13.3
Impact Factor
Theranostics 2024; 14(11):4352-4374. doi:10.7150/thno.97301 This issue Cite
Review
Microfluidic chips in female reproduction: a systematic review of status, advances, and challenges
1. Department of Obstetrics and Gynecology, National Clinical Research Center for Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
2. Key Laboratory of Cancer Invasion and Metastasis (Ministry of Education), Hubei Key Laboratory of Tumor Invasion and Metastasis, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
3. School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan, China.
4. College of Animal Science and Technology, Sichuan Agricultural University, Sichuan, China.
Received 2024-4-13; Accepted 2024-7-6; Published 2024-7-15
Abstract
The female reproductive system is essential to women's health, human reproduction and societal well-being. However, the clinical translation of traditional research models is restricted due to the uncertain effects and low efficiency. Emerging evidence shows that microfluidic chips provide valuable platforms for studying the female reproductive system, while no paper has ever comprehensively discussed the topic. Here, a total of 161 studies out of 14,669 records are identified in PubMed, Scopus, Web of Science, ScienceDirect and IEEE Xplore databases. Among these, 61 studies focus on oocytes, which further involves culture, cell surgeries (oocyte separation, rotation, enucleation, and denudation), evaluation and cryopreservation. Forty studies investigate embryo manipulation via microfluidic chips, covering in vitro fertilization, cryopreservation and functional evaluation. Forty-six studies reconstitute both the physiological and pathological statuses of in vivo organs, mostly involved in placenta and fetal membrane research. Fourteen studies perform drug screening and toxicity testing. In this review, we summarize the current application of microfluidic chips in studying the female reproductive system, the advancements in materials and methods, and discuss the future challenges. The present evidence suggests that microfluidic chips-assisted reproductive system reconstruction is promising and more studies are urgently needed.
Keywords: female reproduction, microfluidic chip, ovary, engineering, fertility
Introduction
The female reproductive system (FRS), mainly comprising the ovary, fallopian tube, uterus, and vagina, plays a crucial role in affecting menstrual cycle, fertility, and pregnancy. The unique morphology and structure of these organs ensure their ability to fulfill individual functions and maintain reciprocal communications (Figure 1). For instance, the ovary synthesizes and secretes steroid hormones that regulate the uterine endometrium [1]. The fallopian tube is responsible for collecting ovulated matured oocytes and transporting zygotes to the uterine cavity. These years, much efforts have been made to advance the knowledge of the FRS, particularly pertaining to oocytes, embryos, and fetal-maternal interfaces. However, translational research to clinical practice faces challenges stemming from structural differences, deficient design, and inadequate reporting [2]. Of significant concern are the research modalities, like animal models, and two-dimensional (2D) in vitro culture approaches [3]. Animal experiments may cause pain, and require substantial labor. The interspecies differences and complexity of certain organs, such as the highly diverse placenta, make it unsuitable to employ animal models [4, 5]. As for 2D culture, its condition is significantly different from the in vivo microenvironment, thus distorting cellular morphology and behavior. Therefore, it is imperative to develop more efficient experimental methods to enhance translational research and promote women's health [6].
With the advancement of biotechnology, the microfluidic chip, also known as organ-on-a-chip, has proven to be more instrumental than traditional experimental approaches in regenerative medicine, drug screening, and toxicity testing. It effectively mimics the in vivo microenvironment by incorporating dynamic fluid flow, thus enabling the long-term survival of cells. Furthermore, it can be coupled with microscopes, electrode arrays, or sensors to support real-time imaging, high-throughput analysis, and biochemical testing, respectively (Figure 2) [7]. Nevertheless, the distinct characteristics of the FRS set it apart from other organs. First, oocytes and follicles are considerably larger than ordinary cells, and they gradually increase in size [8]. Second, the continuous developmental process from follicles to embryos is complicated to achieve. Third, the female reproductive tract undergoes dramatic morphological and biochemical changes in different estrus stages. Consequently, utilizing the microfluidics technique in the FRS holds great importance but remains a challenge.
In this review, we systematically searched five databases using a set of selected keywords based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We offer a comprehensive overview of the microfluidic chip's applications in the FRS, summarizing the functions, experimental designs, fabrication methods, and main findings. We further presented prospects and challenges for advancing microfluidic chips in this domain.
Methods
Data sources
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol [9], and was registered under the Open Science Frame REGISTRIES (ID: osf.io/a4dkn). A comprehensive search was systematically executed across PubMed, Scopus, Web of Science, ScienceDirect, and IEEE Xplore databases, with publication years spanning from January 2010 to December 2023. The search was not limited by language.
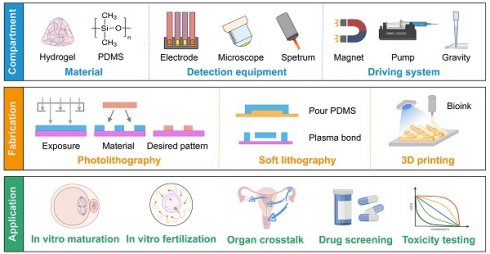
Histological images and schematics of human female reproductive organs. (A) The ovary comprises a central medulla and peripheral cortex. Oocytes are surrounded by granulosa cells and theca cells inside follicles, and their interactions are essential for hormonal secretion and gamete maturation. (B) The ampulla of the fallopian tube has many branching folds, and provides a site for fertilization. Secretory cells produce nutrients, while ciliated cells transport the fertilized egg towards the uterus. (C) The uterus plays a pivotal role in implantation and conceptus development. The uterine endometrium undergoes cyclic growth and regression under the control of ovarian hormones, including the menstrual, proliferative, and secretory phases. (D) The cervix can be divided into the endocervix, transformation zone, and ectocervix. It prevents microorganisms from entering, screens sperm, and lets menstrual blood flow out. (E) The vagina is covered by multiple squamous cell layers that allow bacterial colonization. Lactobacilli are the predominant vaginal microbiota. (F) The placenta-decidual interface mediates metabolic exchange between the developing fetus and the mother. In humans, the placenta consists of the trophoblastic epithelium covering the villi, the chorionic connective tissue and the capillary endothelium, which are all of fetal origin. The human histological images are reproduced with permission from the Human Protein Atlas, copyright 2023 (https://www.proteinatlas.org/humanproteome/tissue).
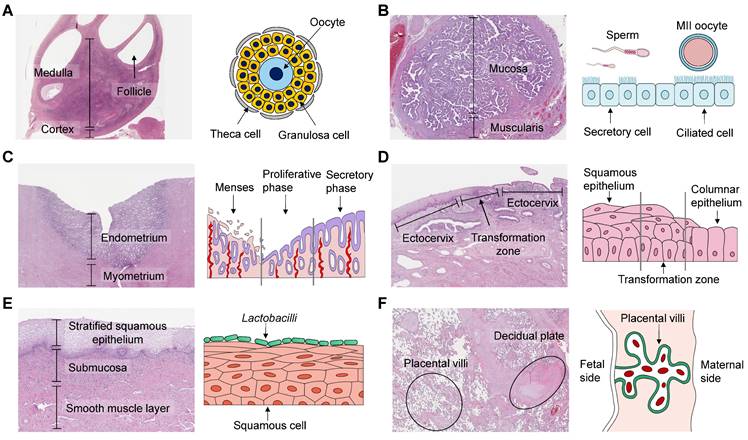
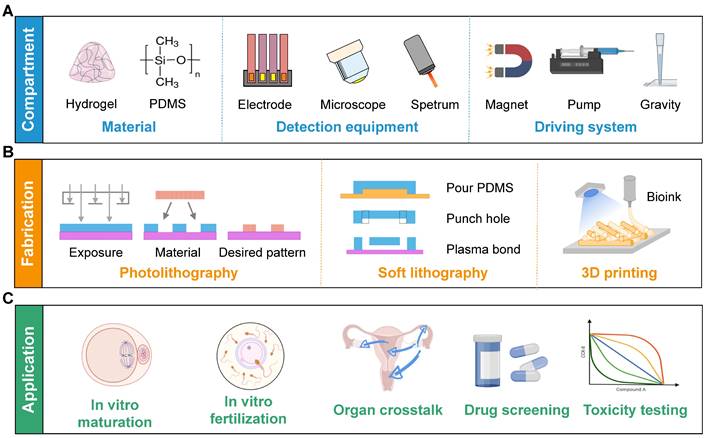
Diagrams of the compartments, fabrication methods, and applications of microfluidic chips in the FRS. (A) Hydrogel and PDMS are frequently used materials. The functions can be extended by various detection equipment. Continuous flow is achieved by a magnetic field, peristaltic pump, or gravity. (B) Commonly-used manufacturing processes include photolithography, soft lithography, and 3D printing. (C) The main applications focus on assisted reproduction techniques, organ crosstalk, drug screening and toxicity testing. Abbreviations: 3D: three-dimensional; FRS: female reproductive system; PDMS: polydimethylsiloxane.
Retrieval strategy
The search terms were formulated based on the widely recognized PICO (Population, Intervention, Comparison, Outcome) principle: (P) all models relevant to the FRS; (I) the microfluidic chip or organ-on-a-chip; (O) viability and function of the female reproductive tract cells, as well as the fabrication information. Eventually, we employed the following retrieval formula: (“ovary” OR “follicle” OR “oocyte” OR “fallopian tube” OR “oviduct” OR “uterus” OR “endometrium” OR “womb” OR “hystera” OR “vagina” OR “cervix” OR “embryo” OR “placenta” OR “fetal membrane” OR “amnion” OR “decidua”) AND (“microfluidic” OR “chip”) (Table S1).
Manually searching for references
While the initial database search is systematic and covers a wide range of sources, it is possible that some relevant studies might not be covered due to variations in indexing, keyword usage, or database scope. Therefore, additional papers were identified by manually looking through the reference lists of identified studies, as well as reviewing related articles that might not have been initially identified [10]. This approach ensures that no relevant research is overlooked, and that the systematic review is as comprehensive as possible.
Eligibility criteria
Documents were included based on the criteria: (i) accessible, peer-reviewed papers published between January 2010 and December 2023; (ii) use of microfluidic platforms or organ-on-a-chip for female reproductive studies; (iii) use of mammalian cells or tissues. Studies were excluded for the following reasons: (i) review papers, conference papers, commentaries, and communications; (ii) non-mammals; (iii) tumor research; (iv) ex vivo models using perfusion systems without chips; (v) mere computational models without experimental validation; (vi) irrelevant to the female reproduction tract. Two authors (T.W. and J.F.Y.) independently selected studies according to the above criteria. The titles and abstracts were manually examined, and the reasons for excluding or including the articles were recorded. Discrepancies between the two authors were discussed with a senior author (S.X.W.), who made the final decision.
Data extraction
Basic information included full titles, first authors, publication years, affiliations, countries, and keywords. Outcomes of interest included the animal models, fabrication methods, used biomaterials, chip designs, types of cells, key groups, and main findings. The above items were carefully reviewed by J.J.Z. and S.X.W.
Visualization and bibliometric analysis
We used the VOSviewer to analyze research papers in medicine, geology, and ecology [11]. All keywords (titles, abstracts, and authors' keywords) of eligible studies from the Web of Science database were used to analyze the co-occurrence. Keywords with the same meaning, such as “chip”, “microfluidic chip”, and “microfluidics” were combined into a single frequency. The word cloud was visualized by the OmicShare online tool.
Results
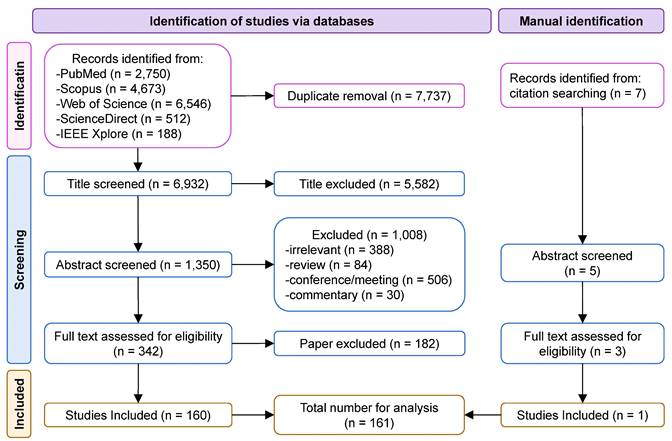
A flowchart of the search procedure is shown in Figure 3. The initial electronic database search resulted in 14,669 papers, of which 6,932 remained after removing duplicates. We excluded 5,582 and 1,008 papers after screening the title and abstract, respectively. The text of 342 studies was carefully assessed, and 182 studies that did not meet the inclusion criteria were excluded. An additional study was retrieved by manually searching the references. Finally, 161 papers were included as eligible for further analysis.
Characteristics of search results
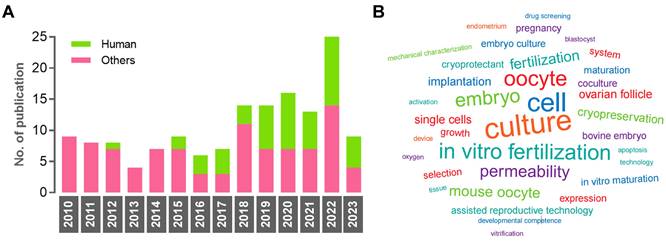
The number of documents showed an increasing trend between 2010 and 2023, while the percentage of studies using human samples fluctuated between 12.5% and 57.1% (Figure 4A). The numbers of non-human samples were as follows: mouse (n = 61), bovine (n = 19), and porcine (n = 15). The USA emerged as the leading country in contributing to this research area (n = 47, 30.3%), followed by China (n = 45, 29.0%) and Japan (n = 20, 12.9%). In terms of institutions, Nagoya University published the highest number of original articles (n = 13). The word cloud map revealed that the most frequently mentioned keywords included culture, cell, oocyte, in vitro fertilization, embryo, mouse oocyte, and permeability (Figure 4B).
In vitro culture and maturation of oocytes
The microfluidics used for oocyte culture and maturation were reported in nine studies (Table 1), employing denuded oocytes (n = 3) [12-14], cumulus-oocyte complexes (n = 3) [15-17], ovarian tissues (n = 2) [18, 19], and preantral follicles (n = 1) [20]. Among these, one study explored the impact of single and group settings on maturation by trapping varying numbers of oocytes (Figure 5A) [12]. Six studies conducted a comparative analysis of viability, diameter, and hormone levels between oocytes matured in microfluidic chips and those matured in traditional 2D dish or static culture systems. The effects of electric fields [13], flow rates [17-19], and collagen/alginate combinations [20] on oocyte growth were also examined.
Flow diagram of the systematic article selection.
Characteristics of the eligible studies. (A) Histogram of publication numbers showing the use of human samples between 2010 and 2023. (B) The research diversity is reflected by a word cloud. A larger font means the word is more frequently used.
In vitro culture and maturation of oocytes using microfluidic chips.
| Reference | Key group | Main finding |
|---|---|---|
| Oocyte | ||
| Choi et al. (2014) | ① Dish ② Chip + hydrogel encapsulation | Using alginate (harder) and collagen (softer) to investigate the mechanical heterogeneity during follicle development and ovulation. |
| Zargari et al. (2016) | Descriptive study without grouping. | Trapping oocytes and tracking the maturation process. |
| Aziz et al. (2017) | ① Dish ② Chip | Similar hormonal trends and similar diameters in ①②. |
| Berenguel-Alonso et al. (2017) | ① Dish ② Chip | No significant differences in nuclear and cytoplasmic maturation in ①②. |
| Huang et al. (2018) | Electric field activation with various duration and intensity. | Higher alternating current electric field improves the parthenogenesis of oocytes without sperm insemination. |
| Nagashima et al. (2018) | ① Dish ② Chip + static flow ③ Chip + various dynamic flow | No significant differences in follicle development markers among ①②③. |
| Healy et al. (2021) | ① 2D culture of ovarian somatic cells ② Chip + encapsulation | Higher androstenedione and lower progesterone in ② than ①. |
| Sadeghzadeh Oskouei et al. (2021) | ① Chip + static flow ② Chip + passive flow ③ Chip + active flow | Lower malondialdehyde concentration and fewer apoptotic cells in ②③ than ①. |
| Del Valle et al. (2022) | ① No treatment ② Chip + static flow ③ Chip + slow flow ④ Chip + dynamic flow | More apoptotic stromal cells in ④ than ①②③. Increased collagen deposition in ②③ than ①. |
Oocyte evaluation
Twenty-three studies aimed to evaluate the properties of oocytes (Table 2). Eight studies deformed oocytes to test their responses to mechanical stimuli [21-28]. Notably, only one study specially applied injection force [21], whereas the remaining seven studies used compression or suction force. Furthermore, six studies investigated cellular membrane permeability [29-34]. Three studies examined the structural characteristics [35-37], and the optical spectrum of oocytes (n = 3; Figure 5B) [38-40]. Less frequently reported properties were electrical impedance (n = 2) [41, 42], and oxygen concentration (n = 1) [43].
Cryopreservation of oocytes
Eight studies used microfluidic chips to minimize osmotic and toxic damages during cryopreservation (Table 3) [44-51]. Four studies employed a manual step-wise protocol to manage oocyte cryopreservation [44, 46, 47, 51]. Five studies compared different methods for loading and removing cryoprotectants (CPAs) [44, 45, 48-50], and found that concave loading and convex unloading of CPAs produced the most favorable results (Figure 5C) [50].
Cell surgeries
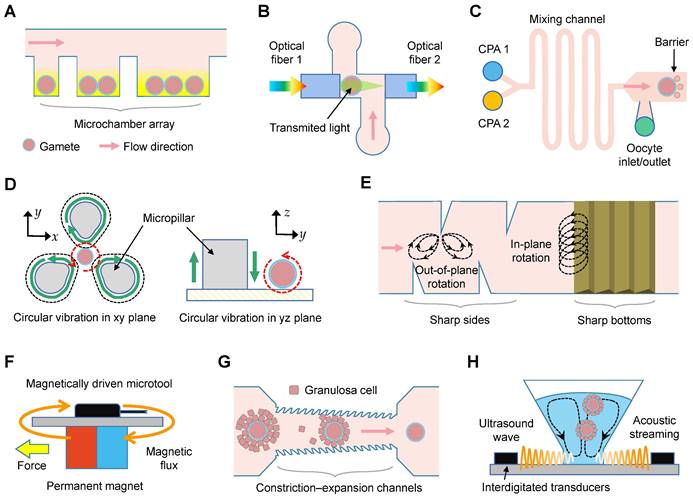
Twenty-one studies examined techniques for oocyte separation (n = 4; Table 4) [52-55], rotation (n = 8) [56-63], enucleation (n = 7) [64-70], and denudation (n = 2) [71, 72]. Three types of microfluidic devices were designed to isolate single oocyte based on optical sensor feedback [52, 68], sedimentation rate [54], or oocyte size [55]. Three studies successfully achieved precise oocyte orientation through permanent magnets and voltage-generated flow streaming [56, 58, 62], while microstructures within the chips were utilized in another four studies for the same purpose (Figure 5D-E) [59-61, 63]. Of the studies focusing on oocyte enucleation, two used electric field-induced driving forces [64, 70], and four used magnetically-driven microtools (Figure 5F) [65, 66, 68, 69]. Microfluidic devices to denudate oocytes within cumulus cells were included in two studies, using either microchannel constriction (Figure 5G) [71], or an acoustic radiation force (Figure 5H) [72].
Culture, observation, and manipulation of oocytes using microfluidics. (A) Oocytes are trapped in an array of microchambers for in vitro maturation. (B) Schematic of the microfluidics for spectrophotometric characterization. (C) Schematic showing the linear cryoprotectant (CPA) addition method using the microfluidics. (D) Oocyte rotation based on vibration-induced flow: (left) vertical plane rotation and (right) focal plane rotation. (E) Streaming is induced by the oscillations of sharp sides and bottoms. (F) Horizontal polar drive using magnetically driven microtools to precisely control oocytes. (G) Schematic of the constriction-expansion channels for oocyte denudation. The height of inlets is taller than the outlets, and constrictions are featured by jagged surface. (H) Streaming inside the microwells is induced by the ultrasound wave, which is produced by digital transducers, to shake and squeeze cells.
Applications of the microfluidics to observe and evaluate oocytes/embryos.
| Reference | Main finding | |
|---|---|---|
| Oocyte | ||
| Mechanics | Liu et al. (2010) | Distinguishing young and old oocytes real time through injection force measurement. |
| Arai and Sakuma (2015) | High-throughput oocyte mechanical characterization using a magnetically driven on-chip probe. | |
| Nakahara et al. (2015) | Simultaneous transportation and mechanical measurement of oocytes through a micropillar array in an open environment. | |
| Nakahara et al. (2018) | The Young's modulus of the zona pellucida of cryopreserved oocytes increases with cultivation time. | |
| Andolfi et al. (2019) | Quantitative estimate of the relaxation times of oocytes using planar atomic force microscopy macro-probes. | |
| Pokrzywnicka et al. (2019) | Oocyte quality classification according to the deformation change. | |
| Saffari et al. (2023) | Abnormal cortical tensions in oocytes lead to unsuccessful growth, and 79% of oocytes with tensions between 1.5 and 3 nN/μm reach the metaphase II stage. | |
| Azarkh et al. (2023) | Utilizing transient electrical impedance spectroscopy to detect the viscoelastic properties of zona pellucida. | |
| Permeability | Zhao et al. (2017) | Characteristics of oocyte permeability under different cryoprotectant and temperature conditions. |
| Chen et al. (2019) | High- and poor-quality oocytes are classified based on the membrane permeability. | |
| Lei et al. (2019) | Testing the membrane permeability of oocytes exposed to various cryoprotectants at different temperatures. | |
| Chen et al. (2020a) | Analysis of multiple oocytes volume under different types cryoprotectants and continuous concentration change. | |
| Guo et al. (2020) | Testing oocyte volume under different types and concentrations of cryoprotectants. | |
| Tu et al. (2022) | Monitoring the osmotic response of oocytes using the experimental and computational techniques. | |
| Structure | Angione et al. (2015) | Real-time, longitudinal imaging of oocytes following fluorescent labeling. |
| Luo et al. (2015) | Evaluating the spindle of oocytes via a constricted microfluidic channel. | |
| Podwin et al. (2020) | Evaluating the expansion ability of oocytes. | |
| Spectrum | Sniadek et al. (2011) | Dividing oocytes into two classes under microspectrometry. |
| Walczak et al. (2012) | Dividing oocytes into large, middle, and small groups using microspectrometry. | |
| Gorecka-Drzazga (2012) | Testing the optical signals of fluorescent-marked oocytes. | |
| Electricity | El Hasni et al. (2017) | Higher impedance for zona pellucida-free oocytes than for those with intact zona pellucida. |
| Cao et al. (2023a) | Calculating the Young's modulus of zona pellucida based on the micropipette aspiration technique. | |
| Oxygen | Tedjo et al. (2021) | Analysis of oxygen consumption rate and oxygen flux density of cumulus-oocyte complexes via an electrochemical sensor. |
| Embryo | ||
| Structure | Jang et al. (2010) | Observations via phase contrast imaging, double interferogram, and optical path length. |
| Bae et al. (2011) | The pressure and duration of applied mechanical stimulus compromise embryonic growth. | |
| Vandormael-Pournin et al. (2021) | An eggbox imaging device designed to observe preimplantation embryos. | |
| Mohagheghian et al. (2023) | Greater tensile and compressive traction force oscillations than shear traction force oscillations in mouse blastocyst. | |
| Viability | Śniadek et al. (2012) | Utilizing miniaturized fluorescence to discriminate between non-apoptotic and apoptotic embryos. |
| Sivelli et al. (2022) | Using nuclear magnetic resonance technology to observe single mammalian embryo for embryo transfer. | |
| Oxygen | Date et al. (2011) | The oxygen consumption increases from morula to blastocyst. |
| Kurosawa et al. (2016) | Estimating the oxygen consumption rate of spheroids, bovine embryos and frozen-thawed human embryos, and it corresponds to the developmental potential of embryos. | |
| Hormone | Heo et al. (2012) | Analysis of embryo glucose production using a mechanical deformation-based actuation. |
| Chen et al. (2020b) | Combination of microfluidic droplets and multicolor fluorescence to accurately detect hCG-β at single embryo level. | |
| Lee et al. (2020) | On-chip immunoassay for human interleukin-1β and TNF-α to distinguish the developmental embryos. | |
Abbreviations: hCG: human chorionic gonadotropin; TNF: tumor necrosis factor.
Oocytes and embryos cryopreservation using microfluidic chips.
| Reference | Group | Main finding |
|---|---|---|
| Oocyte | ||
| Heo et al. (2011) | ① Chip + step-wise ② Chip + linear ③ Chip + complex | Complete equilibration after cryoprotectants loading in <15 min, and with <10% oocyte volume changes. |
| Yang et al. (2012) | Different constant cooling rates | Slow freezing is feasible for cryopreservation of germinal vesicle porcine oocytes. |
| Lai et al. (2015) | ① No treatment ② Manual ③ Chip + gradual addition ④ Chip + abrupt sucrose ⑤ Direct addition | Higher cytoplasmic lipid retention, less cytoplasmic leakage and higher developmental competence in ③ than ②. |
| Zhou et al. (2018) | ① Manual ② Chip | Higher survival rate and cleavage rate of frozen oocytes in ② than ①. |
| Guo et al. (2019) | ① No treatment ② Manual + step-wise ③ Chip + linear | Less osmotic damage of oocytes in ③than ②. |
| Shao et al. (2019) | Different loading times, line types, and concave loading types | Novel parameters and entropy methods are proposed and are highly negative correlated with blastocysts rate. |
| Zhou et al. (2019) | Linear/convex/concave loading/unloading of CPA | The concave loading-convex unloading protocol shows the highest survival rate and morula rate. |
| Miao et al. (2022a) | ① Manual ② Chip | Comparable survival rate, mitochondrial membrane potential and reactive oxygen species levels in ①②. |
| Embryo | ||
| Pyne et al. (2014) | ① No treatment ② Manual ③ Chip | Embryo survival and development rates are comparable in ②③. |
| Tirgar et al. (2021) | ① Manual ② Chip | Comparable re-expansion, hatching rates, and reduced detrimental gene expression levels in ①②. |
| Miao et al. (2022b) | ① No treatment ② Manual ③ Chip | Comparable survival rates and development rates in ①②. |
| Miao et al. (2023) | ① Manual ② Chip | A satisfactory success rate, and high quality of vitrified embryos after thawing in ②. |
Applications of microfluidic chips to observe or manipulate oocytes.
| Reference | Main finding | |
|---|---|---|
| Separation | Kawahara et al. (2012) | Dispensing single oocytes through air-flow based inkjet mechanism. |
| Feng et al. (2013a) | A single enucleated oocyte dispensing system with a 100% success rate, and a 70% survival rate. | |
| Iwasaki et al. (2018) | Separating high-quality oocytes based on sedimentation rate differences for further in vitro fertilization. | |
| Uning et al. (2020) | Combination of a rack-pinion-based loader and a bubble injector to separate single oocyte with a >80% success rate. | |
| Rotation | Hagiwara et al. (2010) | Precise positioning of oocytes by horizontally arranged permanent magnets with two degrees of freedoms. |
| Hagiwara et al. (2012) | Attraction, repulsion, and rotation of oocytes are conducted by adjusting the oscillation parameters of micro-tools. | |
| Benhal et al. (2014) | Three-dimensional cell rotation on an alternating current induced electric field-based platform. | |
| Hayakawa et al. (2015) | Circular vibration induced by a micropillar via a piezoelectric actuator to rotate oocytes. | |
| Feng et al. (2016) | Three-dimensional rotation of a single oocyte with an accuracy of one degree and an average rotation velocity of three rad/s. | |
| Feng et al. (2018) | Trapping and rotation of oocytes using oscillating asymmetrical microstructures with different vibration modes. | |
| Feng et al. (2019) | Precise rotation from in-plane and out-of-plane using acoustic microstreaming generated by oscillating asymmetrical Microstructures. | |
| Bai et al. (2020) | Tunable trapping and rotation of oocytes in an acoustofluidic device. | |
| Enucleation | Clow et al. (2010) | Automated cell positioning by dielectrophoresis and nuclear transfer by electrofusion. |
| Hagiwara et al. (2011) | High-throughput enucleation with two magnetically driven microtool blades. | |
| Inomata et al. (2011) | Cutting an oocyte using the magnetically driven four-leg-type configuration microtool. | |
| Ichikawa et al. (2011) | An automated enucleation by high-precision control using a high-response and high-precision syringe pump. | |
| Feng et al. (2013b) | A microfluidic chip with a magnetically driven microrobot and controlled flow speed for oocyte enucleation | |
| Ichikawa et al. (2014) | A microknife and a microgripper were set to produce enucleated oocytes with a success rate of 100%. | |
| Liu et al. (2020) | Reduced batch process duration, comparable success rate and survival rate when compared to manual process. | |
| Denudation | Weng et al. (2018) | Denudation through jagged-surface constriction microchannels. |
| Mokhtare et al. (2022) | Using acoustic streaming and acoustic radiation force to agitate cumulus-oocyte complexes. |
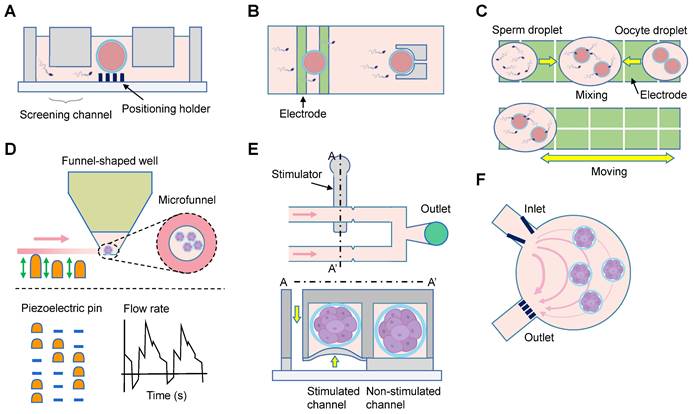
Investigations on in vitro fertilization and embryo culture. (A) The motile sperm swim through the motility screening channels spontaneously to reach the central oocytes. (B) The oocytes and sperm are first trapped on the electrodes for about 1 minute. Afterwards they are co-incubated for 1 hour in the micro-structures for natural insemination. (C) Droplet manipulation on a digital microfluidic platform. The sperm and oocyte droplets approach each other, and then mix for in vitro fertilization. The embryos are dynamically cultured with an automatic program. (D) The embryos are cultured in microfunnels, and the flow is generated by the actuation sequence of piezoelectric pins. (E) The microfluidic device comprises a control module, a stimulated microchannel, and a non-stimulated microchannel. Pressure is applied to the stimulated channel by a syringe pump. (F) Embryos are guided by the V-shaped structure, and trapped in a circular chamber with the help of grids. The flow profile under constant pumping is shown.
In vitro fertilization and culture of primary embryos
Embryo culture is one of the most important steps during in vitro fertilization. The included 25 studies could be categorized into mono- and co-culture systems (Table 5) [73-97]. Among the 18 mono-culture studies, 13 compared the microfluidic system with Petri dish approaches, with nine studies reporting beneficial effects of the microfluidics [74, 76, 79-81, 84-86, 89]. Four studies integrated oocyte capture, sperm selection, in vitro fertilization, and embryo culture functions using specific chamber design (Figure 6A) [75, 78], or dielectrophoretic approach (Figure 6B-C) [81, 88]. Heo et al. provided embryos with fluid mechanical stimulation and the media could be easily loaded and unloaded by simple pipetting (Figure 6D) [76]. One study used Pluronic F127 to conduct surface functionalization and block non-selective adsorption of culture medium, with comparable blastocyst and implantation rates to those in Petri dish [89]. The pre- and post-implantation development has also been achieved in a confined microfluidic environment (Figure 6F) [79]. Improved outcomes of embryonic development were demonstrated in all the seven co-culture systems. Oviductal epithelial cells were employed in four studies [92, 94, 95, 97], while uterine endometrial stromal cells were utilized in two studies [91, 93], and mesenchymal stem cell in one study [96].
Embryo evaluation and cryopreservation
Eleven and four studies were conducted to characterize embryo [98-108], and cryopreserve embryos [109-112], respectively (Table 2-3). The evaluations included morphological changes (n = 3) [98, 100, 101], viability (n = 2) [102, 103], and mechanics (n = 1; Figure 6E) [99]. Two groups investigated the oxygen consumption of embryos [104, 105]. Furthermore, the secretion of human chorionic gonadotropin beta, inflammatory factors, and glucose was studied at a single-embryo level, demonstrating in-depth metabolic and hormonal analysis of the embryo development [106-108]. Lastly, four studies compared embryo cryopreservation using microfluidic chips and manual operation, with similar outcomes [109-112].
Simulation of in vivo organs
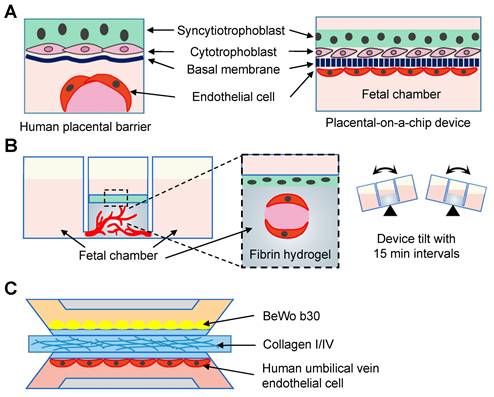
Forty-six studies reconstituted the physiological and pathological statuses of organs using the microfluidic chips, including the vagina (n = 2) [113, 114], cervix (n = 6) [115-120], oviduct (n = 5) [121-125], endometrium and decidua (n = 5) [126-130], placenta-decidual interface (n = 15) [131-145], fetal membrane-decidual or choriodecidual interface (n = 8) [146-153], and multi-organs (n = 5; Table 6) [154-158]. Two vagina-on-chips delved into the behavior of bacteria [114], and the formation of fungal biofilm [113] through vaginal epithelial cells. One microfluidic chip studied the cervical transitional zone [117]. Three cervix chips focused on the reconstitution of the cervical mucus components [115, 118, 122], and one mimicked the geometrical configuration [120]. Interactions between embryonic components and decidua were investigated in three chips [127, 128, 130]. Among the 15 placenta-on-a-chip studies, five explored placental barrier membrane permeability similar to the in vivo condition (Figure 7A) [131, 132, 134-136], and two models explored the placental transferability when exposed to environmental toxins and infection [139, 140]. Additionally, two studies focused on placental development [137, 138], and two tested the effects of inflammatory factors [133, 140]. Commercial microfluidic chips, such as IFlowPlate (Figure 7B) [141], and three-lane OrganoPlate (Figure 7C) [139, 142] have been adopted. Researchers in the University of Texas Medical Branch at Galveston stand out for their prolific work in constructing fetal membrane-decidual interface microfluidic chips (n = 7), and have extensively studied the effects of oxidative stress (n = 3) [146, 147, 153], and infections (n = 3; Figure 8A) [148, 151, 152]. Especially, Yin et al. established a human induced pluripotent stem cell-derived three-dimensional (3D) amnion tissue model on a chip to investigate the intra-uterine inflammatory responses [149]. Integrating multiple organs on a chip is an ideal approach to investigate the bidirectional crosstalk. Xiao et al. fabricated a microfluidic chip featuring the ovary, fallopian tube, endometrium, and ectocervix to stimulate the 28-day menstrual pattern [154]. Tantengco et al. constructed the vagina-cervix-decidua interface to reveal pathogen infection mechanisms [156]. Communications between the ovary and fallopian tube [158], as well as the ovary and uterus have also been studied [155]. One study recapitulated the prototype of polycystic ovary syndrome in ovarian and oviductal tissues [157]. These innovative approaches have paved the way for a deeper understanding of the organ communications in the physical and pathological conditions.
Toxicology and drug screening
A total of 14 studies were conducted to assess toxicology or drug efficacy using microfluidic devices (Table 7) [159-172]. All papers were reported within recent years, indicating a notable increase in research interest in this field. Only four of these studies employed human samples for experimental purposes [168, 169, 171, 172]. The two uterine chips were evaluated with Levonorgestrel [159], and insulin [160]. Placental chips were tested using drugs (n = 4) [161, 163, 166, 168], nanoparticles (n = 2) [162, 165], and pollutants (n = 2) [164, 167]. A fetal membrane microfluidic chip was utilized to identify the function of a transporter protein in transporting Rosuvastatin [169]. Notably, Richardson et al. investigated the drug transport and metabolism on both placenta and fetal membrane chips [170]. The two ovary-on-a-chip models investigated the effects of Doxorubicin [171], and epidermal growth factor inhibitor [172], respectively.
Reconstruction of placental model using the microfluidic chips. (A) Endothelial cells and trophoblasts are co-cultured in close apposition on the opposite sides of the membrane to form the placenta-on-a-chip device. (B) The term placenta is modeled using an IFlowPlate co-culture model, which consists of a differentiated syncytiotrophoblast monolayer cultured on a fibrin hydrogel. The endothelial cells inside the gel self-assemble to form perfusable vasculature. (C) A placental microfluidic chip in the OrganoPlate 3-lane.
In vitro fertilization and culture of primary embryos using microfluidic chips.
| Reference | Key group | Main finding |
|---|---|---|
| Embryo (mono-culture) | ||
| Akagi et al. (2010) | ① Dish ② Chip | Similar developmental outcomes in ①②. |
| Sugimura et al. (2010) | ① Droplet ② Chip + dynamic flow ③ In vivo | Reduced apoptosis and increased pregnancy rate in ② than ①. |
| Han et al. (2010) | ① Dish ② Chip | Similar fertilization rates in ①②. |
| Heo et al. (2010) | ① Dish ② Chip + static flow ③ Chip + dynamic flow ④ In vivo | Enhanced blastocyst development and cell numbers in ③ than ①②. Improved embryo implantation and ongoing pregnancy rates in ③ than ②. |
| Villa et al. (2010) | Descriptive study without grouping. | Supporting the highly proliferative status of pluripotent embryonic stem cells. |
| Ma et al. (2011) | ① Dish ② Chip | Similar embryo growth rate and blastocyst formation between ① and ②. |
| Esteves et al. (2013) | ① Dish ② Chip + static flow ③ Chip + dynamic flow | Improved embryonic development, blastocysts rate, and birth rate in ②③ than ①. |
| Wang et al. (2014) | ① Droplet ② Chip + dynamic flow | Higher rates of 5-8 cell embryo rate, morula rate and blastocyst rate in ② than ①. |
| Huang et al. (2015a) | ① Chip + static ② Chip + dynamic | Higher rate of embryo cleavage to a hatching blastocyst in ② than ①. |
| Huang et al. (2015b) | ① Dish ② Chip | The average rate of fertility is about 52% in ②. |
| Kieslinger et al. (2015) | ① Dish ② Chip | Comparable blastocyst development in ① and ②. |
| Huang et al. (2018) | ① Dish ② Chip | Higher fertilization rate, and more blastocyst stage in ② than ①. |
| Li et al. (2018) | ① Dish ② Chip | Higher rates of development to morulae and blastocysts, and more inner cell mass cells in ② than ①. |
| Yekani et al. (2018) | ① Dish + embryo ② Dish + SB ③ Chip + SB ④ Chip + grouped SB ⑤ Chip + grouped SB + embryo | Improved development of mouse SBs in ③④⑤ than ②. |
| Chiu et al. (2019) | Descriptive study without grouping. | A five-day culture includes emulsion, sorting, expansion and restoration in a droplet. |
| Huang et al. (2020) | ① Dish ② Chip | Comparable blastocyst rate in ①②. |
| Hawkins et al. (2022) | ① Dish ② Chip ③ Chip + pluronic F127 ④ Chip + deionized water | Improved blastocyst rate in ③ than ②④, and comparable apoptosis, DNA breakage and replication in ①②. |
| Karcz et al. (2023) | Descriptive study without grouping. | Utilizing electrowetting on dielectric technology to manipulate bovine embryos in vitro |
| Embryo (co-culture) | ||
| Li et al. (2013) | ① Dish + ESC ② Chip + ESC | Higher four-cell rate, morula rate and blastocyte rate are achieved in ② than ①. |
| Li et al. (2014) | ① Dish + OEC ② Chip + OEC | Higher blastocysts formation rate, and more cell numbers in ② than ①. |
| Chang et al. (2016) | ① Dish + ESC ② Chip + ESC | The blastocyst rate increases along with the increasing progesterone concentration. |
| Ferraz et al. (2017) | ① Dish + OEC ② Chip + OEC | More matured oocytes in ② than ①, and no polyspermy and parthenogenic activation in ②. |
| Ferraz et al. (2018) | ① In vivo ② Dish + OEC ③ Chip + OEC | More physiological zygote genetic reprogramming in ③ than ②. |
| Chen et al. (2021) | ① Dish ② Chip ③ Dish + MSC ④ Chip + MSC | Embryos grow faster, and the blastocyst development rate increased in ④ than ③. |
| Wang et al. (2022) | ① Dish ② Chip without cells ③ Chip + OEC | Lower intracellular reactive oxygen species in four-cell stage embryos in ③ than ①. |
Abbreviations: ESC: endometrial stromal cell; MSC: mesenchymal stem cell; OEC: oviduct epithelial cell; SB: single blastomere.
Fabrication methods and materials
Polydimethylsiloxane (PDMS; n = 91) was the most often used material, followed by polymethyl methacrylate (PMMA). The fabrication methods corresponded to the materials used. The top methods were soft lithography (n = 67), photolithography (n = 11), and 3D printing (n = 6). Eight studies utilized commercial products.
In vitro organ reconstitution under physio- and patho-states.
| Reference | Main finding | |
|---|---|---|
| Vagina | Czechowicz et al. (2022) | Observation of the biofilm formation of Candida on the surface of the vaginal epithelium. |
| Mahajan et al. (2022) | Effects of co-culturing vaginal epithelium with Lactobacillus crispatus and Gardnerella vaginalis. | |
| Cervix | Zhang et al. (2012) | Imitation of the physiological interactions between cervical mucus and sperm. |
| Tung et al. (2014) | Studying the effects of surface topography and fluid flow in bovine cervix. | |
| Tantengco et al. (2021) | Simulating the interactions between the ectocervical and endocervical epithelial layers. | |
| Yu et al. (2022) | Filling cervix channels with hyaluronic acid to study the viscosity and charge of cervical mucus. | |
| Leemans et al. (2022) | Establishing a functional model that resembles the in vivo oviduct epithelium. | |
| Dadkhah et al. (2023) | Serpentine microchannels with different radii of curvature to mimic the tortuous cervical structure. | |
| Oviduct | Xie et al. (2010) | Chemo-attractive function of cumulus-oocyte complexes in the oviduct. |
| Yan et al. (2020) | Mixture of human tubal fluid and methylcellulose to mimic the female viscous environment. | |
| Raveshi et al. (2021) | Droplet microfluidics to create soft curved interfaces with different radii of curvature corresponding to the labyrinthine complexity of human fallopian tube. | |
| Yaghoobi et al. (2022) | A microfluidic platform mimicking the structure of lumen in the uterotubal junction. | |
| Yu et al. (2023) | Forming a progesterone gradient in fibronectin-filled microchannels to mimic the oviductal microenvironment. | |
| Endometrium and decidua | Gnecco et al. (2019) | Stromal decidualization under hemodynamic forces is mediated via cyclooxygenase-2, as well as the paracrine actions of prostaglandin E2 and prostacyclin. |
| Govindasamy et al. (2021) | Trophoblast giant cells migrate collectively and communicate with endothelial cells during implantation. | |
| Park et al. (2022) | Extravillous trophoblasts directly migrate towards the maternal vessel and interact with decidualized stromal cells for vascular remodeling. | |
| Liu et al. (2023) | Follistatin exerts more significant effects on cellular adhesion, wound healing and migration of decidualized endometrial stromal cells than Activin A. | |
| Govindasamy et al. (2023) | Modeling the endothelial cell-embryo interaction in a methacrylated dextran and polyethylene glycol hydrogel environment. | |
| Placenta-decidual interface | Lee et al. (2016) | Confirming the roles of trophoblast and endothelial cells in placental barrier function. |
| Blundell et al. (2016) | Inducing trophoblast cells to form a syncytialized epithelium progressively, and reconstituting the expression and physiological localization of membrane transport proteins. | |
| Abbas et al. (2017) | The granulocyte-macrophage colony-stimulating factor induces the migration of human trophoblast cells. | |
| Mandt et al. (2018) | Fabricating biomimetic placental barriers with optimal structuring parameters, material composition and cultivation conditions. | |
| Zhu et al. (2018) | Employing Escherichia coli. to mimic the bacterial infection and observe transplacental communication. | |
| Pemathilaka et al. (2019) | A steady caffeine state is reached in maternal (0.1513 mg/ mL, 6.5 h) and fetal (0.0033 mg/mL, 5 h) sides. | |
| Mosavati et al. (2020) | Glucose diffusion across the placental barrier in different models and flow rates. | |
| Ko et al. (2022) | Enhanced cell invasion of trophoblast cells in a hypoxic environment to study placental development. | |
| Li et al. (2022) | Follistatin induces migration and invasion of trophoblasts through JNK signaling in placental development. | |
| Mosavati et al. (2022) | Infections resist glucose perfusion and decrease the glucose transfer. | |
| Ghorbanpour et al. (2023) | Investigating the role of inflammatory factors in preeclamptic placenta. | |
| Kouthouridis et al. (2023) | Differentiated placental stem cells produce a more highly fused syncytium that is consistent with in vivo findings. | |
| Rabussier et al. (2023) | Construction of physical and pathological placental barrier on-a-chip models. | |
| Cherubini et al. (2023) | Rising flow in perfusable fetal microvessels shows improved function and enhanced protein expression in placental vascularization model. | |
| Cao et al. (2023b) | Self-assembled human placental model using trophoblast stem cells in a dynamic system. | |
| Fetal membrane-decidual or choriodecidual interface | Richardson et al. (2019) | Testing the effects of oxidative stress on amnion membrane organ-on-chip. |
| Richardson et al. (2020a) | Better membrane permeability regardless of the side of treatment or time point in chips than Transwells when exposed to cigarette smoke extract or dioxin. | |
| Richardson et al. (2020b) | Ascending infection propagates through the chorion, amnion mesenchyme, and reaches the fetal amnion within 72 hours and induces time-dependent and cell-type specific pro-inflammatory cytokine production. | |
| Yin et al. (2020) | Investigating intrauterine inflammation with human induced pluripotent stem cell-derived 3D amnion tissues. | |
| Radnaa et al. (2021) | Fetal membrane-derived exosome mediated paracrine signaling generates inflammation and induces parturition. | |
| Bento et al. (2023) | Ureaplasma parvum causes limited inflammatory response in the choriodecidual interface, but not in the amnion layer. | |
| Richardson et al. (2023a) | Fetal oxidative stress causes more amnion-based cellular pathologies and inflammation, whereas maternal oxidative stress induces localized immune regulatory changes. | |
| Richardson et al. (2023b) | Lipopolysaccharides induce decidual inflammation, infiltration of NK cells and neutrophils, as well as progesterone production in chorion cells. | |
| Multi-organs | Xiao et al. (2017) | Simulating the in vivo female reproductive tract and the endocrine loops between organ modules for the ovary, fallopian tube, uterus, cervix and liver. |
| Park et al. (2020) | Introducing the SERPINB2 luciferase reporter system to test the toxicity in uterine endometrium and ovaries. | |
| Tantengco et al. (2022) | Ureaplasma parvum infection neither promotes cell death nor causes massive inflammation in the vagina-cervix-decidua interface cells, but the damage increases when combined with lipopolysaccharide or directly inoculates the amniotic cavity. | |
| Russo et al. (2022) | Ovary-derived versican is released during ovulation to increase activity of fallopian tube epithelium. | |
| Campo et al. (2023) | Recreating the polycystic ovarian syndrome phenotypes using ovarian and oviductal tissues. |
Discussion
The use of microfluidic chips has significantly transformed basic and clinical research by replicating multicellular architectures, organ crosstalk, and microenvironmental cues. While several recent reviews have outlined their applications in obstetrics and gynecology field, particularly in the assisted reproductive technology (ART) area [6, 173, 174], there has been no investigation into their use across the entire FRS. Thus, this paper represents the first systematic review to comprehensively delve into the multifaceted functions of microfluidic chips from the female reproduction standpoint. We primarily discuss five aspects of the microfluidics: oocyte handling, embryo manipulations, placenta bioengineering, organ communication, and fabrication methods.
Drug screening and toxicity test conducted on microfluidic chip platforms.
| Reference | Key group | Main finding | |
|---|---|---|---|
| Uterus | Ahn et al. (2021) | ① Chip + control ② Chip + Levonorgestrel (10, 100, 1000, and 10000 ng/mL) | More dead cells in ② than ①. |
| Baik et al. (2023) | ① Chip + control ② Chip + vehicle ③ Chip + insulin (10 ng/mL) | Transcriptional comparisons of the endometrial epithelium among ①②③. | |
| Placenta-decidual interface | Blundell et al. (2018) | ① Chip + Glyburide ② Chip without cells + Glyburide | Efflux transporters mediate the active transport function of the human placental barrier. |
| Yin et al. (2019) | ① Chip + vehicle ② Chip + TiO2 (50, 200 μg/mL) | More damage to barrier integrity and impaired immune cells in ② than ①. | |
| Pu et al. (2021) | ① Chip + vehicle ② Chip + folic acid (100 ng/mL) | More cell invasiveness in ② than ①. | |
| Boos et al. (2021) | ① Chip + embryoid body + polystyrene ② Chip without placenta + embryoid body + polystyrene | Reduced polystyrene concentration in fetal side in ① than ②. | |
| Abostait et al. (2022) | ① Static ② Static + forskolin ③ Dynamic ④ Dynamic + forskolin Toxicity: liposome | Increased cell uptake of liposome in ②③④ than ①. | |
| Pemathilaka et al. (2022) | ① Chip without cells + Naltrexone (100 ng/mL) ② Chip without cells + 6β-Naltrexol (100 ng/mL) ③ Chip + Naltrexone ④ Chip + 6β-Naltrexol | Less drug enrichment in ③④ than ①②. | |
| Ticiani et al. (2022) | ① Chip + control ② Chip + Bisphenol S | Bisphenol S prevents EGF mediated extravillous trophoblasts functions. | |
| Kammala et al. (2023) | ① Ex vivo placenta perfusion ② In vivo murine model ③ In silico ④ Chip | Pravastatin transfer in ③④ are similar. | |
| Fetal membrane | Ganguly et al. (2021) | ① Chip + Rosuvastatin + control siRNA ② Chip + Rosuvastatin + OATP2B1 siRNA | Reduced Rosuvastatin propagation from the decidua to the fetal amnion epithelial cell layer in ② than ①. |
| Placenta and fetal membrane | Richardson et al. (2022) | ① Fetal membrane/placenta Chip ② Fetal membrane/placenta Chip + oxidative stress Drug: Pravastatin and Rosuvastatin (200 ng/mL) | The drugs permeate the maternal-fetal interface chips and generate cell- and time-specific statin metabolites. |
| Ovary | Aziz et al. (2020) | ① Chip ② Chip + Doxorubicin | Reduced follicular growth and hormone secretion, more apoptosis in ② than ①. |
| Lee et al. (2022) | ① Chip ② Chip + EGF inhibitor | Higher expansion area in ① than ②. |
Abbreviations: EGF: epidermal growth factor; OATP2B1: organic anion transporting polypeptide 2B1.
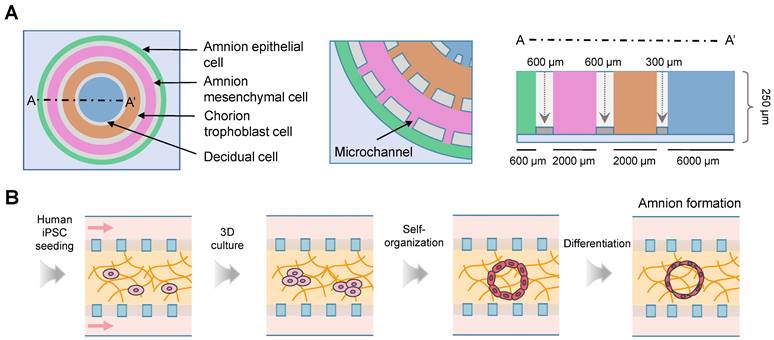
Recreation of fetal membrane-decidual or choriodecidual interface using the microfluidics. (A) The fetal membrane interface organ-on-chip is composed of four circular chambers connected by microchannels. The width of each chamber replicates the natural thickness of the fetal membrane. (B) Schematic of the cavity formation and amnion tissue differentiation on a chip. Abbreviations: iPSC: induced pluripotent stem cell.
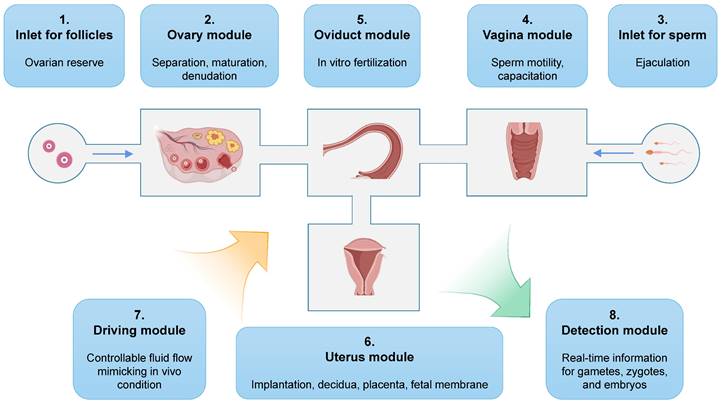
An example of all-in-one microfluidic chips that mimic the entire female reproduction tract.
Using microfluidic chips in ART laboratories
ARTs have revolutionized infertility treatment over the last three decades, enabling millions of individuals to conceive and have children. Annually, more than 2.5 million ART cycles are performed, leading to the birth of over 6.5 million babies worldwide [175]. However, the high costs, time-consuming procedures, and labor-intensive efforts limit its utilization [176]. Moreover, gametes and embryos are exposed to an external environment during traditional ART procedures, which may increase the risks of fetal malformations, epigenetic disorders, and adverse obstetric or perinatal outcomes [177-179]. Therefore, it is essential to provide female reproductive tract cells with a suitable operating environment.
Physical cues in the microenvironment are important yet often overlookded factors. The mechanically heterogeneous extracellular matrix surrounding cumulus cells and oocytes is closely related to follicular growth [180]. A stiff ovarian cortex microenvironment is beneficial for maintaining primordial follicles in a quiescent state, while ovarian fragmentation facilitates primordial follicle loss [181, 182]. These findings suggest that loading ovary-on-a-chip with controllable degradable biomaterials may restrict follicle growth and achieve sequential activation. In the uterus, mechanical stresses are found to interact with the early embryonic development, thus facilitating pregnancy. Therefore, adjusting shear stress via tilting the embryo culture dish [183], or coating the substrate with collagen has been shown to enhance embryo development and blastocyst formation [184]. Despite only a limited number of microfluidic chips have currently improved their performance through modifying mechanical properties [20, 142], utilizing physical cues will greatly enhance the value of the microfluidics. Matrigel, PDMS, collagen, and fibronectin are commonly used extracellular matrix materials [184]. It is also recommended to test the stiffness of the extracellular matrix beforehand, and precoat microchambers with materials of similar hardness to mimic in vivo conditions when culturing cells derived from these tissues.
In terms of the gamete evaluation, general parameters, including structure, maturity, and oxygen levels, are inefficient at detecting subtle disturbances. With the help of microfluidic chips, highly accurate and reproducible in situ assessment can be achieved [21]. Other inherent advantages involve the small volumes required, and the integration of various elements in portable devices [75, 79, 88]. These chips can quantitatively measure follicular development, assess ovulation disorders, and detect gene and protein expression, as well as metabolic characteristics of endometrial and ovarian cancer cells [20, 185, 186]. Indeed, the FRS is understudied when compared with the brain science, bone bioengineering, and the male reproductive system. The microfluidic chips have great potential to revolutionize the diagnosis, and treatment of gynecological and obstetric diseases.
Cell surgery for oocytes and embryos
The modern medical field has experienced a transition from the utilizing large machines to adopting smaller instruments, which significantly enhances the capabilities of minimally invasive surgeries, drug delivery, and diagnostics. Despite this shift, the manipulation and intervention in single cells remains a notable challenge. On this condition, the microfluidic device has emerged as a powerful tool for conducting cell surgeries, offering unique advantages in accurate manipulation, reduced operation time, a shorter learning period, and the exploration of underlying mechanisms.
Cell surgery involves various intricately complex and highly skilled procedures, including cell position, movement, rotation, and enucleation. Single oocytes are commonly manipulated using microneedles and micro-tweezers, which are housed within microfluidic chips. The microtools can be adjusted via manual, optical, electric, magnetic, or acoustic methods to accurately position the spindle, polar body, and inner cell mass (Figure 5F) [56, 69, 70]. The proper positioning of these structures is correlated with higher rates of fertilization and embryonic development [187]. Moreover, an alternative method for cell manipulation known as electrowetting-on-dielectric can be utilized to move oocytes (Figure 6C) [64]. Cell rotation, on the other hand, can be achieved by activating an electric field, acoustic waves, and magnetic force (Figure 5D) [59, 62, 69]. However, the electric field typically results in non-uniform rotation and is challenging to control in and out of the plane. To address this, microstructures are integrated within chips to allow for the rolling of oocytes through oscillating solid microstructures (Figure 5E) [62]. Enucleation, a crucial step in cloning efficiency, involves the removal of the nuclear material from mature metaphase II oocytes. Conventional enucleation methods often involve the use of fluorochromes, ultraviolet light, and chemicals, which may cause varying degrees of damage to the cytoplasm [188]. The microfluidics may conduct enucleation through magnetic field-controlled microtools. It takes less time, but achieves comparable success rates with manual operation [60, 65, 66]. Similarly, cellular cytoplasm can be removed using a micro-knife after an oocyte is grasped with a microgripper [69].
Although automated microfluidic chips for cell surgeries offer many advantages, it is imperative to rigorously examine the potential damage caused by the electric field and optical intensity. Increased laser power from optical devices is shown to disrupt the pH regulation function of cells [189]. The electric field has also been reported to damage the cellular membrane and cytoskeleton, ultimately leading to cell death [190, 191]. Conversely, a static magnetic field enhances the embryo cleavage and blastocyst formation rates, as well as modulates pluripotency in blastocysts during cumulus-oocyte complexes vitrification [192]. The magnetic field induced oocyte damage is associated with the developmental stage [193]. Low-intensity electromagnetic fields can disturb oocyte maturation and impact embryo development [194]. Prevalent challenges encountered in dielectrophoresis devices encompass Joule heating, bubble generation, insulation breakdown, and undesired reactions occurring on the electrodes, which may adversely affect the microchip function. In light of these findings, additional fundamental evidence is warranted to further investigate the intensity and frequency of confounding factors.
Bioengineering for placental and fetal membrane research
Medicinal drug usage during pregnancy is imperative in certain scenarios. Nevertheless, due to the fact that pregnant women are often excluded from clinical trials, the risk of these drugs on fetal development remains largely unknown. Moreover, ethical limits and legal concerns also limit placental research, particularly at early stages [195]. The advent of microfluidic technology has propelled the creation of the placenta and fetal membrane organ-on-chip models [196]. They offer significant potential in investigating embryotoxic effects of drug metabolism and transportation. Among the included studies, glucose is often used as a model substance to test the physiological biomolecular transport [136, 139]. These placenta-on-chip models demonstrate superior capability in replicating glucose transport rates, when compared to the Transwell method. Furthermore, studies have introduced various drugs, such as Naltrexone, Glyburide, and statins, into the maternal micro-channels, subsequently measuring the time-dependent changes in the fetal side [132, 135, 166]. The effects of nanoparticles (such as titanium dioxide, polystyrene etc.) on trophoblast cell activity or early embryonic development are also topics of interest [162, 165].
Studying pregnancy complications, like preeclampsia and intrauterine growth restriction, poses significant challenges. Animal models often fail to accurately replicate the intricate physiological alterations that occur in human organs during pregnancy. In mouse placenta, there is no separation by the endothelium between the trophoblast and maternal circulation [4]. On the contrary to the remodeling of the maternal spiral arteries, the arterial remodeling in rats and guinea pig involves in the endovascular trophoblast cells. The microfluidics technique enables the simulation of in vivo placenta and fetal membrane, and has been adopted to study bacterial infection and preeclampsia of placenta. The innate immune alteration, and inflammatory cytokines production caused by Escherichia coli. were reported [145]. The inflammatory and hypoxic environment acts as an underlying mechanism of preeclampsia. Therefore, tumor necrosis factor alpha and 1%-3% oxygen were employed to mimic the development of preeclampsia [137, 142]. Taken together, the utilization of microfluidics will significantly enhance the capacity to mechanistically comprehend the maternal-fetal interface.
Recapitulating organ communication
In multicellular organisms, organ communications are coordinated through the delivery of bioactive molecules or neurotransmitters via the circulation or neurons, respectively [197]. For instance, in response to meal ingestion, the gastrointestinal tract produces gut peptides, which act on the vagal afferent neurons to inhibit food intake and stimulate the liver to metabolize and store glucose [198]. Likewise, the FRS involves multiple communications with extra-gonadal organs, with the hypothalamus-pituitary-ovarian axis being a typical core. Additionally, circadian oscillators in the central nervous system also regulate steroidogenesis and oocyte development [199, 200]. It was found that gut-derived interleukin-22 improved the disease phenotype in polycystic ovarian syndrome patients [201]. Therefore, a comprehensive understanding of the organ crosstalk is crucial for deepening the knowledge of the FRS and developing novel therapeutic approaches.
Conventional tools used to study inter-organ communications include the Transwell system, coculture, and animal models. Despite being an improvement over traditional 2D culture, they face challenges in available tissue types and imaging. In this case, microfluidic approaches serve as a promising alternative to coculture multiple cell-types and tunable perfusion conditions. In the context of the FRS, microfluidic chips allow for the growth of ciliated and secretory cells with correct spatial organizations [125]. Researchers have successfully fabricated well-defined placental barriers within microfluidic systems by culturing human umbilical vein endothelial cells (HUVECs) and the b30 clone of the BeWo choriocarcinoma cell line (BeWo b30) on a semipermeable membrane (Figure 7). Most importantly, multi-organ microfluidic chips comprising of the ovary, uterus, cervix, and even liver have been achieved, highlighting the potential of microfluidic platforms in overcoming the limitations of conventional tools for studying inter-organ communications [128, 138, 140, 202].
Fabrication techniques and materials
The revolution of microfluidic technology and chip fabrication has been advanced by the rapidly developing manufacturing methods, including soft lithography, photolithography, injection molding, laser ablation, and other direct manufacturing technologies [203]. Soft lithography emerges as the most commonly used method due to its simple yet robust microchannel manufacturing process, varied pattern options, and high optical transparency (Figure 2). However, the high cost of the equipment renders the technology impractical for large-scale production of microfluidic devices [204]. Photolithography offers a high wafer production capacity and is suitable for microscale characteristics. Creating a biomimetic ovarian microstructure through this method facilitates the understanding of ovarian diseases and serves as an in vitro culture system for preserving fertility [20]. Injection molding offers the advantage of easily manufacturing complex geometric shapes with short production cycles. However, the fabrication of large undercut geometric shapes presents challenges due to potential pattern deformation and the introduction of defects [205]. In contrast, laser ablation presents a rapid and scalable method for producing microfluidic devices, but its application is limited by the use of uncommon materials [206]. This was exemplified in the fabrication of a microfluidic device with multi-channels using laser ablation of poly (methyl methacrylate) and poly (ethyl phthalate) plastic laminates, which was applied for the detection of specific DNA sequences and a non-specific binding control related to breast and colorectal cancers [207].
When manufacturing microfluidic chips, several characteristics of the raw material must be taken into consideration, like the durability, ease of use, transparency, biocompatibility, and the potential to meet surface functionalization needs. In the early days, microfluidic chips were made from glass, silicon, metals, and ceramics. However, the frequent use of chemical etching to customize the mask undoubtedly increases the time, cost, and effort [208]. Currently, the most commonly used polymers are PDMS, PMMA, fluoropolymers, polyurethane, and polycarbonate [209]. PDMS, an elastomer with excellent microchip manufacturing performance, is both cheap and easy to mold with good molding outcomes, optically transparent, permeable, biocompatible, low autofluorescence, naturally hydrophobic, and highly elastic [210]. However, when employed in cell culture substrates, PDMS tends to adsorb hydrophobic molecules, resulting in reduced drug concentration and activity [89]. An appealing alternative is polyurethane elastomer. It possesses similar optical transparency, flexibility, and castability as PDMS, but notably, it is resistant to the absorption of small hydrophobic molecules. PMMA exhibits superior solvent compatibility compared to PDMS, and its lack of small-molecule absorption also renders it a favorable material for microchip fabrication [211]. These characteristics are particularly useful for on-chip organ devices and micro-physiological systems. There is ongoing exploration of many other suitable polymers for microfluidic chip fabrication.
The hydrogel is one of the important compartments in constructing microfluidic chips, and has been widely used in the FRS. The cervical mucus serves as a liquid barrier to prevent infection, and screen sperm. To mimic the cervical microenvironment, hyaluronic acid hydrogels have been employed as a surrogate, and share similarity in viscosity and charge with cervical mucus [118]. However, hyaluronic acid may compromise the cellular viability, and methylcellulose hydrogels are preferred by some groups [122, 212]. Extracellular matrix hydrogels containing collagen type I and IV are usually used to create in vitro placenta, and fetal maternal interface models [131, 139]. In these cases, the fetal and maternal sides locate on either side of the hydrogel microchannels. Less frequently used hydrogels, including methacrylamide [134, 137], laminin [135], and Matrigel [135] have also been adopted in some studies.
Features of 3D-printed microfluidic chips
Three-dimensional printing has revolutionized the fabrication of high-precision microfluidic chips, for its characteristics in flexibility, directness, and rapid prototyping [213]. It overcomes the disadvantages of soft lithography and other traditional miniaturization methods, such as the inability to create a true 3D structure, the costly and time-consuming design process, and the challenges in batch manufacturing [214]. Moreover, the use of high printer resolution in 3D printing has enabled the manufacturing of miniaturized and microfluidic systems with significantly reduced equipment and sample requirements [215]. The incorporation of translucent, heat-resistant, and biocompatible materials in 3D printing allows for its future applications in biotechnology.
Among the various 3D printing technologies, fused deposition modeling, inkjet 3D printing, and photopolymerization obtain much popularity due to their well-established procedures and cost-effectiveness with respect to equipment and materials [214]. Fused deposition modeling is characterized by its cost-saving and widespread availability for processing thermoplastics. However, its low accuracy level and nozzle clogging result in mechanically weak devices [216]. On the other hand, inkjet 3D printing offers improved accuracy, and produces multi-material/colored objects with high surface precision. Nonetheless, issues such as poor material durability and low mechanical strength limit its application [217]. Photopolymerization, which encompasses technologies such as stereolithography and digital light processing, stands out for its exceptional accuracy and resolution, making it ideal for constructing complex structural devices with smooth surfaces and versatile printed parts [218]. Nevertheless, concerns related to the compatibility, biocompatibility, and toxicity of the printing resins need to be addressed, particularly in the context of bioprinting. As a whole, the search for a wide range of materials suitable for bioprinting continues to present a challenge in this field.
Future perspectives
Use of commercial products
The commercial development of microfluidic chips is a crucial element in advancing organ chip technology. Unlike those used in laboratories, commercial organ chips require careful consideration of cost, yield, structural dimension tolerance, and manufacturability. To address cost concerns, for instance, injection-molding materials may replace PDMS, and the soft lithography manufacturing method may be substituted with injection molding. Altering material surface characteristics can significantly impact product performance and quality. Therefore, it is essential to take mass production factors into account from the early stages of experimental research. This proactive approach can lead to cost and time reduction in transitioning microfluidic chips into commercial products.
Advancements from the laboratory to commercialization are primarily carried out through start-ups that attract investments to transform the chip-organ industry. At present, several companies produce microfluidic chips and related commercial products. Hesperos has combined lab expertise to develop organ chips for clinical applications. Multi-organ analysis systems with built-in mechanical and chemical biosensors have been produced to tackle rare diseases by a human-on-a-chip approach. Hesperos' key technology is a pumpless four-organ (heart, liver, neurons, and skeletal muscle) system, where toxicology and functional responses of five drugs are clinically diagnosed and analyzed [218]. The start-up company TissUse, established in Germany, is developing two-organ and four-organ microfluidic chips by integrating biological vasculature into a multi-organ chip microsystem. These chips have an open structure, allowing tissues to be externally assembled and prepared before being placed into the device cavity after assembly. Such an approach is compatible with clinically relevant tissue biopsies [219]. ChipSensors focuses on developing a technology to support microfluidic chip-based diagnostics and life science research. Microfluidic-Tech provides various microfluidic chips, ranging from polymerase chain reaction to reagent detection chips. Microfluidic Chip Systems has patents related to microfluidic technology, and their products include microfluidic chips and solutions for biological analysis. Microfluidics UK provides high-performance microfluidic chips and equipment for microfluidic analysis applications such as molecular biology, cell biology, and materials science. Finally, Nordic Microfluidics produces easy-to-use, time-saving, and cost-effective microfluidic chips, helping customers solve microfluidic analysis challenges. The miniaturization, integration, functionalization, and intelligence of the whole organ chips should be considered in clinical translation to expand to individualized medical treatments.
Toward an all-in-one microfluidic chip
The development of a fully functional microfluidic chip that contains a complete FRS presents an intriguing prospect. Operators would simply need to load eligible cumulus-oocyte complexes into the device, and the all-in-one microfluidic chip is capable of automating oocyte manipulation, fertilization, and early embryo culture (Figure 9). After a few days, well-developed embryos would be obtained. The potential of this technology is highlighted by Xiao et al., who developed the EVATAR microfluidic chip containing female reproductive tract explants and peripheral tissues [154]. Their findings demonstrated the reproducibility of a coordinated response to estrogen and progesterone using the microfluidic chip technology. Similarly, Park et al. fabricated a microfluidic device incorporating human endometrial and primary ovarian somatic cells, revealing the facilitation resulting from bidirectional endocrine crosstalk, as well as identifying a predictive marker of reproductive toxicity [155]. It is important to note that each chamber should be designed to support specific functions for each organ, rather than serving as simple supporting platforms. For instance, the spatial separation of theca and granulosa cells could contribute to optimized hormone secretion, while the microchannels could be filled with hyaluronic acid to simulate cervical viscosity [220]. Though the goal of achieving an integrated system is challenging, it represents a substantial opportunity for further advancements in reproductive technology.
Integration of laboratory testing into all-in-one microfluidic systems represents another significant advancement. Conventional hormonal tests typically involve antigen-antibody binding and signaling magnification, which are usually conducted in centralized laboratories with specialized instrumentation and personnel, resulting in low throughput and imposing a heavy burden on medical facilities. However, recent advancements in microfluidic chip technology exhibit promising performance characteristics, such as simultaneous detection, rapid treatment, low consumption, high throughput, and miniaturization. For instance, Lee et al. integrated droplet and bead manipulation together, and detected two growth factors from a single embryo with only 520 nL in less than 40 min [108]. More specifically, the range of testing has been extended to mechanics, cell permeability, viability, and oxygen consumption. To optimize the biomedical and clinical diagnosis, it is essential to integrate the aforementioned components into a fully functional microfluidic chip. By harnessing the power of multiplex detection for reproductive disorders, it is possible to reduce the likelihood of misdiagnosis and incomplete diagnosis of diseases with overlapping symptoms, thereby enhancing the overall efficiency of medical testing procedures.
Selection of human cells
Since animal research findings are typically extrapolated to humans, suitable human cell components should be employed to bring the in vitro systems to the closest possible similarity to the physiological environment. Although the overall utilization rate of human samples reached 58.3%, most studies were limited to pregnancy-related research and only sparsely investigated other organs or tissues. In fact, the human and mouse female reproductive tracts differ significantly [221]. Macroscopically, the gross uterine anatomy of reproductive-age humans is pear-shaped, while the one in mice is Y-shaped. Microscopically, the human cortical region occupies a thin layer of <1 mm in the outmost part of the ovaries [222], while the mouse cortex takes up most of the ovarian volume. The mouse estrous cycle is around 4-5 days with no vaginal bleeding, while the menstrual cycle in humans is around 28 days. Researchers must be aware of the similarities and divergences across species to facilitate clinical translation [223].
Human placental cells are always preferred because they are easily accessible and devoid of ethical issues. A wide range of placental cells, including HUVECs, human placental villous endothelial cells, and several cell lines (such as BeWo b30, HTR8/SVneo, CS2, JEG-3 etc.), have been utilized. In the included studies, HUVECs are the most extensively used endothelial cells. They are well characterized, convenient to identify, as well as relatively easy to obtain and culture [132]. BeWo b30 cells are widely utilized in research on placental permeability, as they possess the unique characteristic of not undergoing contact inhibition [224]. This feature allows them to form multilayers following a one-week culture period, which is important for ensuring the integrity of the biomimetic membrane [225]. Despite the above advantages, these cells may not be the best source due to their choriocarcinoma origin. For example, the primary syncytiotrophoblasts are insensitive to glucose transport inhibition, while BeWo b30 cells show a proportional alteration in transepithelial transport after the treatment [226].
Human trophoblast stem cells (TSCs) have introduced a novel method for replicating placental development in the first-trimester. They can be isolated from human blastocyst outgrowths or primary placentas [227]. The TSCs are capable of long-term proliferation in vitro, and are functionally competent to differentiate into syncytiotrophoblasts and extravillous cytotrophoblasts [227]. The human TSCs can also be derived from human pluripotent stem cells [228], or somatic cells via direct reprogramming [228, 229]. Furthermore, generation of induced trophoblast stem cells is also feasible [229]. Taken together, stem cell technology provides an accessible and patient-specific model system of human placental research. It will greatly facilitate the study of placental biology and diseases, and their treatment.
Human oocytes and embryos are needed to address important scientific questions. Researchers should redesign the culture chambers' size and choose an appropriate medium when shifting from experimental animals to humans. However, human samples are difficult to obtain, resulting in the small number of studies that used human oocytes and embryos. Most importantly, full informed consent should be recorded [230]. Fortunately, one advantage of microfluidic chips is the small number of cells needed for culture. It is essential to consider human cell selection when designing experiments to construct models of the female reproductive system.
Limitations
This systematic review had some limitations. First, the eligible studies lacked rigorous interpretation of the risk of bias because most studies focused on the engineering process. Information on animal randomization, blinding, and biological replicates were not provided. Therefore, we provided detailed information about microfluidic chip designs and biological assessments to fill this void. Then, due to the high technical threshold, the included data relied heavily on small-sample studies, particularly those on vaginal, uterine and oviductal chips, which could potentially decrease the applicability of the findings to other research groups and laboratories. Possibly for the same reason, analysis of drugs, toxins, and chemicals in the female reproduction field was also scarce.
Conclusion
Recreating the organ function of the female reproductive tract and promoting clinical applications are complex and challenging. The microfluidic chip emerges as an enhanced novel technical support to help achieve this goal. This review highlights the feasibility of microfluidic chips in oocyte operation, embryo manipulation, organ stimulation, and drug screening. As microfluidics is developing rapidly, future research should focus on the development of novel materials and the creation of multifunctional devices.
Abbreviations
2D: two-dimensional; 3D: three-dimensional; ART: assisted reproductive technology; CPA: cryoprotectants; FRS: female reproductive system; HUVEC: human umbilical vein endothelial cell; PDMS: polydimethylsiloxane; PMMA: polymethyl methacrylate.
Supplementary Material
Supplementary table.
Acknowledgements
The authors would like to thank Yue Bao from SCI-GO (www.sci-go.com) in enhancing the readability and clarity of the text.
Funding
This work was supported by the grants from the National Key Research and Development Program of China (No. 2022YFC2704100), and National Natural Science Foundation of China (No. 82301849, 82371648).
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used Acadwrite in order to improve readability, grammar and language style. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011;124:229-36
2. Jackson S. The importance of being transparent. J Clin Invest. 2015;125:459
3. Gargus ES, Rogers HB, McKinnon KE, Edmonds ME, Woodruff TK. Engineered reproductive tissues. Nat Biomed Eng. 2020;4:381-93
4. Abbas Y, Turco MY, Burton GJ, Moffett A. Investigation of human trophoblast invasion in vitro. Hum Reprod Update. 2020;26:501-13
5. Myllynen P, Vähäkangas K. Placental transfer and metabolism: an overview of the experimental models utilizing human placental tissue. Toxicol In Vitro. 2013;27:507-12
6. Francés-Herrero E, Lopez R, Hellström M, de Miguel-Gómez L, Herraiz S, Brännström M. et al. Bioengineering trends in female reproduction: a systematic review. Hum Reprod Update. 2022;28:798-837
7. Wu T, Wu Y, Yan J, Zhang J, Wang S. Microfluidic chip as a promising evaluation method in assisted reproduction: a systematic review. Bioeng Transl Med. 2024;9:e10625
8. Griffin J, Emery BR, Huang I, Peterson CM, Carrell DT. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J Exp Clin Assist Reprod. 2006;3:2
9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097
10. Armstrong R, Jackson N, Doyle J, Waters E, Howes F. It's in your hands: the value of handsearching in conducting systematic reviews of public health interventions. J Public Health (Oxf). 2005;27:388-91
11. Perianes-Rodriguez A, Waltman L, van Eck NJ. Constructing bibliometric networks: a comparison between full and fractional counting. J Informetr. 2016;10:1178-95
12. Zargari S, Veladi H, Sadeghzadeh B, Shahabi P, Frounchi J. A microfluidic chip for in vitro oocyte maturation. Sensor Letters. 2016;14:435-40
13. Huang HY, Kao WL, Wang YW, Yao DJ. AC-electric-field-induced parthenogenesis of mouse oocyte. Micro Nano Lett. 2018;13:794-7
14. Healy MW, Dolitsky SN, Villancio-Wolter M, Raghavan M, Tillman AR, Morgan NY. et al. Creating an artificial 3-dimensional ovarian follicle culture system using a microfluidic system. Micromachines (Basel). 2021;12:261
15. Aziz AUR, Fu M, Deng J, Geng C, Luo Y, Lin B. et al. A microfluidic device for culturing an encapsulated ovarian follicle. Micromachines (Basel). 2017;8:335
16. Berenguel-Alonso M, Sabes-Alsina M, Morato R, Ymbern O, Rodriguez-Vazquez L, Tallo-Parra O. et al. Rapid prototyping of a cyclic olefin copolymer microfluidic device for automated oocyte culturing. SLAS Technology. 2017;22:507-17
17. Sadeghzadeh Oskouei B, Zargari S, Shahabi P, Ghaffari Novin M, Pashaiasl M. Design and microfabrication of an on-chip oocyte maturation system for reduction of apoptosis. Cell J. 2021;23:32-9
18. Del Valle JS, Mancini V, Laverde Garay M, Asseler JD, Fan X, Metzemaekers J. et al. Dynamic in vitro culture of cryopreserved-thawed human ovarian cortical tissue using a microfluidics platform does not improve early folliculogenesis. Front Endocrinol (Lausanne). 2022;13:936765
19. Nagashima JB, El Assal R, Songsasen N, Demirci U. Evaluation of an ovary-on-a-chip in large mammalian models: species specificity and influence of follicle isolation status. J Tissue Eng Regen Med. 2018;12:e1926-e35
20. Choi JK, Agarwal P, Huang H, Zhao S, He X. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials. 2014;35:5122-8
21. Liu X, Fernandes R, Jurisicova A, Casper RF, Sun Y. In situ mechanical characterization of mouse oocytes using a cell holding device. Lab Chip. 2010;10:2154-61
22. Arai F, Sakuma S. Cell mechanical characterization based on on-chip robotics. Hyper Bio Assembler for 3D Cellular Systems. Tokyo: Springer. 2015 p. 3-22
23. Nakahara K, Sakuma S, Hayakawa T, Arai F. On-chip transportation and measurement of mechanical characteristics of oocytes in an open environment. Micromachines (Basel). 2015;6:648-59
24. Nakahara K, Sakuma S, Kawahara M, Takahashi M, Arai F. Time-lapse mechanical characterization of zona pellucida using a cell carrier chip. J Microelectromech Syst. 2018;27:464-71
25. Andolfi L, Greco SLM, Tierno D, Chignola R, Martinelli M, Giolo E. et al. Planar AFM macro-probes to study the biomechanical properties of large cells and 3D cell spheroids. Acta Biomater. 2019;94:505-13
26. Pokrzywnicka A, Sniadek P, Malyszka N, Lizanets D, Kubicki W, Pawlak P. et al. MEMS cytometer for porcine oocyte deformation measurement. J Micromech Microeng. 2019;29:095004
27. Saffari H, Hajiaghalou S, Hajari MA, Gourabi H, Fathi D, Fathi R. Design and fabrication of aspiration microfluidic channel for oocyte characterization. Talanta. 2023;254:124098
28. Azarkh D, Cao Y, Floehr J, Schnakenberg U. Viscoelastic properties of zona pellucida of oocytes characterized by transient electrical impedance spectroscopy. Biosensors (Basel). 2023;13:442
29. Zhao G, Zhang Z, Zhang Y, Chen Z, Niu D, Cao Y. et al. A microfluidic perfusion approach for on-chip characterization of the transport properties of human oocytes. Lab Chip. 2017;17:1297-305
30. Chen Z, Zhang Z, Guo X, Memon K, Panhwar F, Wang M. et al. Sensing cell membrane biophysical properties for detection of high quality human oocytes. ACS Sens. 2019;4:192-9
31. Lei Z, Xie D, Mbogba MK, Chen Z, Tian C, Xu L. et al. A microfluidic platform with cell-scale precise temperature control for simultaneous investigation of the osmotic responses of multiple oocytes. Lab Chip. 2019;19:1929-40
32. Chen Z, Memon K, Cao Y, Zhao G. A microfluidic approach for synchronous and nondestructive study of the permeability of multiple oocytes. Microsyst Nanoeng. 2020a; 6: 55.
33. Guo X, Chen Z, Memon K, Chen X, Zhao G. An integrated microfluidic device for single cell trapping and osmotic behavior investigation of mouse oocytes. Cryobiology. 2020;92:267-71
34. Tu F, Bhat M, Benson JD. Real-time computer assisted measurement of oocyte and embryo volume for assessment of transport parameters. Cryobiology. 2022;108:19-26
35. Angione SL, Oulhen N, Brayboy LM, Tripathi A, Wessel GM. Simple perfusion apparatus for manipulation, tracking, and study of oocytes and embryos. Fertil Steril. 2015;103:281-90.e5
36. Luo Z, Guven S, Gozen I, Chen P, Tasoglu S, Anchan RM. et al. Deformation of a single mouse oocyte in a constricted microfluidic channel. Microfluid Nanofluidics. 2015;19:883-90
37. Podwin A, Lizanets D, Przystupski D, Kubicki W, Śniadek P, Kulbacka J. et al. Lab-on-chip platform for culturing and dynamic evaluation of cells development. Micromachines (Basel). 2020;11:196
38. Sniadek P, Walczak R, Dziuban J, Jackowska M, Antosik P, Jaskowski J. et al. Lab-on-a-chip for quality classification of pig oocytes. Optica Applicata. 2011;41:417-22
39. Walczak R, Sniadek P, Dziuban JA, Kempisty B, Jackowska M, Antosik P. et al. Lab-on-a-chip spectrophotometric characterization of porcine oocytes. Sens Actuators B Chem. 2012;165:38-43
40. Górecka-Drzazga A, Cichy B, Szczepańska P, Walczak R, Dziuban J. Field-emission light sources for lab-on-a-chip microdevices. Bulletin of the Polish Academy of Sciences: Technical Sciences. 2012;60:13-7
41. El Hasni A, Schmitz C, Bui-Göbbels K, Bräunig P, Jahnen-Dechent W, Schnakenberg U. Electrical impedance spectroscopy of single cells in hydrodynamic traps. Sens Actuators B Chem. 2017;248:419-29
42. Cao Y, Floehr J, Azarkh D, Schnakenberg U. Microfluidic aspiration-assisted electrical impedance spectroscopy system is a reliable tool for the characterization of oocyte hardening. Sens Actuators B Chem. 2023a; 380: 133316.
43. Tedjo W, Obeidat Y, Catandi G, Carnevale E, Chen T. Real-time analysis of oxygen gradient in oocyte respiration using a high-density microelectrode array. Biosensors (Basel). 2021;11:256
44. Heo YS, Lee HJ, Hassell BA, Irimia D, Toth TL, Elmoazzen H. et al. Controlled loading of cryoprotectants (CPAs) to oocyte with linear and complex CPA profiles on a microfluidic platform. Lab Chip. 2011;11:3530-7
45. Yang CY, Chen MC, Lee PT, Lin TT. Cryopreservation of germinal vesicle stage porcine oocytes based on intracellular ice formation assessment. Cryo Letters. 2012;33:349-62
46. Lai D, Ding J, Smith GW, Smith GD, Takayama S. Slow and steady cell shrinkage reduces osmotic stress in bovine and murine oocyte and zygote vitrification. Hum Reprod. 2015;30:37-45
47. Zhou XL, Guo YY, Yi XY, Dai JJ, Zhang DF. Experimental study of microfluidic chip for cryopreservation of oocytes. Progress in Biochemistry and Biophysics. 2018;45:763-71
48. Guo Y, Yang Y, Yi X, Zhou X. Microfluidic method reduces osmotic stress injury to oocytes during cryoprotectant addition and removal processes in porcine oocytes. Cryobiology. 2019;90:63-70
49. Shao WQ, Guo YY, Dai JJ, Zhang DF, Zhou XL. The injury evaluation during cryoprotectant loading with microfluidic chip. Progress in Biochemistry and Biophysics. 2019;46:296-304
50. Zhou X, Du Y, Yi X, Dai J, Zhang D. Optimization of cryoprotectants addition and removal protocols using microfluidic chip. Chinese Journal of Biomedical Engineering. 2019;38:719-25
51. Miao S, Guo C, Jiang Z, Wei H-X, Jiang X, Gu J. et al. Development of an open microfluidic platform for oocyte one-stop vitrification with cryotop method. Biosensors (Basel). 2022;12:766
52. Kawahara T, Ohashi S, Hagiwara M, Yamanishi Y, Arai F. Air-flow-based single-cell dispensing system. Adv Robot. 2012;26:291-306
53. Feng L, Sun Y, Ohsumi C, Arai F. Accurate dispensing system for single oocytes using air ejection. Biomicrofluidics. 2013a; 7: 54113.
54. Iwasaki W, Yamanaka K, Sugiyama D, Teshima Y, Briones-Nagata MP, Maeki M. et al. Simple separation of good quality bovine oocytes using a microfluidic device. Sci Rep. 2018;8:14273
55. Uning KT, Ichikawa K, Hirao A, Michimoto T, Sato T, Kume H. et al. Simultaneous loading and injection chip to automate single-cell injections for bovine oocytes. Sensors and Materials. 2020;32:4151-67
56. Hagiwara M, Kawahara T, Yamanishi Y, Arai F. Driving method of microtool by horizontally arranged permanent magnets for single cell manipulation. Appl Phys Lett. 2010;97:013701
57. Hagiwara M, Kawahara T, Arai F. Local streamline generation by mechanical oscillation in a microfluidic chip for noncontact cell manipulations. Appl Phys Lett. 2012;101:074102
58. Benhal P, Chase JG, Gaynor P, Oback B, Wang W. AC electric field induced dipole-based on-chip 3D cell rotation. Lab Chip. 2014;14:2717-27
59. Hayakawa T, Sakuma S, Arai F. On-chip 3D rotation of oocyte based on a vibration-induced local whirling flow. Microsyst Nanoeng. 2015;1:15001
60. Feng L, Di P, Arai F. High-precision motion of magnetic microrobot with ultrasonic levitation for 3-D rotation of single oocyte. Int J Rob Res. 2016;35:1445-58
61. Feng L, Song B, Zhang D, Jiang Y, Arai F. On-chip tunable cell rotation using acoustically oscillating asymmetrical microstructures. Micromachines (Basel). 2018;9:596
62. Feng L, Song B, Chen Y, Liang S, Dai Y, Zhou Q. et al. On-chip rotational manipulation of microbeads and oocytes using acoustic microstreaming generated by oscillating asymmetrical microstructures. Biomicrofluidics. 2019;13:064103
63. Bai X, Bin S, Yuguo D, Wei Z, Yanmin F, Yuanyuan C. et al. Parallel trapping, patterning, separating and rotating of micro-objects with various sizes and shapes using acoustic microstreaming. Sens Actuators A Phys. 2020;315:112340
64. Clow AL, Gaynor PT, Oback BJ. A novel micropit device integrates automated cell positioning by dielectrophoresis and nuclear transfer by electrofusion. Biomed Microdevices. 2010;12:777-86
65. Hagiwara M, Kawahara T, Yamanishi Y, Arai F. Precise control of magnetically driven microtools for enucleation of oocytes in a microfluidic chip. Adv Robot. 2011;25:991-1005
66. Inomata N, Mizunuma T, Yamanishi Y, Arai F. Omnidirectional actuation of magnetically driven microtool for cutting of oocyte in a chip. J Microelectromech Syst. 2011;20:383-8
67. Ichikawa A, Tanikawa T, Akagi S, Ohba K. Automatic cell cutting by high-precision microfluidic control. Journal of Robotics and Mechatronics. 2011;23:X13-8
68. Feng L, Hagiwara M, Ichikawa A, Arai F. On-chip enucleation of bovine oocytes using microrobot-assisted flow-speed control. Micromachines. 2013b; 4: 272-85.
69. Ichikawa A, Sakuma S, Sugita M, Shoda T, Tamakoshi T, Akagi S. et al. On-chip enucleation of an oocyte by untethered microrobots. J Micromech Microeng. 2014;24:095004
70. Liu Y, Wang X, Zhao Q, Zhao X, Sun M. Robotic batch somatic cell nuclear transfer based on microfluidic groove. IEEE Trans Autom Sci Eng. 2020;17:2097-106
71. Weng L, Lee GY, Liu J, Kapur R, Toth TL, Toner M. On-chip oocyte denudation from cumulus-oocyte complexes for assisted reproductive therapy. Lab Chip. 2018;18:3892-902
72. Mokhtare A, Davaji B, Xie P, Yaghoobi M, Rosenwaks Z, Lal A. et al. Non-contact ultrasound oocyte denudation. Lab Chip. 2022;22:777-92
73. Akagi S, Hosoe M, Matsukawa K, Ichikawa A, Tanikawa T, Takahashi S. Culture of bovine embryos on a polydimethylsiloxane (PDMS) microwell plate. J Reprod Dev. 2010;56:475-9
74. Sugimura S, Akai T, Somfai T, Hirayama M, Aikawa Y, Ohtake M. et al. Time-lapse cinematography-compatible polystyrene-based microwell culture system: a novel tool for tracking the development of individual bovine embryos. Biol Reprod. 2010;83:970-8
75. Han C, Zhang Q, Ma R, Xie L, Qiu T, Wang L. et al. Integration of single oocyte trapping, in vitro fertilization and embryo culture in a microwell-structured microfluidic device. Lab Chip. 2010;10:2848-54
76. Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod. 2010;25:613-22
77. Villa M, Pope S, Conover J, Fan TH. Growth of primary embryo cells in a microculture system. Biomed Microdevices. 2010;12:253-61
78. Ma R, Xie L, Han C, Su K, Qiu T, Wang L. et al. In vitro fertilization on a single-oocyte positioning system integrated with motile sperm selection and early embryo development. Anal Chem. 2011;83:2964-70
79. Esteves TC, van Rossem F, Nordhoff V, Schlatt S, Boiani M, Le Gac S. A microfluidic system supports single mouse embryo culture leading to full-term development. RSC Advances. 2013;3:26451-8
80. Wang W, Peng YY, Liang GT, Liao ZW, Liu DY. A dynamic method of embryo culture based on a microfluidic chip. Reprod Contracept. 2014;22:730-3 +7
81. Huang H-Y, Shen H-H, Tien C-H, Li C-J, Fan S-K, Liu C-H. et al. Digital microfluidic dynamic culture of mammalian embryos on an electrowetting on dielectric (EWOD) chip. PLoS One. 2015a; 10: e0124196.
82. Huang HY, Huang YH, Kao WL, Yao DJ. Embryo formation from low sperm concentration by using dielectrophoretic force. Biomicrofluidics. 2015b; 9: 022404.
83. Kieslinger DC, Hao Z, Vergouw CG, Kostelijk EH, Lambalk CB, Le Gac S. In vitro development of donated frozen-thawed human embryos in a prototype static microfluidic device: a randomized controlled trial. Fertil Steril. 2015;103:680-U393
84. Huang H-Y, Lai Y-L, Yao D-J. Dielectrophoretic microfluidic device for in vitro fertilization. Micromachines (Basel). 2018;9:135
85. Li J, Li Q, Wei G, Liu S, Zhang J, Li Y. Application of a microfluidic chip with mechanical stimuli for culturing mouse embryos in vitro. International Journal of Bioautomation. 2018;22:107-16
86. Yekani F, Fazel-Tabar M, Kowsari-Esfahan R, Renaud P, Kavand H, Esfandiari F. et al. Enhancing developmental rate and quality of mouse single blastomeres into blastocysts using a microplatform. J Cell Physiol. 2018;233:9070-6
87. Chiu YL, Yadav RAK, Huang HY, Wang YW, Yao DJ. Unveiling the potential of droplet generation, sorting, expansion, and restoration in microfluidic biochips. Micromachines (Basel). 2019;10:756
88. Huang HY, Kao WL, Wang YW, Yao DJ. Using a dielectrophoretic microfluidic biochip enhanced fertilization of mouse embryo in vitro. Micromachines (Basel). 2020;11:714
89. Hawkins J, Miao X, Cui W, Sun Y. Surface functionalization of poly(dimethylsiloxane) substrates facilitates culture of pre-implantation mouse embryos by blocking non-selective adsorption. J R Soc Interface. 2022;19:20210929
90. Karcz A, Van Soom A, Smits K, Van Vlierberghe S, Verplancke R, Pascottini OB. et al. Development of a microfluidic chip powered by EWOD for in vitro manipulation of bovine embryos. Biosensors (Basel). 2023;13:419
91. Li WX, Liang GT, Yan W, Zhang Q, Wang W, Zhou XM. et al. Artificial uterus on a microfluidic chip. Fenxi Huaxue/ Chinese Journal of Analytical Chemistry. 2013;41:467-72
92. Li Z, Chen W, Liu W, Liu R. Construction of a microfluidic device for single embryo positioning and co-cultures with three-dimensional cells. Journal of Tsinghua University Science and Technology. 2014;54:1112-6
93. Chang KW, Chang PY, Huang HY, Li CJ, Tien CH, Yao DJ. et al. Womb-on-a-chip biomimetic system for improved embryo culture and development. Sens Actuators B Chem. 2016;226:218-26
94. Ferraz MAMM, Rho HS, Hemerich D, Henning HHW, van Tol HTA, Hölker M. et al. An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat Commun. 2018;9:4934
95. Ferraz MAMM, Henning HHW, Costa PF, Malda J, Melchels FP, Wubbolts R. et al. Improved bovine embryo production in an oviduct-on-a-chip system: prevention of poly-spermic fertilization and parthenogenic activation. Lab Chip. 2017;17:905-16
96. Chen YS, Lo TW, Huang HY, Li LM, Wang YW, Yao DJ. et al. A microfluidic lab chip for the manipulation and co-culturing of embryos with stromal cells. Sens Actuators B Chem. 2021;349:130820
97. Wang M, Zhu T, Liu C, Jin L, Fei P, Zhang B. Oviduct-mimicking microfluidic chips decreased the ROS concentration in the in vitro fertilized embryos of CD-1 mice. Biomed Pharmacother. 2022;154:113567
98. Jang J, Bae CY, Park JK, Ye JC. Self-reference quantitative phase microscopy for microfluidic devices. Opt Lett. 2010;35:514-6
99. Bae CY, Kim MS, Park J-K. Mechanical stimulation of bovine embryos in a microfluidic culture platform. Biochip J. 2011;5:106-13
100. Vandormael-Pournin S, Frachon E, Gobaa S, Cohen-Tannoudji M. Microfabricated device for high-resolution imaging of preimplantation embryos. Methods Mol Biol. 2021;2214:11-30
101. Mohagheghian E, Luo J, Yavitt FM, Wei F, Bhala P, Amar K. et al. Quantifying stiffness and forces of tumor colonies and embryos using a magnetic microrobot. Sci Robot. 2023;8:eadc9800
102. Śniadek P, Walczak R, Dziuban J, Kluger J, Chełmońska-Soyta A. Detection of apoptosis in mice embryos by using lab-on-a-chip device. Procedia Eng. 2012;47:1334-7
103. Sivelli G, Conley GM, Herrera C, Marable K, Rodriguez KJ, Bollwein H. et al. NMR spectroscopy of a single mammalian early stage embryo. J Magn Reson. 2022;335:107142
104. Date Y, Takano S, Shiku H, Ino K, Ito-Sasaki T, Yokoo M. et al. Monitoring oxygen consumption of single mouse embryos using an integrated electrochemical microdevice. Biosens Bioelectron. 2011;30:100-6
105. Kurosawa H, Utsunomiya H, Shiga N, Takahashi A, Ihara M, Ishibashi M. et al. Development of a new clinically applicable device for embryo evaluation which measures embryo oxygen consumption. Hum Reprod. 2016;31:2321-30
106. Heo YS, Cabrera LM, Bormann CL, Smith GD, Takayama S. Real time culture and analysis of embryo metabolism using a microfluidic device with deformation based actuation. Lab Chip. 2012;12:2240-6
107. Chen P, Sun Q, Xiong F, Zhong H, Yao Z, Zeng Y. A method for the detection of hCG β in spent embryo culture medium based on multicolor fluorescence detection from microfluidic droplets. Biomicrofluidics. 2020b; 14: 024107.
108. Lee MS, Hsu W, Huang HY, Tseng HY, Lee CT, Hsu CY. et al. Simultaneous detection of two growth factors from human single-embryo culture medium by a bead-based digital microfluidic chip. Biosens Bioelectron. 2020;150:111851
109. Pyne DG, Liu J, Abdelgawad M, Sun Y. Digital microfluidic processing of mammalian embryos for vitrification. PLoS One. 2014;9:e108128
110. Tirgar P, Sarmadi F, Najafi M, Kazemi P, AzizMohseni S, Fayazi S. et al. Toward embryo cryopreservation-on-a-chip: a standalone microfluidic platform for gradual loading of cryoprotectants to minimize cryoinjuries. Biomicrofluidics. 2021;15:034104
111. Miao S, Jiang Z, Luo J, Zhong F, Wei H, Sun X. et al. A robotic system with embedded open microfluidic chip for automatic embryo vitrification. IEEE Trans Biomed Eng. 2022b; 69: 3562-71.
112. Miao S, Xu J, Jiang Z, Luo J, Sun X, Jiang X. et al. Microfluidics-enabled robotic system for embryo vitrification with real-time observation: design, method, and evaluation. IEEE ASME Trans Mechatron. 2023:1-11
113. Czechowicz P, Nowicka J, Neubauer D, Chodaczek G, Krzyżek P, Gościniak G. Activity of novel ultrashort cyclic lipopeptides against biofilm of candida albicans isolated from VVC in the ex vivo animal vaginal model and bioflux biofilm model-a pilot study. Int J Mol Sci. 2022;23:14453
114. Mahajan G, Doherty E, To T, Sutherland A, Grant J, Junaid A. et al. Vaginal microbiome-host interactions modeled in a human vagina-on-a-chip. Microbiome. 2022;10:201
115. Zhang QC, Wang W, Li WX, Zhang Q, Liang GT, Yan W. et al. Sperm sorting based on the imitation of the physiological process on the microfluidic chip. Zhonghua Nan Ke Xue. 2012;18:803-6
116. Tung CK, Ardon F, Fiore AG, Suarez SS, Wu M. Cooperative roles of biological flow and surface topography in guiding sperm migration revealed by a microfluidic model. Lab Chip. 2014;14:1348-56
117. Tantengco OAG, Richardson LS, Medina PMB, Han A, Menon R. Organ-on-chip of the cervical epithelial layer: a platform to study normal and pathological cellular remodeling of the cervix. Faseb j. 2021;35:e21463
118. Yu S-X, Liu Y, Wu Y, Luo H, Huang R, Wang Y-J. et al. Cervix chip mimicking cervical microenvironment for quantifying sperm locomotion. Biosens Bioelectron. 2022;204:114040
119. Leemans B, Bromfield EG, Stout TAE, Vos M, Van der Ham H, Van Beek R. et al. Developing a reproducible protocol for culturing functional confluent monolayers of differentiated equine oviduct epithelial cells. Biol Reprod. 2022;106:710-29
120. Dadkhah E, Hajari MA, Abdorahimzadeh S, Shahverdi A, Esfandiari F, Ziarati N. et al. Development of a novel cervix-inspired tortuous microfluidic system for efficient, high-quality sperm selection. Lab Chip. 2023;23:3080-91
121. Xie L, Ma R, Han C, Su K, Zhang Q, Qiu T. et al. Integration of sperm motility and chemotaxis screening with a microchannel-based device. Clin Chem. 2010;56:1270-8
122. Yan Y, Liu H, Zhang B, Liu R. A PMMA-based microfluidic device for human sperm evaluation and screening on swimming capability and swimming persistence. Micromachines (Basel). 2020;11:793
123. Raveshi MR, Abdul Halim MS, Agnihotri SN, O'Bryan MK, Neild A, Nosrati R. Curvature in the reproductive tract alters sperm-surface interactions. Nat Commun. 2021;12:3446
124. Yaghoobi M, Azizi M, Mokhtare A, Javi F, Abbaspourrad A. Rheotaxis quality index: a new parameter that reveals male mammalian in vivo fertility and low sperm DNA fragmentation. Lab Chip. 2022;22:1486-97
125. Yu SX, Wu Y, Luo H, Liu Y, Chen YC, Wang YJ. et al. Escaping behavior of sperms on the biomimetic oviductal surface. Anal Chem. 2023;95:2366-74
126. Gnecco JS, Ding T, Smith C, Lu J, Bruner-Tran KL, Osteen KG. Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin E2 and prostacyclin in a microfluidic model of the human endometrium. Hum Reprod. 2019;34:702-14
127. Govindasamy N, Long H, Jeong HW, Raman R, Özcifci B, Probst S. et al. 3D biomimetic platform reveals the first interactions of the embryo and the maternal blood vessels. Dev Cell. 2021;56:3276-87.e8
128. Park JY, Mani S, Clair G, Olson HM, Paurus VL, Ansong CK. et al. A microphysiological model of human trophoblast invasion during implantation. Nat Commun. 2022;13:1252
129. Liu G, Qi Y, Wu J, Lin F, Liu Z, Cui X. Follistatin is a crucial chemoattractant for mouse decidualized endometrial stromal cell migration by JNK signalling. J Cell Mol Med. 2023;27:127-40
130. Govindasamy N, Long H, Ranga A, Trappmann B, Bedzhov I. 3D biomimetic environment enabling ex utero trophoblast invasion and co-culture of embryos and somatic cells. STAR Protoc. 2023;4:102456
131. Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS. et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med. 2016;29:1046-54
132. Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S. et al. A microphysiological model of the human placental barrier. Lab Chip. 2016;16:3065-73
133. Abbas Y, Oefner CM, Polacheck WJ, Gardner L, Farrell L, Sharkey A. et al. A microfluidics assay to study invasion of human placental trophoblast cells. J R Soc Interface. 2017;14:20170131
134. Mandt D, Gruber P, Markovic M, Tromayer M, Rothbauer M, Kratz SRA. et al. Fabrication of biomimetic placental barrier structures within a microfluidic device utilizing two-photon polymerization. Int J Bioprint. 2018;4:144
135. Pemathilaka RL, Caplin JD, Aykar SS, Montazami R, Hashemi NN. Placenta-on-a-chip: in vitro study of caffeine transport across placental barrier using liquid chromatography mass spectrometry. Glob Chall. 2019;3:1800112
136. Mosavati B, Oleinikov AV, Du E. Development of an organ-on-a-chip-device for study of placental pathologies. Int J Mol Sci. 2020;21:8755
137. Ko G, Jeon TJ, Kim SM. Trophoblast migration with different oxygen levels in a gel-patterned microfluidic system. Micromachines (Basel). 2022;13:2216
138. Li J, Qi Y, Yang K, Zhu L, Cui X, Liu Z. Follistatin is a novel chemoattractant for migration and invasion of placental trophoblasts of mice. Cells. 2022;11:3816
139. Mosavati B, Oleinikov A, Du E. 3D microfluidics-assisted modeling of glucose transport in placental malaria. Sci Rep. 2022;12:15278
140. Ghorbanpour SM, Richards C, Pienaar D, Sesperez K, Aboulkheyr Es H, Nikolic VN. et al. A placenta-on-a-chip model to determine the regulation of FKBPL and galectin-3 in preeclampsia. Cell Mol Life Sci. 2023;80:44
141. Kouthouridis S, Sotra A, Khan Z, Alvarado J, Raha S, Zhang B. Modeling the progression of placental transport from early- to late-stage pregnancy by tuning trophoblast differentiation and vascularization. Adv Healthc Mater. 2023;12:e2301428
142. Rabussier G, Bünter I, Bouwhuis J, Soragni C, van Zijp T, Ng CP. et al. Healthy and diseased placental barrier on-a-chip models suitable for standardized studies. Acta Biomater. 2023;164:363-76
143. Cherubini M, Erickson S, Padmanaban P, Haberkant P, Stein F, Beltran-Sastre V. et al. Flow in fetoplacental-like microvessels in vitro enhances perfusion, barrier function, and matrix stability. Sci Adv. 2023;9:eadj8540
144. Cao R, Wang Y, Liu J, Rong L, Qin J. Self-assembled human placental model from trophoblast stem cells in a dynamic organ-on-a-chip system. Cell Prolif. 2023b; 56: e13469.
145. Zhu Y, Yin F, Wang H, Wang L, Yuan J, Qin J. Placental barrier-on-a-chip: modeling placental inflammatory responses to bacterial infection. ACS Biomater Sci Eng. 2018;4:3356-63
146. Richardson L, Jeong S, Kim S, Han A, Menon R. Amnion membrane organ-on-chip: an innovative approach to study cellular interactions. Faseb j. 2019;33:8945-60
147. Richardson L, Gnecco J, Ding T, Osteen K, Rogers LM, Aronoff DM. et al. Fetal membrane organ-on-chip: an innovative approach to study cellular interactions. Reprod Sci. 2020a; 27: 1562-9.
148. Richardson LS, Kim S, Han A, Menon R. Modeling ascending infection with a feto-maternal interface organ-on-chip. Lab Chip. 2020;20:4486-501
149. Yin F, Zhu Y, Wang H, Wang Y, Li D, Qin J. Microengineered hiPSC-derived 3D amnion tissue model to probe amniotic inflammatory responses under bacterial exposure. ACS Biomater Sci Eng. 2020;6:4644-52
150. Radnaa E, Richardson LS, Sheller-Miller S, Baljinnyam T, de Castro Silva M, Kumar Kammala A. et al. Extracellular vesicle mediated feto-maternal HMGB1 signaling induces preterm birth. Lab Chip. 2021;21:1956-73
151. Bento GFC, Richardson LS, da Silva MG, Tantengco OAG, Menon R. Modeling an ascending infection by Ureaplasma parvum and its cell signaling and inflammatory response at the feto-maternal interface. Am J Reprod Immunol. 2023;90:e13770
152. Richardson L, Radnaa E, Lintao RCV, Urrabaz-Garza R, Maredia R, Han A. et al. A microphysiological device to model the choriodecidual interface immune status during pregnancy. J Immunol. 2023b; 210: 1437-46.
153. Richardson LS, Kammala AK, Kim S, Lam PY, Truong N, Radnaa E. et al. Development of oxidative stress-associated disease models using feto-maternal interface organ-on-a-chip. Faseb j. 2023a; 37: e23000.
154. Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA. et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584
155. Park SR, Kim SR, Lee JW, Park CH, Yu WJ, Lee SJ. et al. Development of a novel dual reproductive organ on a chip: recapitulating bidirectional endocrine crosstalk between the uterine endometrium and the ovary. Biofabrication. 2020;13:015001
156. Tantengco OAG, Richardson LS, Radnaa E, Kammala AK, Kim S, Medina PMB. et al. Modeling ascending Ureaplasma parvum infection through the female reproductive tract using vagina-cervix-decidua-organ-on-a-chip and feto-maternal interface-organ-on-a-chip. Faseb j. 2022;36:e22551
157. Campo H, Zha D, Pattarawat P, Colina J, Zhang D, Murphy A. et al. A new tissue-agnostic microfluidic device to model physiology and disease: the lattice platform. Lab Chip. 2023;23:4821-33
158. Russo A, Yang Z, Heyrman GM, Cain BP, Lopez Carrero A, Isenberg BC. et al. Versican secreted by the ovary links ovulation and migration in fallopian tube derived serous cancer. Cancer Lett. 2022;543:215779
159. Ahn J, Yoon MJ, Hong SH, Cha H, Lee D, Koo HS. et al. Three-dimensional microengineered vascularised endometrium-on-a-chip. Hum Reprod. 2021;36:2720-31
160. Baik SY, Miani A, Tinning H, Wang D, Adlam DJ, Ruane P. et al. Transcriptional response of endometrial cells to insulin, cultured using microfluidics. Reprod Fertil. 2023;4:e210120
161. Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A. et al. Placental drug transport-on-a-chip: a microengineered in vitro model of transporter-mediated drug efflux in the human placental barrier. Adv Healthc Mater. 2018;7:1700786
162. Yin F, Zhu Y, Zhang M, Yu H, Chen W, Qin J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol In Vitro. 2019;54:105-13
163. Pu Y, Gingrich J, Veiga-Lopez A. A 3-dimensional microfluidic platform for modeling human extravillous trophoblast invasion and toxicological screening. Lab Chip. 2021;21:546-57
164. Boos JA, Misun PM, Brunoldi G, Furer LA, Aengenheister L, Modena M. et al. Microfluidic co-culture platform to recapitulate the maternal-placental-embryonic axis. Adv Biol (Weinh). 2021;5:e2100609
165. Abostait A, Tyrrell J, Abdelkarim M, Shojaei S, Tse WH, El-Sherbiny IM. et al. Placental nanoparticle uptake-on-a-chip: the impact of trophoblast syncytialization and shear stress. Mol Pharm. 2022;19:3757-69
166. Pemathilaka RL, Alimoradi N, Reynolds DE, Hashemi NN. Transport of maternally administered pharmaceutical agents across the placental barrier in vitro. ACS Appl Bio Mater. 2022;5:2273-84
167. Ticiani E, Pu Y, Gingrich J, Veiga-Lopez A. Bisphenol S impairs invasion and proliferation of extravillous trophoblasts cells by interfering with epidermal growth factor receptor signaling. Int J Mol Sci. 2022;23:671
168. Kammala AK, Richardson LS, Radnaa E, Han A, Menon R. Microfluidic technology and simulation models in studying pharmacokinetics during pregnancy. Front Pharmacol. 2023;14:1241815
169. Ganguly E, Kammala AK, Benson M, Richardson LS, Han A, Menon R. Organic anion transporting polypeptide 2B1 in human fetal membranes: a novel gatekeeper for drug transport during pregnancy? Front Pharmacol. 2021;12:771818
170. Richardson LS, A KK, Costantine MM, Fortunato SJ, Radnaa E, Kim S. et al. Testing of drugs using human feto-maternal interface organ-on-chips provide insights into pharmacokinetics and efficacy. Lab Chip. 2022;22:4574-92
171. Aziz AuR, Yu X, Jiang Q, Zhao Y, Deng S, Qin K. et al. Doxorubicin-induced toxicity to 3D-cultured rat ovarian follicles on a microfluidic chip. Toxicol In Vitro. 2020;62:104677
172. Lee JH, Linden Cvd, Diaz FJ, Wong PK. A reconfigurable microfluidic building block platform for high-throughput nonhormonal contraceptive screening. Lab Chip. 2022;22:2531-9
173. Stejskalová A, Vankelecom H, Sourouni M, Ho MY, Götte M, Almquist BD. In vitro modelling of the physiological and diseased female reproductive system. Acta Biomater. 2021;132:288-312
174. Young RE, Huh DD. Organ-on-a-chip technology for the study of the female reproductive system. Adv Drug Deliv Rev. 2021;173:461-78
175. Fauser BC. Towards the global coverage of a unified registry of IVF outcomes. Reprod Biomed Online. 2019;38:133-7
176. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R. et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393-406
177. Sullivan-Pyke CS, Senapati S, Mainigi MA, Barnhart KT. In vitro fertilization and adverse obstetric and perinatal outcomes. Semin Perinatol. 2017;41:345-53
178. Berntsen S, Söderström-Anttila V, Wennerholm UB, Laivuori H, Loft A, Oldereid NB. et al. The health of children conceived by ART: 'the chicken or the egg?'. Hum Reprod Update. 2019;25:137-58
179. Giorgione V, Parazzini F, Fesslova V, Cipriani S, Candiani M, Inversetti A. et al. Congenital heart defects in IVF/ICSI pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51:33-42
180. Chen X, Bonfiglio R, Banerji S, Jackson DG, Salustri A, Richter RP. Micromechanical analysis of the hyaluronan-rich matrix surrounding the oocyte reveals a uniquely soft and elastic composition. Biophys J. 2016;110:2779-89
181. Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1-24
182. Vo KCT, Kawamura K. Ovarian fragmentation and AKT stimulation for expansion of fertile lifespan. Front Reprod Health. 2021;3:636771
183. Hara T, Matsuura K, Kodama T, Sato K, Kikkawa Y, Muneto T. et al. A tilting embryo culture system increases the number of high-grade human blastocysts with high implantation competence. Reprod Biomed Online. 2013;26:260-8
184. Kolahi KS, Donjacour A, Liu X, Lin W, Simbulan RK, Bloise E. et al. Effect of substrate stiffness on early mouse embryo development. PLoS One. 2012;7:e41717
185. Chung YD, Liu TH, Liang YL, Lin CN, Hsu KF, Lee GB. An integrated microfluidic platform for detection of ovarian clear cell carcinoma mRNA biomarker FXYD2. Lab Chip. 2021;21:2625-32
186. Law KS, Huang CE, Chen SW. Detection of circulating tumor cell-related markers in gynecologic cancer using microfluidic devices: a pilot study. Int J Mol Sci. 2023;24:2300
187. Abu Ajamieh I, Benhabib B, Mills JK. Automatic system for the blastocyst embryo manipulation and rotation. Ann Biomed Eng. 2020;48:426-36
188. Li GP, White KL, Bunch TD. Review of enucleation methods and procedures used in animal cloning: state of the art. Cloning Stem Cells. 2004;6:5-13
189. Rasmussen MB, Oddershede LB, Siegumfeldt H. Optical tweezers cause physiological damage to Escherichia coli and Listeria bacteria. Appl Environ Microbiol. 2008;74:2441-6
190. Thompson GL, Roth CC, Kuipers MA, Tolstykh GP, Beier HT, Ibey BL. Permeabilization of the nuclear envelope following nanosecond pulsed electric field exposure. Biochem Biophys Res Commun. 2016;470:35-40
191. Stacey M, Fox P, Buescher S, Kolb J. Nanosecond pulsed electric field induced cytoskeleton, nuclear membrane and telomere damage adversely impact cell survival. Bioelectrochemistry. 2011;82:131-4
192. Baniasadi F, Hajiaghalou S, Shahverdi A, Pirhajati V, Fathi R. Static magnetic field halves cryoinjuries of vitrified mouse COCs, improves their functions and modulates pluripotency of derived blastocysts. Theriogenology. 2021;163:31-42
193. Panagopoulos DJ, Karabarbounis A, Lioliousis C. ELF alternating magnetic field decreases reproduction by DNA damage induction. Cell Biochem Biophys. 2013;67:703-16
194. Chen JS, Tsai LK, Yeh TY, Li TS, Li CH, Wei ZH. et al. Effects of electromagnetic waves on oocyte maturation and embryonic development in pigs. J Reprod Dev. 2021;67:392-401
195. Karvas RM, Khan SA, Verma S, Yin Y, Kulkarni D, Dong C. et al. Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens. Cell Stem Cell. 2022;29:810-25.e8
196. Elzinga FA, Khalili B, Touw DJ, Prins JR, Olinga P, Leuvenink HGD. et al. Placenta-on-a-chip as an in vitro approach to evaluate the physiological and structural characteristics of the human placental barrier upon drug exposure: a systematic review. J Clin Med. 2023;12:4315
197. Castillo-Armengol J, Fajas L, Lopez-Mejia IC. Inter-organ communication: a gatekeeper for metabolic health. EMBO Rep. 2019;20:e47903
198. Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL. et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650-4
199. Chen H, Zhao L, Kumazawa M, Yamauchi N, Shigeyoshi Y, Hashimoto S. et al. Downregulation of core clock gene Bmal1 attenuates expression of progesterone and prostaglandin biosynthesis-related genes in rat luteinizing granulosa cells. Am J Physiol Cell Physiol. 2013;304:C1131-40
200. Amano T, Tokunaga K, Kakegawa R, Yanagisawa A, Takemoto A, Tatemizo A. et al. Expression analysis of circadian genes in oocytes and preimplantation embryos of cattle and rabbits. Anim Reprod Sci. 2010;121:225-35
201. Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y. et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25:1225-33
202. Cherubini M, Haase K. A bioengineered model for studying vascular-pericyte interactions of the placenta. Methods Mol Biol. 2023;2608:409-23
203. Hu Z, Yang L, Xu B, Zhang C, Zhong H, Xin C. et al. Integration of functional microstructures inside a microfluidic chip by direct femtosecond laser writing. 9th International Symposium on Advanced Optical Manufacturing and Testing Technologies - Subdiffraction-Limited Plasmonic Lithography and Innovative Manufacturing Technology. Chengdu, PEOPLES R CHINA. 2018
204. Zheng G, Lee SA, Yang S, Yang C. Sub-pixel resolving optofluidic microscope for on-chip cell imaging. Lab Chip. 2010;10:3125-9
205. Borchers A, Pieler T. Programming pluripotent precursor cells derived from xenopus embryos to generate specific tissues and organs. Genes. 2010;1:413-26
206. Mansour H, Soliman EA, El-Bab AMF, Abdel-Mawgood AL. Development of epoxy resin-based microfluidic devices using CO2 laser ablation for DNA amplification point-of-care (POC) applications. Int J Adv Manuf Technol. 2022;120:4355-72
207. Edwards TL, Harper JC, Polsky R, Lopez DM, Wheeler DR, Allen AC. et al. A parallel microfluidic channel fixture fabricated using laser ablated plastic laminates for electrochemical and chemiluminescent biodetection of DNA. Biomicrofluidics. 2011;5:44115-4411514
208. Hwang J, Cho YH, Park MS, Kim BH. Microchannel fabrication on glass materials for microfluidic devices. International Journal of Precision Engineering and Manufacturing. 2019;20:479-95
209. Niculescu A-G, Chircov C, Birca AC, Grumezescu AM. Fabrication and applications of microfluidic devices: a review. Int J Mol Sci. 2021;22:2011
210. Pan LJ, Tu JW, Ma HT, Yang YJ, Tian ZQ, Pang DW. et al. Controllable synthesis of nanocrystals in droplet reactors. Lab Chip. 2018;18:41-56
211. Campbell SB, Wu Q, Yazbeck J, Liu C, Okhovatian S, Radisic M. Beyond polydimethylsiloxane: alternative materials for fabrication of organ-on-a-chip devices and microphysiological systems. ACS Biomater Sci Eng. 2021;7:2880-99
212. Ivic A, Onyeaka H, Girling A, Brewis IA, Ola B, Hammadieh N. et al. Critical evaluation of methylcellulose as an alternative medium in sperm migration tests. Hum Reprod. 2002;17:143-9
213. Gonzalez G, Roppolo I, Pirri CF, Chiappone A. Current and emerging trends in polymeric 3D printed microfluidic devices. Addit Manuf. 2022;55:102867
214. Nielsen AV, Beauchamp MJ, Nordin GP, Woolley AT. 3D printed microfluidics. Annu Rev Anal Chem (Palo Alto Calif). 2020;13:45-65
215. Bhattacharjee N, Urrios A, Kang S, Folch A. The upcoming 3D-printing revolution in microfluidics. Lab Chip. 2016;16:1720-42
216. Stansbury JW, Idacavage MJ. 3D printing with polymers: challenges among expanding options and opportunities. Dent Mater. 2016;32:54-64
217. Li F, Macdonald NP, Guijt RM, Breadmore MC. Increasing the functionalities of 3D printed microchemical devices by single material, multimaterial, and print-pause-print 3D printing. Lab Chip. 2019;19:35-49
218. Oleaga C, Bernabini C, Smith AST, Srinivasan B, Jackson M, McLamb W. et al. Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep. 2016;6:20030
219. Ataç B, Wagner I, Horland R, Lauster R, Marx U, Tonevitsky AG. et al. Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip. 2013;13:3555-61
220. Sittadjody S, Saul JM, Opara EC. Compartmentalization of two cell types in multilayered alginate microcapsules. Methods Mol Biol. 2017;1479:225-35
221. Laffan SB, Posobiec LM, Uhl JE, Vidal JD. Species comparison of postnatal development of the female reproductive system. Birth Defects Res. 2018;110:163-89
222. Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016;33:1595-603
223. Cunha GR, Sinclair A, Ricke WA, Robboy SJ, Cao M, Baskin LS. Reproductive tract biology: of mice and men. Differentiation. 2019;110:49-63
224. Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol. 1997;273:C1596-604
225. Cartwright L, Poulsen MS, Nielsen HM, Pojana G, Knudsen LE, Saunders M. et al. In vitro placental model optimization for nanoparticle transport studies. Int J Nanomedicine. 2012;7:497-510
226. Vardhana PA, Illsley NP. Transepithelial glucose transport and metabolism in BeWo choriocarcinoma cells. Placenta. 2002;23:653-60
227. Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K. et al. Derivation of human trophoblast stem cells. Cell Stem Cell. 2018;22:50-63.e6
228. Castel G, Meistermann D, Bretin B, Firmin J, Blin J, Loubersac S. et al. Induction of human trophoblast stem cells from somatic cells and pluripotent stem cells. Cell Rep. 2020;33:108419
229. Liu X, Ouyang JF, Rossello FJ, Tan JP, Davidson KC, Valdes DS. et al. Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature. 2020;586:101-7
230. Lo B, Chou V, Cedars MI, Gates E, Taylor RN, Wagner RM. et al. Informed consent in human oocyte, embryo, and embryonic stem cell research. Fertil Steril. 2004;82:559-63
Author contact
![]() Corresponding authors: Department of Obstetrics and Gynecology, Tongji Hospital, No. 1095, Jiefang Avenue, 430030 Wuhan, China. E-mail: shixuanwangtjmu.edu.cn (S.X.W.); jinjinzhangtjmu.edu.cn (J.J.Z.).
Corresponding authors: Department of Obstetrics and Gynecology, Tongji Hospital, No. 1095, Jiefang Avenue, 430030 Wuhan, China. E-mail: shixuanwangtjmu.edu.cn (S.X.W.); jinjinzhangtjmu.edu.cn (J.J.Z.).
 Global reach, higher impact
Global reach, higher impact