabolism in Hepatocellular Carcinoma Cells. Cancer Research. 2015;75:1445-56
29. Battaglia S, Karasik E, Gillard B, Williams J, Winchester T, Moser MT. et al. LSD1 dual function in mediating epigenetic corruption of the vitamin D signaling in prostate cancer. Clin Epigenetics. 2017;9:82
30. Liu J, Feng J, Li L, Lin L, Ji J, Lin C. et al. Arginine methylation-dependent LSD1 stability promotes invasion and metastasis of breast cancer. EMBO Rep. 2020;21:e48597
31. Mamun MAA, Zhang Y, Zhao J-Y, Shen D-D, Guo T, Zheng Y-C. et al. LSD1: an emerging face in altering the tumor microenvironment and enhancing immune checkpoint therapy. Journal of Biomedical Science. 2023;30:60
32. Fang Y, Liao G, Yu B. LSD1/KDM1A inhibitors in clinical trials: advances and prospects. Journal of Hematology & Oncology. 2019;12:129
33. Shen DD, Pang JR, Bi YP, Zhao LF, Li YR, Zhao LJ. et al. LSD1 deletion decreases exosomal PD-L1 and restores T-cell response in gastric cancer. Mol Cancer. 2022;21:75
34. Sheng W, LaFleur MW, Nguyen TH, Chen S, Chakravarthy A, Conway JR. et al. LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade. Cell. 2018;174:549-63.e19
35. Xu S, Wang X, Yang Y, Li Y, Wu S. LSD1 silencing contributes to enhanced efficacy of anti-CD47/PD-L1 immunotherapy in cervical cancer. Cell Death Dis. 2021;12:282
36. Hiatt JB, Sandborg H, Garrison SM, Arnold HU, Liao SY, Norton JP. et al. Inhibition of LSD1 with bomedemstat sensitizes small cell lung cancer to immune checkpoint blockade and T cell killing. Clin Cancer Res. 2022;28:4551-4564
37. Nguyen EM, Taniguchi H, Chan JM, Zhan YA, Chen X, Qiu J. et al. Targeting Lysine-Specific Demethylase 1 Rescues Major Histocompatibility Complex Class I Antigen Presentation and Overcomes Programmed Death-Ligand 1 Blockade Resistance in SCLC. J Thorac Oncol. 2022;17:1014-31
38. Cai W, Xiao C, Fan T, Deng Z, Wang D, Liu Y. et al. Targeting LSD1 in cancer: Molecular elucidation and recent advances. Cancer Lett. 2024;598:217093
39. Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941-53
40. Saleque S, Kim J, Rooke HM, Orkin SH. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol Cell. 2007;27:562-72
41. Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19:857-64
42. Forneris F, Binda C, Battaglioli E, Mattevi A. LSD1: oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem Sci. 2008;33:181-9
43. Forneris F, Binda C, Vanoni MA, Battaglioli E, Mattevi A. Human histone demethylase LSD1 reads the histone code. J Biol Chem. 2005;280:41360-5
44. Forneris F, Binda C, Vanoni MA, Mattevi A, Battaglioli E. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005;579:2203-7
45. Lan F, Nottke AC, Shi Y. Mechanisms involved in the regulation of histone lysine demethylases. Curr Opin Cell Biol. 2008;20:316-25
46. Yang GJ, Lei PM, Wong SY, Ma DL, Leung CH. Pharmacological Inhibition of LSD1 for Cancer Treatment. Molecules. 2018;23:3194
47. Duteil D, Tosic M, Schüle R. Lsd1, a metabolic sensor of environment requirements that prevents adipose tissue from aging. Adipocyte. 2017;6:298-303
48. Ambrosio S, Saccà CD, Majello B. Epigenetic regulation of epithelial to mesenchymal transition by the Lysine-specific demethylase LSD1/KDM1A. Biochim Biophys Acta Gene Regul Mech. 2017;1860:905-10
49. Li Q, Shi L, Gui B, Yu W, Wang J, Zhang D. et al. Binding of the JmjC demethylase JARID1B to LSD1/NuRD suppresses angiogenesis and metastasis in breast cancer cells by repressing chemokine CCL14. Cancer Res. 2011;71:6899-908
50. Zhang W, Ruan X, Li Y, Zhi J, Hu L, Hou X. et al. KDM1A promotes thyroid cancer progression and maintains stemness through the Wnt/β-catenin signaling pathway. Theranostics. 2022;12:1500-17
51. Chen L, Xu Y, Xu B, Deng H, Zheng X, Wu C. et al. Over-expression of lysine-specific demethylase 1 predicts tumor progression and poor prognosis in human esophageal cancer. Int J Clin Exp Pathol. 2014;7:8929-34
52. Jie D, Zhongmin Z, Guoqing L, Sheng L, Yi Z, Jing W. et al. Positive expression of LSD1 and negative expression of E-cadherin correlate with metastasis and poor prognosis of colon cancer. Dig Dis Sci. 2013;58:1581-9
53. Lim S, Janzer A, Becker A, Zimmer A, Schüle R, Buettner R. et al. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31:512-20
54. Liu YD, Dai M, Yang SS, Xiao M, Meng FL, Chen XW. Overexpression of Lysine-Specific Demethylase 1 Is Associated With Tumor Progression and Unfavorable Prognosis in Chinese Patients With Endometrioid Endometrial Adenocarcinoma. Int J Gynecol Cancer. 2015;25:1453-60
55. Nagasawa S, Sedukhina AS, Nakagawa Y, Maeda I, Kubota M, Ohnuma S. et al. LSD1 overexpression is associated with poor prognosis in basal-like breast cancer, and sensitivity to PARP inhibition. PLoS One. 2015;10:e0118002
56. Zhao ZK, Yu HF, Wang DR, Dong P, Chen L, Wu WG. et al. Overexpression of lysine specific demethylase 1 predicts worse prognosis in primary hepatocellular carcinoma patients. World J Gastroenterol. 2012;18:6651-6
57. Chen Y, Jie W, Yan W, Zhou K, Xiao Y. Lysine-specific histone demethylase 1 (LSD1): A potential molecular target for tumor therapy. Crit Rev Eukaryot Gene Expr. 2012;22:53-9
58. Lynch JT, Harris WJ, Somervaille TC. LSD1 inhibition: a therapeutic strategy in cancer? Expert Opin Ther Targets. 2012;16:1239-49
59. Ambrosio S, Ballabio A, Majello B. Histone methyl-transferases and demethylases in the autophagy regulatory network: the emerging role of KDM1A/LSD1 demethylase. Autophagy. 2019;15:187-96
60. Ma T, Li A, Guo Y, Li S, Li M, Feng S. et al. KDM1A/LSD1 as a promising target in various diseases treatment by regulating autophagy network. Biomed Pharmacother. 2022;148:112762
61. Sheng W, Liu Y, Chakraborty D, Debo B, Shi Y. Simultaneous Inhibition of LSD1 and TGFβ Enables Eradication of Poorly Immunogenic Tumors with Anti-PD-1 Treatment. Cancer Discov. 2021;11:1970-81
62. Hong Y, Li X, Zhu J. LSD1-mediated stabilization of SEPT6 protein activates the TGF-β1 pathway and regulates non-small-cell lung cancer metastasis. Cancer Gene Ther. 2022;29:189-201
63. Li Y, Tian X, Sui C-G, Jiang Y-H, Liu Y-P, Meng F-D. Interference of lysine-specific demethylase 1 inhibits cellular invasion and proliferation in vivo in gastric cancer MKN-28 cells. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2016;82:498-508
64. Saccà CD, Gorini F, Ambrosio S, Amente S, Faicchia D, Matarese G. et al. Inhibition of lysine-specific demethylase LSD1 induces senescence in Glioblastoma cells through a HIF-1α-dependent pathway. Biochim Biophys Acta Gene Regul Mech. 2019;1862:535-46
65. Yang SJ, Park YS, Cho JH, Moon B, An HJ, Lee JY. et al. Regulation of hypoxia responses by flavin adenine dinucleotide-dependent modulation of HIF-1α protein stability. Embo j. 2017;36:1011-28
66. Mora VP, Loaiza RA, Soto JA, Bohmwald K, Kalergis AM. Involvement of trained immunity during autoimmune responses. J Autoimmun. 2023;137:102956
67. Yu L-L, Xiao Q, Yu B, Lv Q-L, Liu Z-Q, Yin J-Y. CircRNAs in tumor immunity and immunotherapy: Perspectives from innate and adaptive immunity. Cancer Lett. 2023;564:216219
68. Guillerey C. NK Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1273:69-90
69. Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. NK Cell Metabolism and Tumor Microenvironment. Front Immunol. 2019;10:2278
70. Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S. et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. 2018;172:1022-1037.e14
71. Liu M, Du M, Yu J, Qian Z, Gao Y, Pan W. et al. CEBPA mutants down-regulate AML cell susceptibility to NK-mediated lysis by disruption of the expression of NKG2D ligands, which can be restored by LSD1 inhibition. Oncoimmunology. 2022;11:2016158
72. Bailey CP, Figueroa M, Gangadharan A, Yang Y, Romero MM, Kennis BA. et al. Pharmacologic inhibition of lysine-specific demethylase 1 as a therapeutic and immune-sensitization strategy in pediatric high-grade glioma. Neuro Oncol. 2020;22:1302-14
73. Bailey CP, Figueroa M, Gangadharan A, Lee DA, Chandra J. Scaffolding LSD1 Inhibitors Impair NK Cell Metabolism and Cytotoxic Function Through Depletion of Glutathione. Front Immunol. 2020;11:2196
74. Dai X, Lu L, Deng S, Meng J, Wan C, Huang J. et al. USP7 targeting modulates anti-tumor immune response by reprogramming Tumor-associated Macrophages in Lung Cancer. Theranostics. 2020;10:9332-47
75. Wu Y-T, Fang Y, Wei Q, Shi H, Tan H, Deng Y. et al. Tumor-targeted delivery of a STING agonist improvescancer immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2022;119:e2214278119
76. Xue G, Wang Z, Zheng N, Fang J, Mao C, Li X. et al. Elimination of acquired resistance to PD-1 blockade via the concurrent depletion of tumour cells and immunosuppressive cells. Nature Biomedical Engineering. 2021;5:1306-19
77. Tan AHY, Tu W, McCuaig R, Hardy K, Donovan T, Tsimbalyuk S. et al. Lysine-Specific Histone Demethylase 1A Regulates Macrophage Polarization and Checkpoint Molecules in the Tumor Microenvironment of Triple-Negative Breast Cancer. Frontiers In Immunology. 2019;10:1351
78. Boulding T, McCuaig RD, Tan A, Hardy K, Wu F, Dunn J. et al. LSD1 activation promotes inducible EMT programs and modulates the tumour microenvironment in breast cancer. Sci Rep. 2018;8:73
79. Yang RF, Zhao GW, Liang ST, Chen HZ, Liu DP. Lysine-specific demethylase 1 represses THP-1 monocyte-to-macrophage differentiation. Chin Med Sci J. 2013;28:82-7
80. Huang Z, Efthymiadou A, Liang N, Fan R, Treuter E. Antagonistic action of GPS2 and KDM1A at enhancers governs alternative macrophage activation by interleukin 4. Nucleic Acids Res. 2023;51:1067-86
81. Díez-Sánchez A, Lindholm HT, Vornewald PM, Ostrop J, Yao R, Single AB. et al. LSD1 drives intestinal epithelial maturation and controls small intestinal immune cell composition independent of microbiota in a murine model. Nature Communications. 2024;15:3412
82. Soldani C, De Simone G, Polidoro MA, Morabito A, Franceschini B, Colombo FS. et al. Riboflavin-LSD1 axis participates in the in vivo tumor-associated macrophage morphology in human colorectal liver metastases. Cancer Immunol Immunother. 2024;73:63
83. Sobczak M, Strachowska M, Gronkowska K, Karwaciak I, Pułaski Ł, Robaszkiewicz A. LSD1 Facilitates Pro-Inflammatory Polarization of Macrophages by Repressing Catalase. Cells. 2021;10:2465
84. Srivastava P, Tzetzo SL, Gomez EC, Eng KH, Jani Sait SN, Kuechle JB. et al. Inhibition of LSD1 in MDS progenitors restores differentiation of CD141(Hi) conventional dendritic cells. Leukemia. 2020;34:2460-72
85. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792-804
86. Liu C, Liu L, Chen X, Cheng J, Zhang H, Zhang C. et al. LSD1 Stimulates Cancer-Associated Fibroblasts to Drive Notch3-Dependent Self-Renewal of Liver Cancer Stem-like Cells. Cancer Research. 2018;78:938-49
87. Pan X, Li J, Tu X, Wu C, Liu H, Luo Y. et al. Lysine-specific demethylase-1 regulates fibroblast activation in pulmonary fibrosis via TGF-β1/Smad3 pathway. Pharmacological Research. 2020;152:104592
88. Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023;56:2188-205
89. Castro CD, Luoma AM, Adams EJ. Coevolution of T-cell receptors with MHC and non-MHC ligands. Immunol Rev. 2015;267:30-55
90. He Q, Jiang X, Zhou X, Weng J. Targeting cancers through TCR-peptide/MHC interactions. J Hematol Oncol. 2019;12:139
91. Dusenbery AC, Maniaci JL, Hillerson ND, Dill EA, Bullock TN, Mills AM. MHC Class I Loss in Triple-negative Breast Cancer: A Potential Barrier to PD-1/PD-L1 Checkpoint Inhibitors. Am J Surg Pathol. 2021;45:701-7
92. Garrido C, Paco L, Romero I, Berruguilla E, Stefansky J, Collado A. et al. MHC class I molecules act as tumor suppressor genes regulating the cell cycle gene expression, invasion and intrinsic tumorigenicity of melanoma cells. Carcinogenesis. 2012;33:687-93
93. Mahadevan NR, Knelson EH, Wolff JO, Vajdi A, Saigí M, Campisi M. et al. Intrinsic Immunogenicity of Small Cell Lung Carcinoma Revealed by Its Cellular Plasticity. Cancer Discov. 2021;11:1952-69
94. Zhou Z, Van der Jeught K, Fang Y, Yu T, Li Y, Ao Z. et al. An organoid-based screen for epigenetic inhibitors that stimulate antigen presentation and potentiate T-cell-mediated cytotoxicity. Nat Biomed Eng. 2021;5:1320-35
95. Mardani F, Saad W, El-Hachem N, Bikorimana JP, Kurdi M, Shammaa R. et al. LSD1 Inhibition Enhances the Immunogenicity of Mesenchymal Stromal Cells by Eliciting a dsRNA Stress Response. Cells. 2022;11:1816
96. Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17:349-62
97. Suzuki J, Maruyama S, Tamauchi H, Kuwahara M, Horiuchi M, Mizuki M. et al. Gfi1, a transcriptional repressor, inhibits the induction of the T helper type 1 programme in activated CD4 T cells. Immunology. 2016;147:476-87
98. Gu T, Vasilatos SN, Yin J, Qin Y, Zhang L, Davidson NE. et al. Restoration of TFPI2 by LSD1 inhibition suppresses tumor progression and potentiates antitumor immunity in breast cancer. Cancer Lett. 2024;600:217182
99. Qin Y, Vasilatos SN, Chen L, Wu H, Cao Z, Fu Y. et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene. 2019;38:390-405
100. Han Y, Xu S, Ye W, Wang Y, Zhang X, Deng J. et al. Targeting LSD1 suppresses stem cell-like properties and sensitizes head and neck squamous cell carcinoma to PD-1 blockade. Cell Death Dis. 2021;12:993
101. Qiu F, Jiang P, Zhang G, An J, Ruan K, Lyu X. et al. Priming with LSD1 inhibitors promotes the persistence and antitumor effect of adoptively transferred T cells. Nature Communications. 2024;15:4327
102. Liu Y, Debo B, Li M, Shi Z, Sheng W, Shi Y. LSD1 inhibition sustains T cell invigoration with a durable response to PD-1 blockade. Nat Commun. 2021;12:6831
103. Tang F, Lu C, He X, Lin W, Xie B, Gao X. et al. E3 ligase Trim35 inhibits LSD1 demethylase activity through K63-linked ubiquitination and enhances anti-tumor immunity in NSCLC. Cell Rep. 2023;42:113477
104. Li Y, Han M, Wei H, Huang W, Chen Z, Zhang T. et al. Id2 epigenetically controls CD8+ T-cell exhaustion by disrupting the assembly of the Tcf3-LSD1 complex. Cell Mol Immunol. 2024;21:292-308
105. Dai X-J, Liu Y, Wang N, Chen H-X, Wu J-W, Xiong X-P. et al. Novel acridine-based LSD1 inhibitors enhance immune response in gastric cancer. European Journal of Medicinal Chemistry. 2023;259:115684
106. Dai X-J, Zhao L-J, Yang L-H, Guo T, Xue L-P, Ren H-M. et al. Phenothiazine-Based LSD1 Inhibitor Promotes T-Cell Killing Response of Gastric Cancer Cells. J Med Chem. 2023;66:3896-916
107. Liu H-M, Xiong X-P, Wu J-W, Chen H-X, Zhou Y, Ji S-K. et al. Discovery of acridine-based LSD1 inhibitors as immune activators targeting LSD1 in gastric cancer. European Journal of Medicinal Chemistry. 2023;251:115255
108. Wang B, Wang S-W, Zhou Y, Wang S-P, Gao Y, Liu H-M. et al. Discovery of 2-Aryl-4-aminoquinazolin-Based LSD1 Inhibitors to Activate Immune Response in Gastric Cancer. J Med Chem. 2024;67:16165-84
109. Laumont CM, Nelson BH. B cells in the tumor microenvironment: Multi-faceted organizers, regulators, and effectors of anti-tumor immunity. Cancer Cell. 2023;41:466-89
110. Haines RR, Barwick BG, Scharer CD, Majumder P, Randall TD, Boss JM. The Histone Demethylase LSD1 Regulates B Cell Proliferation and Plasmablast Differentiation. J Immunol. 2018;201:2799-811
111. Su S-T, Ying H-Y, Chiu Y-K, Lin F-R, Chen M-Y, Lin K-I. Involvement of histone demethylase LSD1 in Blimp-1-mediated gene repression during plasma cell differentiation. Mol Cell Biol. 2009;29:1421-31
112. Hatzi K, Geng H, Doane AS, Meydan C, LaRiviere R, Cardenas M. et al. Histone demethylase LSD1 is required for germinal center formation and BCL6-driven lymphomagenesis. Nat Immunol. 2019;20:86-96
113. Bandini C, Mereu E, Paradzik T, Labrador M, Maccagno M, Cumerlato M. et al. Lysin (K)-specific demethylase 1 inhibition enhances proteasome inhibitor response and overcomes drug resistance in multiple myeloma. Exp Hematol Oncol. 2023;12:71
114. Wei X, Calvo-Vidal MN, Chen S, Wu G, Revuelta MV, Sun J. et al. Germline Lysine-Specific Demethylase 1 (LSD1/KDM1A) Mutations Confer Susceptibility to Multiple Myeloma. Cancer Research. 2018;78:2747-59
115. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807-21
116. Leonard WJ, Lin J-X. Strategies to therapeutically modulate cytokine action. Nat Rev Drug Discov. 2023;22:827-54
117. Cambier S, Gouwy M, Proost P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol. 2023;20:217-51
118. Kirchhammer N, Trefny MP, Auf der Maur P, Läubli H, Zippelius A. Combination cancer immunotherapies: Emerging treatment strategies adapted to the tumor microenvironment. Sci Transl Med. 2022;14:eabo3605
119. Dwyer BJ, Macmillan MT, Brennan PN, Forbes SJ. Cell therapy for advanced liver diseases: Repair or rebuild. J Hepatol. 2021;74:185-99
120. Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD. et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3:911-26
121. Kang S, Narazaki M, Metwally H, Kishimoto T. Historical overview of the interleukin-6 family cytokine. J Exp Med. 2020;217:e20190347
122. Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2:194-9
123. Siveen KS, Prabhu K, Krishnankutty R, Kuttikrishnan S, Tsakou M, Alali FQ. et al. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumour Vascularization: Potential and Challenges. Curr Vasc Pharmacol. 2017;15:339-51
124. Vilgelm AE, Richmond A. Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front Immunol. 2019;10:333
125. Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220-7
126. Mao J, Li J, Chen J, Wen Q, Cao M, Zhang F. et al. CXCL10 and Nrf2-upregulated mesenchymal stem cells reinvigorate T lymphocytes for combating glioblastoma. J Immunother Cancer. 2023;11:e007481
127. Larson C, Oronsky B, Carter CA, Oronsky A, Knox SJ, Sher D. et al. TGF-beta: a master immune regulator. Expert Opin Ther Targets. 2020;24:427-38
128. Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65:599-608
129. Walshe TE. TGF-beta and microvessel homeostasis. Microvasc Res. 2010;80:166-73
130. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156-72
131. Hao Y, Baker D, Ten Dijke P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int J Mol Sci. 2019;20:2767
132. Xie F, Ling L, van Dam H, Zhou F, Zhang L. TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai). 2018;50:121-32
133. Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W. et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660-72
134. Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248-64
135. Niu M, Yi M, Wu Y, Lyu L, He Q, Yang R. et al. Synergistic efficacy of simultaneous anti-TGF-β/VEGF bispecific antibody and PD-1 blockade in cancer therapy. Journal of Hematology & Oncology. 2023;16:94
136. Zhou C, Ma L, Xu H, Huo Y, Luo J. Meningeal lymphatics regulate radiotherapy efficacy through modulating anti-tumor immunity. Cell Research. 2022;32:543-54
137. Pan HML. et al. shRNA-interfering LSD1 inhibits proliferation and invasion of gastric cancer cells via VEGF-C/PI3K/AKT signaling pathway. World journal of gastrointestinal oncology. 2019;11:622-33
138. Kashyap VA. et al. The lysine specific demethylase-1 (LSD1/KDM1A) regulates VEGF-A expression in prostate cancer. Molecular oncology. 2013;7:555-66
139. Lee JY, Park JH, Choi HJ, Won HY, Joo HS, Shin DH. et al. LSD1 demethylates HIF1α to inhibit hydroxylation and ubiquitin-mediated degradation in tumor angiogenesis. Oncogene. 2017;36:5512-21
140. Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675-705
141. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705-16
142. Strazza M, Mor A. The Complexity of Targeting Chemokines to Promote a Tumor Immune Response. Inflammation. 2020;43:1201-8
143. Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1-17
144. Soldi R, Ghosh Halder T, Weston A, Thode T, Drenner K, Lewis R. et al. The novel reversible LSD1 inhibitor SP-2577 promotes anti-tumor immunity in SWItch/Sucrose-NonFermentable (SWI/SNF) complex mutated ovarian cancer. PLoS One. 2020;15:e0235705
145. Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237-53
146. Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A. et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186:1652-69
147. Bally APR, Neeld DK, Lu P, Majumder P, Tang Y, Barwick BG. et al. PD-1 Expression during Acute Infection Is Repressed through an LSD1-Blimp-1 Axis. J Immunol. 2020;204:449-58
148. Alhousami T, Diny M, Ali F, Shin J, Kumar G, Kumar V. et al. Inhibition of LSD1 attenuates oral cancer development and promotes therapeutic efficacy of immune checkpoint blockade and Yap/Taz inhibition. Molecular cancer research: MCR. 2022;20:712-21
149. Wang Y, Cao K. KDM1A Promotes Immunosuppression in Hepatocellular Carcinoma by Regulating PD-L1 through Demethylating MEF2D. J Immunol Res. 2021;2021:9965099
150. He P, Du L, Hao P, Yang H, Ren Y, Kang H. et al. Inhibition of lysine-specific demethylase 1 (LSD1) prevented tumor growth and metastasis by downregulating PD-L1 expression in lung adenocarcinoma. Genes Dis. 2023;10:1779-82
151. Koerner J, Horvath D, Herrmann VL, MacKerracher A, Gander B, Yagita H. et al. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nature Communications. 2021;12:2935
152. Mei Y, Wang X, Zhang J, Liu D, He J, Huang C. et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat Cancer. 2023;4:1273-91
153. Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A. et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019;177:414-427.e13
154. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382-6
155. Dong B, Wang X, Song X, Wang J, Liu X, Yu Z. et al. RNF20 contributes to epigenetic immunosuppression through CDK9-dependent LSD1 stabilization. Proceedings of the National Academy of Sciences of the United States of America. 2024;121:e2307150121
156. Peng J-J, Wang L, Li Z, Ku C-L, Ho P-C. Metabolic challenges and interventions in CAR T cell therapy. Sci Immunol. 2023;8:eabq3016
157. Larson RC, Kann MC, Bailey SR, Haradhvala NJ, Llopis PM, Bouffard AA. et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature. 2022;604:563-70
158. Sulejmani O, Grunewald L, Andersch L, Schwiebert S, Klaus A, Winkler A. et al. Inhibiting Lysine Demethylase 1A Improves L1CAM-Specific CAR T Cell Therapy by Unleashing Antigen-Independent Killing via the FAS-FASL Axis. Cancers. 2021;13:5489
159. Zhang J, Zhu J, Zheng G, Wang Q, Li X, Feng Y. et al. Co-Expression of miR155 or LSD1 shRNA Increases the Anti-Tumor Functions of CD19 CAR-T Cells. Frontiers In Immunology. 2021;12:811364
160. Pallavicini I, Frasconi TM, Catozzi C, Ceccacci E, Tiberti S, Haas D. et al. LSD1 inhibition improves efficacy of adoptive T cell therapy by enhancing CD8+ T cell responsiveness. Nature Communications. 2024;15:7366
161. van der Leun AM, Thommen DS, Schumacher TN. CD8+ T cell states in human cancer: insights from single-cell analysis. Nature Reviews Cancer. 2020;20:218-32
162. Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462-72
163. Liu G, Rui W, Zhao X, Lin X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol Immunol. 2021;18:1085-95
164. Hung H-C, Fan M-H, Wang D, Miao CH, Su P, Liu C-L. Effect of chimeric antigen receptor T cells against protease-activated receptor 1 for treating pancreatic cancer. BMC Med. 2023;21:338
165. Qiao Y, Chen J, Wang X, Yan S, Tan J, Xia B. et al. Enhancement of CAR-T cell activity against cholangiocarcinoma by simultaneous knockdown of six inhibitory membrane proteins. Cancer Commun (Lond). 2023;43:788-807
166. Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF. et al. Dominant-Negative TGF-β Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation And Augments Prostate Cancer Eradication. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2018;26:1855-66
167. Narayan V, Barber-Rotenberg JS, Jung I-Y, Lacey SF, Rech AJ, Davis MM. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2022;28:724-34
Author contact
![]() Corresponding authors: Email: xiaoye1695651408com (Lijuan Zhao), siplei2013edu.cn (Pilei Si).
Corresponding authors: Email: xiaoye1695651408com (Lijuan Zhao), siplei2013edu.cn (Pilei Si).
 Global reach, higher impact
Global reach, higher impact