13.3
Impact Factor
Theranostics 2024; 14(9):3674-3692. doi:10.7150/thno.98178 This issue Cite
Review
Trop2-targeted therapies in solid tumors: advances and future directions
1. The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao 266071, China.
2. Qingdao Cancer Institute, Qingdao, 266071, China.
3. School of Life Sciences, Tsinghua University, Beijing, 100084, China.
Received 2024-5-7; Accepted 2024-6-4; Published 2024-6-11
Abstract
Trophoblast cell surface antigen 2 (Trop2) is overexpressed in a range of solid tumors and participants in multiple oncogenic signaling pathways, making it an attractive therapeutic target. In the past decade, the rapid development of various Trop2-targeted therapies, notably marked by the advent of the antibody-drug conjugate (ADC), revolutionized the outcome for patients facing Trop2-positive tumors with limited treatment opinions, such as triple-negative breast cancer (TNBC). This review provides a comprehensive summary of advances in Trop2-targeted therapies, including ADCs, antibodies, multispecific agents, immunotherapy, cancer vaccines, and small molecular inhibitors, along with in-depth discussions on their designs, mechanisms of action (MOAs), and limitations. Additionally, we emphasize the clinical research progress of these emerging Trop2-targeted agents, focusing on their clinical application and therapeutic efficacy against tumors. Furthermore, we propose directions for future research, such as enhancing our understanding of Trop2's structure and biology, exploring the best combination strategies, and tailoring precision treatment based on Trop2 testing methodologies.
Keywords: Trop2, targeted therapies, solid tumor, therapeutic efficacy
Introduction
Trophoblast cell surface antigen 2 (Trop2), identified in 1981, was a type I surface glycoprotein that has garnered attention in oncology [1]. It is also known by various other names including epithelial glycoprotein-1 (EGP-1), gastrointestinal tumor-associated antigen GA7331, pancreatic carcinoma marker protein GA733-1/GA733, CAA1, membrane component chromosome 1 surface marker 1 (M1S1), and TTD2 [2-4]. Trop2 is critical in embryonic organ development, and its expression is limited in normal tissues [5]. Accumulating evidence suggests that Trop2 is overexpressed in a range of solid tumors, significantly impacting tumor growth, invasion, and metastasis [6, 7]. Consequently, Trop2 has become an attractive prognostic marker and therapeutic target for solid tumors. The approval of the first Trop2-targeted antibody-drug conjugate (ADC), TrodelvyTM (sacituzumab govitecan), which enhances survival in metastatic breast cancer (mBC) and metastatic urothelial cancer (mUC), has encouraged further research on Trop2 biology and the expansion of Trop2-targeted therapeutic opinions. Despite its potential, poor response rates and unfavorable risk-benefit profiles present ongoing challenges. As a result, extensive research continues in preclinical, translational, and clinical settings to innovate and enhance targeted therapies for Trop2.
Progress in Trop2 targeting involves advancing ADC technology, modifying linkers, payloads, or antibody scaffolds to optimize therapeutic efficacy [8]. Other approaches encompass the development of antibodies, multispecific agents, immunotherapies, cancer vaccines, and small molecule inhibitors. In this review, we present an unprecedented overview of Trop2-targeted therapies, with a focus on their designs, mechanisms of action (MOAs), and limitations. We also highlight the clinical advancements of novel therapies, which could alter the treatment landscape of Trop2-positive tumors. Finally, we maintain an optimistic view regarding the future of Trop2-targeted therapies, envisioning a growing emphasis on Trop2's structure and biology, combination strategies, and biomarker-driven precision treatment that will contribute to improved clinical outcomes for patients with Trop2-positive tumors.
Trop2-targeted therapies
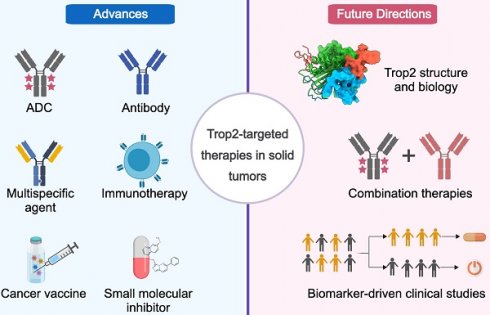
Since the initial approval of TrodelvyTM for the treatment of triple-negative breast cancer (TNBC) and mUC, a range of Trop2-directed therapies with different MOAs and safety profiles have been developed (Figure 1). Next, we summarize their therapeutic outcomes and discuss the designs, MOAs, and limitations of each strategy.
Trop2-directed ADCs
In light of TrodelvyTM's success, over fifteen Trop2-directed ADCs are currently undergoing clinical investigation with the goal of improving therapeutic efficacy. Below, we discuss in detail key examples of novel ADCs, and additional ADCs in clinical development are listed in Table 1.
Sacituzumab govitecan (TrodelvyTM). Sacituzumab govitecan (SG), developed by Immunomedics and Gilead Science, is the only approved Trop2-targeted agent. It consists of a humanized IgG1κ anti-Trop2 antibody (hRS7), a hydrolyzable CL2A linker, and the active metabolite of irinotecan (SN-38) [9, 10]. The preclinical study demonstrated that SG exerts significant and specific antitumor effects against various Trop2-positive tumor types. Additionally, this first-in-class (FIC) Trop2-targeted ADC is well tolerated in monkeys at clinically relevant doses [11]. Multiple unique attributes, including a high drug-to-antibody ratio (DAR) of 7.6, a moderately potent payload, and a high dosing regimen, contribute to the favorable therapeutic index of SG [12]. Numerous clinical trials have shown favorable outcomes with SG, leading the approval from the Food and Drug Administration (FDA) [13-20]. To date, SG has been approved for three indications, including unresectable locally advanced or metastatic TNBC, unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer (HR+/HER2- BC), and locally advanced or mUC.
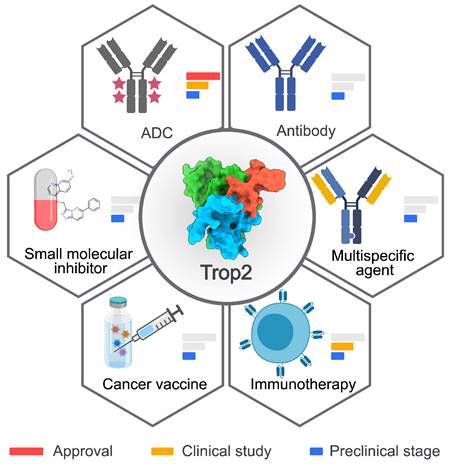
Summary of Trop2-targeted therapies. Six types of Trop2-targeted therapeutics have been developed. The development phases are depicted with a red bar for approval, a yellow bar for clinical studies, and a blue bar for the preclinical stage. The crystal structure of Trop2-ECD (7E5M) was obtained from Protein Data Bank (PDB). The image was created with MedPeer.cn.
Additional Trop2-targeted ADCs in clinical development
| Name | Institute/Company | Phase/Clinical trial ID | Trial design/Patient population | Reference |
|---|---|---|---|---|
| BIO-106 | BiOneCure Therapeutics | Phase 1/2/ NCT05320588 | BIO-106 or BIO-106 + pembrolizumab in advanced cancers | Not applicable |
| BL-M02D1 | Biokin Pharmaceutical | Phase 1/2/ NCT05339685, NCT05385692, NCT05949619 | BL-M02D1 in locally advanced or metastatic TNBC or other solid tumors (NCT05339685); BL-M02D1 in locally advanced or metastatic gastroenteric tumors or other solid tumors (NCT05385692); BL-M02D1 in locally advanced or metastatic NSCLC or other solid tumors (NCT05949619) | [99] |
| 9MW2921 | Mabwell (Shanghai) Bioscience | Phase 1/2/ NCT05990452 | 9MW2921 in locally advanced or metastatic solid tumors refractory to all standard therapies | Not applicable |
| DB-1305 | Yinen Biopharmaceutical | Phase 1/2/ NCT05438329 | DB-1305 in advanced/unresectable, recurrent, or metastatic malignant solid tumors | [100] |
| IBI130 | Innovent Biologics | Phase 1/2/ NCT05923008 | IBI130 in unresectable, locally advanced, or metastatic solid tumors | Not applicable |
| HS-20105 | Hansoh Pharma | Phase 1/ NCT06144723 | HS-20105 in advanced solid tumors | Not applicable |
| BAT8008 | Bio-Thera Solutions | Phase 1/ NCT05620017 | BAT8008 in advanced solid tumors | [101] |
| FDA018 | Shanghai Fudan Zhangjiang Bio Pharmaceutical | Phase 1/ NCT05174637 | FDA018 in advanced solid tumors | Not applicable |
| FZ-AD004 | Shanghai Fudan-Zhangjiang Bio-Pharmaceutical | Phase 1/ NCT05914545 | FZ-AD004 in advanced/metastatic solid tumors | Not applicable |
| JS108 | Hangzhou DAC Biotechnology | Phase 1/ NCT04601285 | JS108 in advanced solid tumors | Not applicable |
| BAT8003 | Bio-Thera Solutions | Phase 1/ NCT03884517, terminated | BAT8003 in advanced epithelial cancer | [102] |
TNBC, triple-negative breast cancer; NSCLC, non-small cell lung cancer.
Currently, more than 40 clinical trials are ongoing or planned to explore the efficacy of SG, alone or in combination with other therapies, across various solid tumor types, especially those with limited treatment opinions. It is heartening to note that an impressive objective response rate (ORR) of 70% was achieved in the Double Antibody Drug Conjugate (DAD) phase I trial, which was designed to evaluate the combination of SG and a nectin-4-directed ADC enfortumab vedotin (EV) in mUC. Given the promising clinical results of SG + EV, further trials have been planned to investigate triplet therapy of SG in combination with EV and pembrolizumab in treating mUC. Although SG has enhanced responses and outcomes for patients with metastatic TNBC and mUC, the prevalence of SG-related adverse events (AEs) has significantly risen between 2020 and 2023, coinciding with its expanded clinical use. The predominant AEs reported in mTNBC clinical trials involving SG treatment included diarrhea, neutropenia, leukopenia, nausea, anemia, constipation, fatigue, alopecia, and vomiting. Approximately 60% of patients treated with SG experienced diarrhea, with around 10% suffering from severe (grade 3) episodes, likely due to the drug dissociating early from its antibody, leading to off-target off-tumor toxicity [16, 20]. Liu et al. identified an association between SG and an increased risk of blood lymphatic system disorders and hepatobiliary disorders [21]. Additionally, they noted that the median onset time for SG-related AEs was 14 days, with the bulk of these events emerging within the first month of treatment, although some AEs continued to manifest up to six months later. Consequently, extended follow-up periods will be essential to monitor SG-related AEs in upcoming clinical trials. Likewise, resistance to SG continues to be a significant challenge. One reason for SG treatment failure is the lack of Trop2 expression. The T256R mutation of Trop2 can lead to decreased plasma membrane localization and reduced cell-surface binding by hRS7, resulting in acquired resistance to SG [22]. In January 2024, Gilead announced that the phase 3 EVOKE-01 study did not meet its primary endpoint of overall survival (OS) in previously treated metastatic non-small cell lung cancer (NSCLC). In May 2024, the TROPiCS-04 phase 3 confirmatory study focusing on locally advanced or mUC failed to meet the primary endpoint of OS in the intention-to-treat population. The experience with NSCLC and mUC indicates that replicating the success seen in breast cancer treatment is challenging due to differences in tumor biology and Trop2 expression levels.
Datopotamab deruxtecan (Dato-DXd). Dato-DXd, a novel Trop2-directed ADC, has been developed by Daiichi Sankyo and AstraZeneca. It consists of a recombinant humanized anti-Trop2 IgG1 monoclonal antibody (mAb) conjugated with a topoisomerase I inhibitor (DXd) via a tetrapeptide-based linker, which attaches to reduce cysteine residues at the interchain disulfide bonds of datopotamab [23]. High internalization activity, DNA damage, and apoptosis induced by Dato-DXd were observed in Trop2-expressing tumor cells in vitro. Dato-DXd further showed potent antitumor efficacy in a broad range of xenograft tumors in vivo, including patient-derived xenograft (PDX) models of NSCLC. Additionally, Dato-DXd exhibited well tolerability in rat and monkey models. Unlike other ADCs, Dato-DXd was designed with an optimized DAR of 4, which helps to maximize the therapeutic window in preclinical studies. To date, 18 clinical trials have been initiated to evaluate the therapeutic efficacy and safety of Dato-DXd in various solid tumors, including TNBC, HR-positive, HER2-low or -negative breast cancer (BC), and NSCLC. The primary results of a first-in-human (FIH) phase 1 clinical study (ClinicalTrials.gov identifier: NCT03401385) revealed that Dato-DXd showed promising antitumor activity and a manageable safety profile in heavily pretreated patients with advanced NSCLC [24]. The ORR was 26%, with an OS of 6.9 months. The TROPION-Lung01 phase 3 study (NCT04656652) indicated a significant improvement in progression-free survival (PFS) with Dato-DXd compared to docetaxel (DTX) in previously treated advanced/metastatic NSCLC [25]. Additionally, the phase 1b clinical study TROPION-Lung02 (NCT04526691) demonstrated that the combination of Dato-DXd and pembrolizumab exerted promising first-line (1L) activity with an ORR of 60% in advanced NSCLC [26]. Initial results from the phase 2 TROPION-Lung05 trial (NCT04484142) showed that Dato-DXd exhibited encouraging antitumor activity with a confirmed ORR (cORR) of 35.8% in heavily pretreated NSCLC patients with actionable genomic alterations (AGAs) [27]. Overall, Dato-DXd has demonstrated tremendous potential and a manageable safety profile in the treatment of various Trop2-positive solid tumors, with particular promise shown in NSCLC. While the clinical potential of Dato-DXd is promising, its side effects require diligent attention and management. Interstitial lung disease (ILD) is a significant identified risk associated with Dato-DXd, especially in patients with NSCLC. During the TROPION-PanTumor01 clinical trial, 11% of patients experienced drug-related ILD [28]. Careful monitoring and prompt intervention are necessary for ILDs to minimize the risk of serious health outcomes. Prophylactic measures are essential to prevent infusion-related reactions (IRRs), oral mucositis, stomatitis, ocular surface events (OSEs), and nausea and vomiting.
SKB264. SKB264 is a Trop2-targeted ADC that is developed by Kelun-Biotech. Sharing the same mAb as SG, SKB264 uses a sulfonyl pyrimidine-CL2A-carbonate linker to conjugate the mAb with seven to eight molecules of a payload named KL610023, a topoisomerase I inhibitor derived from Belotecan. Like other ADCs, SKB264 is engineered to release a free payload that inhibits tumor cell proliferation following internalization prompted by Trop2-specific binding [29]. Preclinical data showed that SKB264 exhibited potent inhibitory activity with a nanomolar level of inhibitory concentration IC50 in various Trop2-positive tumor cell lines. Furthermore, SKB264 significantly inhibited tumor growth in a dose-dependent manner in both cell-derived xenograft (CDX) and PDX models in vivo. The pharmacokinetic-pharmacodynamic (PK-PD) study demonstrated that SKB264, with its longer half-life, had better antitumor efficacy than SG. This data suggests that SKB264 has achieved a favorable balance between stability in circulation and release of the payload within tumor cells. Building on these positive outcomes, eight clinical trials are underway or planned to assess the therapeutic effect of SKB264, alone or in combination with other agents, in multiple advanced solid tumors, such as NSCLC, TNBC, and HR+/HER2- BC. Recently, the primary results from the clinical trial of SKB264 in advanced NSCLC patients (NCT04152499) were presented at the 2023 ASCO conference [30]. In the study, the ORR of 44% and median duration of response (DoR) of 9.3 months were observed among the 39 response-valuable patients. Notably, in the subgroup with tyrosine kinase inhibitor (TKI) -resistant epidermal growth factor receptor (EGFR) -mutant NSCLC, an ORR of 60% and a median PFS of 11.1 months were recorded. Additionally, there were no reported cases of neuropathy or drug-related ILD/pneumonitis. The phase 2 clinical study of SKB264 in patients with locally advanced or metastatic TNBC also showed encouraging antitumor activity, demonstrated by an ORR of 42.4% and a manageable safety profile. Results from the phase 3 OptiTROP-Breast01 study (NCT05347134) in patients with advanced TNBC showed that SKB264 monotherapy significantly improves PFS and ORR compared to chemotherapy. Additionally, it maintains a manageable safety profile in patients with heavily pretreated TNBC and few treatment alternatives. Kelun-Biotech has submitted a listing application of SKB264 in China for the treatment of adult patients with unresectable locally advanced or metastatic TNBC who have received at least two prior systemic therapies, including at least one for advanced or metastatic disease.
PF-06664178 (RN927C). PF-06664178, a site-specific Trop2-targeted ADC with enhanced stability, was first reported by Pfizer in 2016 [31]. This ADC is composed of a humanized anti-Trop2 IgG1 antibody (RN926) conjugated to an AcLys-VCAur0101 linker-payload. Aur0101 is a potent tubulin polymerization inhibitor with an IC50 in the picomolar range. In consideration of the balance of efficacy and toxicity, a low DAR of 2 was chosen for PF-06664178. Preclinical studies demonstrated robust in vitro cytotoxicity of PF-06664178 against Trop2-positive tumor cell lines and promising in vivo antitumor efficacy in a range of PDX models, including TNBC, ovarian, and lung cancers [31]. Toxicity signals, it should be noted, were observed in Trop2-expressing normal tissues of cynomolgus monkeys. Increased necrosis was observed in various epithelial tissues, including skin, upper gastrointestinal (GI) mucosa, and vaginal tissues. In 2014, a phase 1 dose-escalation study (NCT02122146) was initiated to evaluate the safety and tolerability of PF-06664178 in patients with advanced solid tumors. However, patients treated with PF-06664178 showed no objective responses, and the drug exhibited toxicity at high doses with only modest antitumor activity [32]. Seven patients experienced at least one dose-limiting toxicity (DLT), such as neutropenia or rash. Owing to the potential lack of a therapeutic window, further development of PF-06664178 was discontinued. The excess toxicities of PF-06664178 are thought to be attributed to its highly potent payload and stable linker.
SHR-A1921. SHR-A1921, developed by Hengrui, is a novel Trop2-directed ADC with an optimized DAR of 4. It comprises a proprietary IgG1 mAb conjugated to DNA topoisomerase I inhibitor via a tetrapeptide-based cleavable linker [33]. The payload, named SHR9265, is a novel exatecan derivative characterized by great liposolubility and cellular permeability. Compared to SG and SKB264, SHR-A1921 demonstrated stronger binding affinity, improved plasma stability, a more effective bystander effect, superior in vivo efficacy, and a longer half-life. Preliminary data from a FIH phase 1 study (NCT05154604) of SHR-A1921 showed an ORR of 33.3% and a disease control rate (DCR) of 80% in patients with pretreated advanced cancer [34]. In all dose cohorts, the most frequent treatment-related adverse events (TRAEs; occurring in ≥30% of participants) included nausea (71.1%), stomatitis (65.8%), anemia (42.1%), and vomiting, decreased appetite, decreased weight, and rash, each affecting 36.8% of patients. Additional ongoing clinical trials (NCT05765032, NCT06394492, and NCT05594875) aim to assess the safety and antitumor activity of SHR-A1921, either alone or in combination with other anticancer agents, in patients with advanced solid tumors.
ESG-401 (STI-3258). ESG-401 consists of a humanized Trop2-directed IgG1 mAb, conjugated to SN-38 via a highly stable and cleavable linker, featuring a DAR of 8. Upon binding to Trop2-expressing tumor cells, ESG-401 could release its payload to inhibit cell proliferation after rapid endocytosis. Preclinical models showed minimal free toxins and high enrichment in tumor tissues. Furthermore, ESG-401 exerted potent in vivo inhibitory effects in various Trop2-expressing tumor models at a low effective dosage and with prolonged tumor growth suppression. Regarding the safety profile, no off-target or off-tumor toxicities were observed in non-human primates that repetitively received ESG-401. The preliminary results of a FIH phase 1 trial (NCT04892342) with ESG-401 demonstrated good safety and tolerability with promising signals of efficacy in heavily pretreated patients with locally advanced or metastatic solid tumors [35]. The most frequently reported TRAEs included leukopenia (80%), neutropenia (69%), anemia (66%), fatigue (54%), nausea (51%), and vomiting (46%). No instances of Grade 3 thrombocytopenia, diarrhea, skin rash, or oral mucositis were observed. No cases of ILD were detected. Further clinical evaluations are necessary to determine its therapeutic potential.
LCB84. LCB84, developed by LegoChem Biosciences, is a next-generation ADC designed for the treatment of solid tumors that express Trop2. It consists of monomethyl auristatin E (MMAE) as the payload and a humanized IgG1 mAb (Hu2G10) that can selectively target ADAM10-activated Trop2 on transformed tumor cells [36]. LegoChem Biosciences engineered LCB84 with a plasma-stable cleavable linker to allow for efficient and traceless payload release in a cancer-specific manner [37]. Compared with SG and Dato-DXd, LCB84 exhibited significantly enhanced in vivo antitumor activity in multiple CDX models, such as those for TNBC, NSCLC, and pancreatic ductal adenocarcinoma (PDAC). Additionally, toxicity testing indicated that LCB84 was well-tolerated with a therapeutic index (TI) of approximately 30 for single dosing and 40 for repeat dosing in cynomolgus monkeys. Encouraged by positive preclinical results, a FIH phase 1/2 clinical trial (NCT05941507) has commenced to evaluate the efficacy of LCB84 as both a single agent or in combination with programmed cell death 1 (PD-1) inhibitor for the treatment of advanced solid tumors.
While ADCs hold immense therapeutic promise in Trop2-positive tumors, actualizing this potential demands overcoming several critical challenges, including intratumor and intratumor heterogeneity, the risks of TRAEs, and drug resistance. The physicochemical and biophysical properties of an ADC are profoundly influenced by its components, including the naked antibody, chemical linker, cytotoxic payload, and conjugation chemistry [38]. To develop next-generation Trop2-targeted ADCs with an ideal efficacy-safety balance, comprehensive optimization of each component is necessary. Notably, Trop2 is expressed on both tumor cells and certain nonmalignant tissues, leading to unavoidable on-target off-tumor toxicities in many clinical trials, resulting in some being discontinued. Therefore, improving tumor-specific binding activity is a crucial objective for developing novel Trop2-targeted ADCs. Moreover, the identification of biomarkers and thorough patient stratification might enhance the clinical outcomes of Trop2-directed ADC therapies. Beyond the traditional ADC format featuring small-molecule drug payloads, alternative conjugation strategies like immunoconjugates and nanoparticles are emerging in preclinical development [39, 40]. Liu et al. engineered a Trop2-targeted tetrakis-ranpirnase conjugate, composed of hRS7 mAb fused with four copies of ranpirnase [41]. This novel IgG-Rap immunoRNase provided a significant survival benefit in TNBC mouse models.
Trop2-directed monoclonal antibodies
Although Trop2-targeted ADCs have shown clinical benefits, their broader utility is still limited by issues such as off-target payload-mediated toxicities [32, 42-44]. Consequently, there is a need to develop safer and more effective targeted agents that leverage the biological functions of Trop2 itself. Recent advances in structural biology have uncovered the unique oligomerization properties of Trop2, which are critical in oncogenic signaling pathways and associated with poor prognosis [45-47]. Furthermore, the large extracellular domain (ECD) makes it an ideal therapeutic target for Trop2-targeted drugs that are designed to block its oncogenic activity [48]. As a result, the development of functional Trop2-directed mAb has become a focal point of recent research. In the following discussion, we will explore recently identified anti-Trop2 mAbs that exhibit potent antitumor activity and possess unique MOAs. Additional mAbs in preclinical development are listed in Table 2.
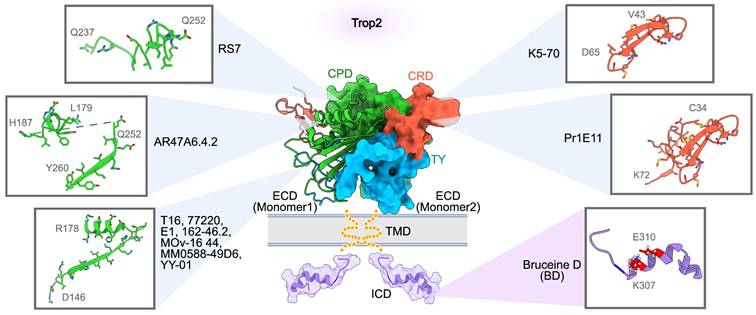
RS7-3G11 (RS7), the antibody component of SG, is an extensively studied anti-Trop2 mAb from early research. Stein et al. obtained RS7 using the hybridoma technique [49, 50]. On its own, RS7 demonstrates strong internalization activity but lacks therapeutic efficacy [11, 51]. In 2021, Sun and colleagues constructed domain-substituted Trop2 mutants to map the epitopes of RS7 [46]. Binding analysis showed that RS7 recognizes a linear polypeptide, Q237-Q252, within the cysteine-poor domain (CPD) of Trop2-ECD (Figure 2). This stretched loop is far from the interfaces of the Trop2-ECD dimer, suggesting that RS7 is unlikely to disrupt the oligomeric assembly of Trop2. This could explain why RS7, in its unconjugated form, does not exert a suppressive effect on Trop2-positive tumors. Similarly, a group of mAbs, such as T16, 77220, E1 [52], 162-46.2, MOv-16 [53], MM0588-49D6, and YY-01 [54] exhibit limited therapeutic effects.
Additional Trop2-targeted monoclonal antibodies in preclinical development
| Name | Institute/ Company | Reference/ Patent |
|---|---|---|
| OXG-64 | Oncoxx Biotech SRL | WO201089782A1 |
| KD065 | Nanjing KAEDI Biotech | Not applicable |
| Harbour Biomed patent anti-Trop2 antibody | Harbour Biomed | WO2022143670 |
| Kisoji Bio patent anti-Trop2 antibody | Kisoji Biotechnology | WO2023049999 |
| WuXi Biologics patent anti-Trop2 antibody | WuXi Biologics (Shanghai) | WO2022152308 |
| Chia Tai Tianqing Pharma patent anti-Trop2 antibody | Chia Tai Tianqing Pharmaceutical Group | WO2023104080 |
| Biosion patent anti-Trop2 antibody | Biosion | WO2022222992 |
| Zhejiang Nanomab Tech. patent anti-Trop2-antibody | Zhejiang Nanomab Technology Center | WO2023274365 |
Binding sites of Trop2-targeted antibodies and small molecular inhibitors. A composite model of full-length Trop2 dimer, projected based on the structures of individual functional modules. PDB identifier 7E5N for ECD, and PDB identifier 2MVK for ICD. RS7 recognizes Q237-Q252 of CPD. AR47A6.4.2 recognizes L179-H187 and Q252-Y260 of CPD. T16, 77220, E1, 162-46.2, MOv-16, MM0588-49D6, and YY-01 recognize D146-R178 of CPD. K5-70 recognizes V43-D65 of CRD. Pr1E11 recognizes C34-K72 of CRD. The only small molecule inhibitor, Bruceine D (BD), binds to the key sites K307 and E310 of Trop2-ICD.
This limitation is partly because their binding epitopes (D146-R178) are distant from the interaction region essential for Trop2 dimerization. These results suggest that the CPD region of Trop2 may comprise multiple immunodominant epitopes, enabling easy screening of numerous antibodies that, however, lack therapeutic functions. Additional research has indicated that these immunodominant epitopes appear to be equally accessible in both tumors and normal cells, raising concerns that Trop2-directed mAbs targeting these epitopes may cause unmanageable off-tumor toxicity [6, 53, 55]. Consequently, the approach of targeting tumor-specific epitopes on Trop2 seems more promising.
Alberti's team identified the cleavage of Trop2 by ADAM metallopeptidase domain 10 (ADAM10) as a critical switch activating tumor growth and metastasis [52]. The proteolysis of Trop-2 at R87-T88, mediated by ADAM10, could induce a profound structural rearrangement, potentially exposing binding sites that are typically inaccessible [56]. To discover mAbs sensitive to these tumor-specific sites, Alberti et al. employed a deletion mutagenesis strategy to remove the immunodominant regions of Trop2 and then selected mAbs by multiple methods [36]. The 2G10 mAb, which selectively recognizes cleaved Trop2 in transformed cells, was obtained and exhibited inhibitory activity on tumor cell proliferation in vitro. Humanized 2G10 (Hu2G10) binds cancer-specific, cleaved/activated Trop2 with a high affinity (KD < 10-12 M), and effectively suppresses the growth of Trop2-positive tumors in vivo, including breast, colon, and prostate cancers [36, 57]. The ADC version of Hu2G10, known as LCB84, is being evaluated in an FIH phase 1/2 clinical trial (NCT05941507) [37]. Given that the combination of two mAbs, binding non-overlapping epitopes, could offer complementary MOAs and lead to synergistic therapeutic efficacy, Alberti's team explored the combined in vivo activity of 2G10 and 2EF-an anti-Trop2 mAb recognizing N-terminal epitopes of Trop2-ECD with proven antitumor activity across multiple tumor models [58]. Analysis of Trop2-expressing CDX models demonstrated that 2EF can serve as a potent synergistic partner, significantly boosting the in vivo tumor-killing effect of 2G10. Furthermore, Hu2G10 and humanized 2EF (Hu2EF) were well-tolerated from 5 to 10 mg/kg, with no evidence of systemic toxicity observed in cynomolgus monkeys [59]. No significant neurological, respiratory, digestive, or urinary symptoms, including vomiting, diarrhea, and anorexia, nor any biochemical or hematological toxicities were observed during the 28-day period. These findings support the therapeutic potential of a tumor-selective strategy targeting the activated Trop2 in cancer cells.
ARIUS Research utilized the FunctionFIRST platform to generate a mAb, AR47A6.4.2, which targets two liner epitopes (L179-H187 and Q252-Y260) within Trop2-CPD (Figure 2). AR47A6.4.2 can inhibit Trop2 signaling and mediate the downregulation of mitogen-activated protein kinase (MAPK) pathway in response to serum stimulation [60]. It also induces complement-dependent cytotoxicity (CDC) in human pancreatic cells. The dual MOAs empower AR47A6.4.2 to inhibit tumor growth in human models of breast (90%, p<0.00001), colon (60%, p<0.001), and prostate (60.9%, p<0.001) cancer. In addition, an enhanced therapeutic efficacy was observed when AR47A6.4.2 was combined with Gemcitabine, a chemotherapy drug for pancreatic cancer, in the pancreatic tumor model [61]. Notably, the Q252-Y260 epitope is located at the interfaces of both the cis-dimer and the trans-dimer of Trop2, implying that AR47A6.4.2 may inhibit tumor growth by preventing Trop2 dimerization [46, 48].
Crystallographic studies of Trop2-ECD have revealed that the N-terminal region, especially the cysteine-rich domain (CRD), is essential for the tetrameric assembly [45, 46]. Therefore, targeting the epitopes within the CRD could influence the dynamic behavior of Trop2, potentially disrupting its activation. Pr1E11, an early reported CRD-targeted mAb, showed low internalization activity but great cell surface retention in Trop2-expressing tumor cells [54, 62]. While Pr1E11 did not show an antiproliferative effect in vitro, it inhibited tumor growth more effectively than AR47A6.4.2 in vivo, aided in part by its potent antibody-dependent cellular cytotoxicity (ADCC). Further research is needed to ascertain if the CRD-binding of Pr1E11 affects the oligomeric state of Trop2 on the tumor cell surface, a crucial factor in Trop2-mediated tumor progression. K5-70, developed by Chiome Bioscience (patent CN107236043B), is another CRD-directed mAb. Chemically linked peptides on scaffolds technology (CLIPS) elucidated that the key epitope of K5-70 is located on V43-D65 polypeptide within Trop2-CRD (Figure 2). This binding region overlaps with the tetramerization interface of Trop2, hinting that K5-70' potential to disrupt the Trop2 clustering on the tumor cell surface and subsequently interfere with its oncogenic signaling pathways. Potent inhibitory effects of K5-70 were observed in vivo across different tumor models such as SW480, DU-145, and PK-59. Additionally, K5-70 effectively suppressed a recurrent tumor model previously treated with irinotecan hydrochloride. These findings confirm the therapeutic potential of CRD-targeted mAb in Trop2-positive tumors.
Besides the aforementioned antibodies with well-defined epitopes, many antibodies with unknown binding sites also exhibit promising antitumor properties. Zhou and colleagues recently reported a novel Trop2-directed mAb, IMB1636, notable for its high affinity and specificity for Trop2-expressing tumors [63]. IMB1636 demonstrated potent internalization activity and sustained tumor-targeting capability in vivo, with an impressive retention of 264 hours. The humanized form of IMB1636 (hIMB1636) recognizes non-competitive binding sites compared to hRS7 and retains robust antitumor activity in MDA-MB-468 xenograft tumors. The promising in vivo antitumor effects are attributable to at least three MOAs: ADCC, the inhibition of cancer cell proliferation and migration, and the induction of cell cycle arrest and apoptosis. Sun et al. further constructed an ADC version of hIMB1636 (hIMB1636-MMAE), using a Valine-Citrulline linker to conjugate mAb with three to four molecules of MMAE [64]. Not only does hIMB1636 retain the MOAs of its parent antibody, but it also achieves enhanced tumor inhibition via bystander effect in pancreatic cancer. TANAKA et al. generated an anti-Trop2 mAb TrMab‑6 via the Cell‑Based Immunization and Screening (CBIS) method [65]. TrMab‑6 strongly triggers ADCC and CDC, leading to pronounced tumor reduction in breast cancer mouse xenograft models. Beyond the conventional IgG antibodies, Trop2-targeted Fab and nanobodies (Nb) may also serve as therapeutic agents in Trop2-expressing tumors. A fully human Fab against Trop2, isolated from a phage library by Lin and colleagues, inhibits breast cancer growth in vitro and in vivo by inducing the downregulation of Bcl-2 and upregulation of Bax in Trop2-expressing tumor cells [66]. This Fab was subsequently used to construct an anti-Trop2 IgG mAb, which inhibits tumor cell growth, migration, and invasion in vitro, and suppresses tumor proliferation in ovarian cancer xenografts [67]. Hu et al. recently described a Trop2-targeted nanobody, N60, that is capable of inhibiting tumor cell proliferation and migration [68].
Overall, antibodies targeting Trop2, particularly those that recognize tumor-specific Trop2, have demonstrated promising therapeutic potential and safety profiles. However, a key challenge hinders their clinical translation: the fundamental relationship between antibodies' binding epitopes and MOAs that contribute to its antitumor effects. It should also be noted that most reported anti-Trop2 mAbs primarily target the CRD and CPD domains, with fewer directed against the thyroglobulin type-1 (TY) domain (Figure 2). Structurally, the TY region is extensively involved in the oligomeric assembly of Trop2 and includes the conserved sites crucial for tumor-specific proteolytic cleavage. Thus, it is reasonable to speculate that targeting TY may interfere with Trop2-mediated intracellular and intercellular communication, potentially hindering the progression of Trop2-positive tumors. Consequently, the discovery of novel mAbs targeting the TY region could offer an alternative therapeutic approach for managing Trop2-positive tumors. Given the widespread expression of Trop2 in normal epithelial tissues, employing non-human primate (NHP) models to assess the safety profile is essential for the preclinical development of the next generation of Trop2-targeted antibodies.
Multispecific agents
Drug resistance and tumor heterogeneity are well-known factors that can limit the clinical outcomes of targeted therapies directed toward a single antigen [8]. To address this challenge, the development of multispecific agents has gained attention as a technique allowing concurrent binding to two or multiple distinct targets, either molecules or cells. A variety of emerging multispecific agents associated with Trop2, such as bispecific antibodies, trispecific antibodies, and bispecific ADCs, have been evaluated in preclinical settings (Table 3). Below, we discuss their MOAs, antitumor efficacy, and limitations.
Bispecific antibodies
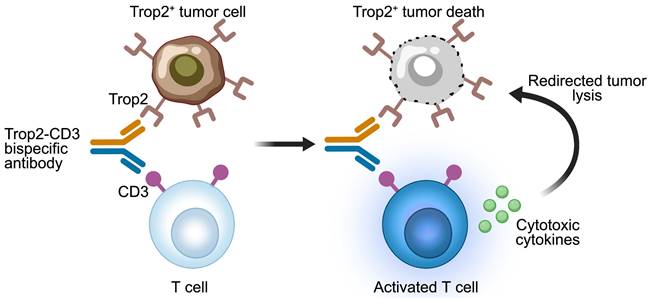
Various formats of bispecific antibodies have been engineered to enhance the antitumor activity and tumor specificity in Trop2-positive solid tumors. The early reported anti-Trop2 bispecific antibodies are bispecific T cell engagers (BiTEs) that use CD3 as a target to redirect T cells towards Trop2-expressing tumor cells (Figure 3). An early BiTEs candidate, (E1)-3s, comprised an anti-CD3 single-chain variable fragment (scFv) fused to a stabilized dimer of a Trop2-targeted Fab (derived from hRS7) via Dock-and-Lock (DNL) technique [69]. Surface plasmon resonance analysis showed that (E1)-3s maintained high Trop2-binding affinity (KD = 1.03 ± 0.19 nM). Bidirectional trogocytosis and functional immunologic synapses induced by (E1)-3s were observed between T cells and target cells. (E1)-3s effectively mediated T-cell activity, leading to the potent lysis of target tumor cells by T-cell. Promising T-cell-mediated killing of Trop2-expressing pancreatic and gastric cancers triggered by (E1)-3s were observed in vitro and in vivo. Moreover, this T-cell redirection against solid cancers was significantly enhanced by interferon-α (INFα). Furthermore, combining (E1)-3s with an antagonistic anti-PD-1 antibody boosted the antitumor potency of T-cells, leading to enhanced cell death in 3D spheroid cultures and prolonged the survival in mice with MDA-MB-231 tumors [70]. These preclinical findings highlight the potential benefits of combining T-cell-redirecting bispecific antibodies with agents that counteract T-cell inhibition in the tumor microenvironment (TME), potentially improving the effectiveness of immunotherapy in patients with solid tumors.
Liu and colleagues engineered an alternative Trop2-targeted BiTE, named F7AK3, designed to bind both CD3 and Trop2, with a predominant affinity for Trop2 [71]. This design aims to prevent non-specific peripheral activation of T-cells and to localize T-cells within the tumors. The tetravalent F7AK3 is composed of an anti-CD3 scFv fused to the Fc region of a human anti-Trop2 IgG4. The in vitro analysis showed that F7AK3 triggered T cell-mediated cytotoxicity against several Trop2-expressing tumor cell types, with the level of activation depending on Trop2 antigen expression. In TNBC xenograft models, F7AK3 was shown to inhibit tumor growth via multiple MOAs. First, F7AK3 treatment resulted in a significant expansion of human immune cells within the spleens and tumors. Second, the treatment enhanced the expression of CD69 and PD1 in both CD8+ and CD4+ T cells.
Third, F7AK3 induced significant upregulation of IL-2 and Granzyme B in CD4+ T cells. Considering the increased risk of cytokine release syndrome due to excessive T cell activation, Wang's team constructed a Trop2-CD3 BiTE with a reduced affinity for CD3, called Trop2/CD3b [72]. Trop2/CD3b consists of an anti-CD3 scFv (from SP34) inserted into the heavy chain of an anti-Trop2 mAb (from Pr1E11). Crucially, Trop2/CD3b elicited a notably lower level of Th1 cytokines production but still effectively maintained tumor cell cytotoxicity in vitro and tumor growth suppression in vivo. To reduce the risk of on-target off-tumor effects, Arbele developed a novel Trop2-CD3 BiTE with weaker binding affinity to cells expressing Trop2 [73]. Comparable to F7AK3 and (E1)-3s, this Trop2-CD3 BiTE was created by fusing a humanized anti-Trop2 mAb with an anti-CD3 scFv. Unlike F7AK3 and (E1)-3s, which both bind tightly to Trop2 antigen, the modified Trop2-CD3 BiTE has a lower affinity with a KD of 214 nM. This is achieved by altering a complementarity-determining region (CDR) on the heavy chain of the anti-Trop2 mAb component. This bispecific antibody candidate merits further exploration, with additional toxicity assessments in non-human primates. These results suggest that optimizing a Trop2-CD3 bispecific antibody requires careful calibration of safety and efficacy, aiming to achieve tumor-specific cytotoxicity without eliciting excessive T-cell activation. Besides the Trop2-CD3 bispecific antibodies, additional bispecific formats like Trop2-HER2, Trop2-PD-L1, and Trop2-CD28 are currently under preclinical investigation (Table 3).
Bispecific or trispecific agents in preclinical development for Trop2-expressing tumors
| Name | Institute/ Company | Type | Target | Reference/ Patent |
|---|---|---|---|---|
| (E1)-3s | Immunomedics | Bispecific antibody | Trop2, CD3 | [69, 70] |
| F7AK3 | Huazhong University of Science and Technology Tongji Medical College | Bispecific antibody | Trop2, CD3 | [71] |
| Trop2/CD3 bispecific antibody | Arbele Corp | Bispecific antibody | Trop2, CD3 | [73] |
| Sunshine Guojian anti-Trop2/CD3 bispecific antibody | Sunshine Guojian Pharmaceutical (Shanghai) | Bispecific antibody | Trop2, CD3 | [72] |
| EX108 | Excelmab | Bispecific antibody | Trop2, CD3 | WO2019184549A1 |
| Janux patent anti-Trop2/CD3 bispecific antibody | Janux Therapeutics | Bispecific antibody | Trop2, CD3 | WO2023164513 |
| BiOneCure patent anti-Trop2/HER2 bispecific antibody | BiOneCure Therapeutics | Bispecific antibody | Trop2, HER2 | WO2022159984 |
| Zeda Bio patent anti-Trop2/PD-L1 bispecific antibody | Zeda Biopharmaceuticals | Bispecific antibody | Trop2, PD-L1 | WO2023098770 |
| Xencor patent anti-Trop2/CD28 bispecific antibody | Xencor | Bispecific antibody | Trop2, CD28 | WO2023164640 |
| TF12 | Radboud University Medical Center | Bispecific antibody | Trop2, HSG | [90] |
| Trop2 ProTriTAC | Harpoon Therapeutics | Trispecific antibody | Trop2, CD3, HSA | [74] |
| Boehringer patent anti-Trop2/CD3/CDH-17 trispecific antibody | Boehringer Ingelheim | Trispecific antibody | Trop2, CD3, CDH-17 | WO2022263507 |
| YH012 | Beijing Biocytogen | Bispecific ADC | Trop2, HER2 | [76] |
| BCG033 | Beijing Biocytogen | Bispecific ADC | Trop2, PTK7 | [79] |
| BCG011 | Beijing Biocytogen | Bispecific ADC | Trop2, EGFR | WO2023046061A1 |
| DM001 | Xadcera Biopharmaceutical | Bispecific ADC | Trop2, EGFR | [75] |
| JSKN016 | Alphamab Oncology | Bispecific ADC | Trop2, HER3 | Not applicable |
| HuNbTROP2-HSA-MMAE | Shanghai Novamab Biopharmaceuticals | Bispecific ADC | Trop2, HSA | [80] |
HER2, human epidermal growth factor receptor 2; PD-L1, programmed death-ligand 1; HSG, histamine-succinyl-glycine; CDH-17, cadherin-17; PTK7, protein tyrosine kinase-7; EGFR, epidermal growth factor receptor; HER3, human epidermal growth factor receptor 3;HSA human serum albumin.
Mechanisms of action of Trop2-CD3 bispecific T cell engagers. The Trop2-CD3 bispecific antibody binds to CD3 on T cells and Trop2 antigen on tumor cells. The activated T cells release cytotoxic cytokines, resulting in the lysis of Trop2-positive tumor cells. The image was created with MedPeer.cn.
Trispecific antibodies
To circumvent the potential for on-target off-tumor toxicities leading to treatment failure, HARPOON Therapeutics has developed a Trop2-targeted ProTriTAC™ (Protease-activated Tri-specific T cell Activating Construct), a prodrug designed for selective activation within tumor tissues. This trispecific T cell engager comprises three antibody-derived binding domains on a single polypeptide chain: one to extend half-life through albumin binding, a second for T cell engagement via CD3 binding, and a third targeting Trop2-positive tumor cells [74]. The anti-albumin domain, equipped with a masking moiety and a protease-cleavable linker, renders the prodrug inactive by preventing the nearby anti-CD3 domain from binding to T cells. Once tumor-associated proteases cleave the linker, the anti-albumin domain and the masking moiety are detached, uncovering a highly active drug that redirects T cells to lyse tumor cells. In vitro experiments revealed that the protease-activated T cell engager exhibits over a 1000-fold increase in binding affinity to primary human T cells and approximately a 100-fold enhancement in T cell cytotoxicity compared to the masked prodrug. In human T cell-reconstituted immunodeficient mice, this anti-Trop2 ProTriTAC displayed potent antitumor effects across various xenograft models, such as HPAFII (pancreatic cancer), CAL27 (head and neck cancer), and HCC70 (breast cancer), with complete tumor regression at doses as low as 30 µg/kg. ProTriTAC showed favorable pharmacokinetics and was well-tolerated up to 180 µg/kg in cynomolgus monkeys. Boehringer Ingelheim has recently patented a novel trispecific T-cell engager that targets Trop2, CD3, and cadherin-17 (CDH-17). The trispecific Trop2/CD3/CDH-17 binding molecule effectively mediated the lysis of Trop2+ CDH-17+ cells in vitro and induced significant tumor regression in vivo in human xenograft mouse models (HPAF-II) reconstituted with human T cells. Additional clinical investigation is required to assess the efficacy and safety of these FIC conditionally active, Trop2-directed trispecific antibodies.
Bispecific ADCs
Bispecific ADCs are emerging targeted agent that simultaneously targets two different antigens. Compared to traditional ADC drugs, bispecific ADCs may present several benefits. First, they can identify and eliminate a wider range of tumor cells, including those in heterogeneous tumor tissues. Second, by selecting optimal targets, bispecific ADCs could enhance tumor-specific binding, given the restricted expression of the target antigens on nonmalignant cells, potentially boosting payload delivery while minimizing toxicities in healthy tissues [8]. Moreover, the simultaneous targeting of multiple antigens or cells could exert a synergistic effect, which may not be attenable by traditional ADCs recognizing a single antigen. Considering these potential advantages, researchers have developed a variety of anti-Trop2 bispecific ADCs, predominantly choosing tumor-associated kinases as their secondary targets.
EGFR is overexpressed in numerous solid tumors and has become a well-established therapeutic target. However, drug resistance and low cytotoxicity remain challenges. Trop2 and EGFR are co-expressed in various solid tumors, such as those of the head and neck, esophagus, lungs, and pancreas, suggesting the potential for a combined therapeutic approach to benefit a diverse set of tumors. Li and colleagues engineered a FIC Trop2-EGFR bispecific ADC, named DM001, using a protease-cleavable linker to conjugate antibody with MMAE [75]. In tumor cells expressing both Trop2 and EGFR, DM001 exhibited comparable internalization and tumor-killing efficacy to that of its parent anti-Trop2 and anti-EGFR mAbs. Furthermore, DM001 demonstrated enhanced selectivity and potency in binding and killing cells with co-expression of Trop2 and EGFR, in comparison to those expressing single targets. The potent in vitro antitumor effect of DM001 is attributable to at least two MOAs: the induction of cell cycle arrest and the elevation of apoptosis rates in an antigen-dependent manner. Notably, DM001 outperformed SG and its parental ADCs in a range of CDX and PDX models, including those for lung and pancreatic tumors. The prior efficacy of DM001 in PDX models indicated that it may be more effective in targeting heterogeneous tumors, which better mimics the TME of patients. Beijing Biocytogen has also developed a Trop2-EGFR bispecific ADC, called BCG011, which is currently undergoing preclinical evaluation.
HER2, another well-established therapeutic target, is often found co-expressed with Trop2 across various solid tumor types, including NSCLC, breast cancer (BC), gastric cancer (GC), and bladder cancer. Shang et al. recently reported a FIC Trop2-HER2 bispecific ADC (YH012), which consists of a bispecific antibody conjugated with MMAE via a protease-cleavable linker [76]. Compared to its parental anti-Trop2 or anti-HER2 mAbs, YH012 exhibited enhanced affinity, internalization activity, and tumor selectivity in vitro. The in vivo studies demonstrated that YH012 effectively suppressed tumor growth in a range of CDX and PDX models, including NSCLC, BC, GC, and pancreatic cancer. Furthermore, YH012 outperformed benchmark antibodies in reducing tumors in both HER2-high and HER2-low-expressing xenograft models, suggesting a robust and expansive therapeutic impact. As a member of the EGFR family, human epidermal growth factor receptor 3 (HER3) plays a key role in regulating the proliferation of epithelial cells [77]. HER3 is a potent activator of the phosphatidylinositide-3 kinase (PI3K)/Akt survival pathway, which is vital for tumor expansion. Alphamab Oncology has recently developed a Trop2-HER3 bispecific ADC, named JSKN016, that was engineered using a Glycan-specific conjugation platform. JSKN016 can induce apoptosis in tumor cells that express either Trop2 or HER3. Additionally, it can penetrate the cell membrane of antigen-negative tumor cells, exerting a bystander effect that helps to inhibit tumor growth through topoisomerase I inhibitor mechanism. The clinical investigation of JSKN016 for treating advanced malignant solid tumors has been approved by China's Center for Drug Evaluation (CDE).
Protein tyrosine kinase-7 (PTK7) exhibits overexpression in breast cancer, with notably higher levels found in TNBC than in non-TNBC. Overexpressed PTK7 is associated with the progression of TNBC and a worse prognosis [78]. Yao and colleagues generated a novel Trop2-PTK7 bispecific ADC (BCG033) composed of a fully human bispecific antibody conjugated with MMAE [79]. Preliminary data indicated that BCG033 had favorable tumor selectivity and exhibited potent antitumor efficacy in both TNBC and non-TNBC CDX models, which co-expressed Trop2 and PTK7. Beyond human IgG1-based scaffolds, the nanobody format has been utilized in constructing bispecific ADCs. HuNbTROP2-HSA-MMAE is a nanobody-drug conjugate that simultaneously targets Trop2 and human serum albumin (HSA) [80]. The humanized anti-Trop2 nanobody was fused to an anti-HSA nanobody and conjugated to MMAE by a cleavable linker with a DAR of 1. HuNbTROP2-HSA-MMAE retained high affinity and binding specificity for Trop2 and HSA. The potent internalization activity of HuNbTROP2-HSA-MMAE facilitated its involvement in lysosomal trafficking, leading to the release of cytotoxic payload. Its tumor-killing efficacy in Trop2-expressing pancreatic cancer is attributed to caspase-3/9-induced cell apoptosis. The in vivo studies using pancreatic cancer CDX models revealed that doses of HuNbTROP2-HSA-MMAE at 0.2 mg/kg and 1 mg/kg achieved notable antitumor effects, with complete tumor eradication achieved at a dose of 5 mg/kg.
CAR-T and CAR-NK
Chimeric antigen receptors (CARs) are engineered constructs that combine the targeting precision of antibodies with the effector function signaling of T cells, forming a pivotal subset of cellular immunotherapies. While CAR-T therapies have received FDA approval for treating hematological malignancies, challenges with the directed migration and infiltration of T cells into the TME impede their effectiveness against solid tumors. Nonetheless, some Trop2-specific CARs have demonstrated promising efficacy in preclinical or early clinical stages (Table 4). Cancer Hospital Chinese Academy of Medical Sciences has developed a Trop2 CAR-T that has been evaluated with advanced solid tumors in a phase 1/2 clinical trial (NCT06082557). For cellular preparation, peripheral blood mononuclear cells (PBMCs) are utilized. Using the blood cell apheresis technique, T cells expressing PD-1 are separated from the peripheral blood and transduced with a lentivirus carrying both an "enhanced receptor" and a Trop2-CAR. These resultant T60c cells are then administered through a single intravenous infusion. In 2019, Zhao and colleagues engineered a bispecific Trop2/PD-L1 CAR-T therapy with a potent cytotoxic effect against GC [81]. These bispecific CAR-T cells induced higher cytokine levels in vitro and showed more potent inhibitory activity in vivo compared to monospecific CAR-T cells. However, as both Trop2 and PD-L1 are expressed in normal tissues, further research is necessary to determine whether the bispecific strategy might lead to off-tumor toxicities.
CAR-T and CAR-NK therapies in preclinical and early clinical stage
| Name | Institute/ Company | Type | Phase/ clinical trial ID | Reference/ Patent |
|---|---|---|---|---|
| T60c | Cancer Hospital Chinese Academy of Medical Sciences | CAR-T | Phase 1/2/ NCT06082557 | Not applicable |
| MT-302 | Myeloid Therapeutics | CAR-T | Phase 1/ NCT05969041 | Not applicable |
| Immunomedics anti-Trop-2 CAR | Immunomedics | CAR-T | Preclinical | US20160361360 |
| Trop2/PD-L1 CAR-T | Nanjing Medical University | CAR-T | Preclinical | [81] |
| Trop2-CAR-NK | M.D. Anderson Cancer Center | CAR-NK | Phase 1/2/ NCT05922930 | Not applicable |
| Trop2-CAR | German Cancer Research Center | CAR-NK | Preclinical | [82] |
NK, natural killer; CAR, chimeric antigen receptor.
In addition to T cells, alternative immune cell types, specifically natural killer (NK) cells, have been genetically engineered to express chimeric antigen receptors (CAR-NK), redirecting their cytotoxic abilities toward solid tumors. In 2023, the M.D. Anderson Cancer Center initiated two clinical studies (NCT05922930 and NCT06066424) to evaluate Trop2 CAR-engineered IL15-transduced cord blood-derived NK cells in patients with platinum-resistant ovarian cancer, mesonephric-like adenocarcinoma, and pancreatic cancer. Poorebrahim and colleagues recently described an NK cell line designed for constitutively expressing the human CD3 and CD8 genes. Furthermore, the NK cell was engineered with co-expression of a human papillomavirus type 16 oncoprotein E7-reactive T cell receptor (E7-TCR) and a costimulatory Trop2-CAR [82]. The resultant E7-TCR/Trop2-CAR NK cells demonstrated increased antigen-specific activation and exhibited enhanced cytotoxicity against tumor cells than NK cells equipped with only the E7-TCR. The inclusion of a costimulatory Trop2-CAR indeed synergizes with E7-TCR in NK cells, enhancing their signaling capabilities and targeted cytotoxic response against antigen-presenting tumor cells. Beyond varying immune cell types, researchers are refining Trop2-CAR therapies by optimizing expression methodologies. Myeloid Therapeutic has designed a novel Trop2-CAR, named MT-302, engineered to be expressed within the myeloid compartment. Treatment with MT-302 has shown effectiveness as a monotherapy in a Trop2-positive TNBC model, highlighting the efficacy of the engineered myeloid cells without the need for T-cell involvement. A FIH phase 1 clinical study has been initiated to assess the safety, tolerability, and recommended phase 2 dosing (RP2D) of MT-302 in patients facing advanced or metastatic epithelial tumors.
Cancer vaccines targeting Trop2
Cancer vaccines are formulated to activate a patient's immune system, empowering it to detect and eliminate tumor cells through the induction of B cells and elicitation of CD8+/CD4+ T cell responses against tumor-specific antigens [83]. Several Trop2-specific cancer vaccines are currently under development at the preclinical stage using different platforms.
In 2011, Cubas and colleagues used virus-like particles (VLPs) platform to generate murine Trop2 (mTrop2) VLP by incorporating full-length mTrop2 proteins into the membrane envelope of simian immunodeficiency virus (SIV) VLPs [84]. Vaccination with chimeric mTrop2 VLPs can break self-tolerance and initiate robust cellular and humoral immune responses, markedly enhancing the presence of CD4+ and CD8+ T cells, along with NK and natural killer T (NKT) cells, within tumor tissues. The tumor-infiltrating lymphocytes (TILs) elicited by this vaccine not only maintained an activated effector phenotype but also modulated numerous suppressive elements within the TME, including regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs), and cytokines like Interleukin 10 (IL-10) and transforming growth factor-β (TGF-β). These MOAs translated into a noteworthy decrease in tumor growth and an increased survival rate for mice bearing Trop2-expressing pancreatic cancer. Combining mTrop2 VLP vaccination with gemcitabine therapy, a prevalent chemotherapeutic agent for pancreatic cancer, resulted in a remarkable improvement in the survival rates of mice, surpassing the outcomes achieved with either treatment alone. These findings suggest a potential immunotherapeutic strategy for Trop2-positive tumors using VLPs as a vector to provoke a full immune response against Trop2 antigen, leading to the activation of a diverse range of immune effector cells and the modulation of crucial immunosuppressive factors within the TME. In 2018, Xi et al. constructed a Trop2-CD40 ligand VLPs (Trop2-CD40L VLPs) employing a human immunodeficiency virus (HIV) gag‑based strategy [85]. The in vivo analysis revealed that the incorporation of CD40 ligand significantly boosted the immunogenicity of Trop2-CD40L VLPs against lung cancer, resulting in more effective tumor control and an increased survival rate in Lewis (Trop2+) tumor-bearing mice. Potent humoral and cellular immune responses triggered by Trop2-CD40L VLPs demonstrated the adjuvant potential of CD40L in VLP‑based cancer vaccines.
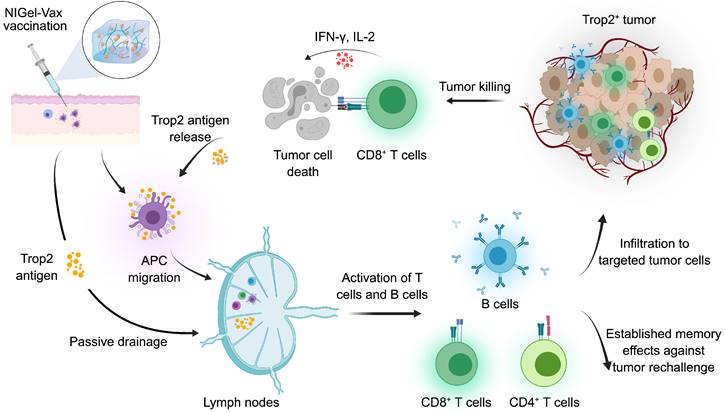
To achieve the precise spatial and temporal control of vaccine kinetics, Liu and colleagues recently designed a novel one-shot injectable nano-in-gel vaccine (NIGel-Vax) for postoperative breast cancer therapy [86]. The Trop2 antigens were combined with a polyethylenimine-benzoimidazole-4-carboxylic acid-2-isothiocyanatoethyl-α-Dmannopyranoside (PEI-4BImi-Man) adjuvant, known for its inherent immunostimulatory properties and the capacity to target dendritic cells (DCs) via mannose receptors. Subsequently, the resulting nanoparticles were encapsulated in a dynamic covalent hydrogel with 4-arm-PEG-ONH2 and oxidized dextran (ODEX) via oxime-linkages. Using a 4T1 postoperative TNBC recurrence model, NIGel-Vax accomplished a 96% tumor suppression rate and a 50% cure rate due to enhanced immune activation. Furthermore, the injectable nano-in-gel vaccine system could establish a long-lasting immune memory effect, providing sufficient protection against tumor rechallenges (Figure 4). Overall, while Trop2-specific cancer vaccines can elicit immunogenicity and improve long-term survival in mouse models, their efficacy and safety profile must be validated through clinical trials.
Mechanisms of action of a Trop2-targeted Nano-in-Gel Vaccine (NIGel-Vax) for treating Trop2-expressing tumors. The Trop2 antigen is mixed with PEI-4BImi-Man adjuvant to form nanoparticles, then incorporated into an injectable hydrogel to construct the NIGel-Vax. After subcutaneous vaccination, the Trop2-containing nanoparticles are sustainably released and are transported to lymph nodes by dendritic cells (DCs) or through passive drainage. Subsequently, activated B cells produce antigen-specific antibodies, and activated T cells migrate to the TME, leading to direct tumor killing or tumor cell apoptosis. Furthermore, NIGel-Vax induces the generation of central memory and effector memory T cells, primed for potential tumor rechallenge. Immunogenic dead tumor cells can release Trop2 antigens and danger signaling molecules, amplifying the intensity and breadth of the subsequent immune response. The image was created with MedPeer.cn.
Small molecular inhibitor
Antibodies are typically characterized by their high specificity and affinity for cell surface targets, which often restricts their scope of action. Small molecule agents are capable of inhibiting tumor growth by targeting an extensive array of extracellular and intracellular proteins, positioning them as a viable alternative for targeted cancer therapy. Bruceine D (BD) is currently the sole small molecule inhibitor known to exhibit antitumor activity in Trop2-positive tumor cells. Tang and colleagues discovered BD's inhibitory effects using techniques such as cell membrane chromatography (CMC) and cellular thermal shift assay (CETSA) [87]. Molecular docking and site-directed mutagenesis studies revealed that BD recognized the intracellular domain (ICD) of Trop2, identifying K307 and E310 as key binding sites (Figure 2). The in vitro and in vivo studies focusing on Trop2-dependent inhibitory activity further confirmed the target specificity of BD. Mechanistically, BD suppressed Trop2-induced cancer metastasis by interfering with the Trop2/β-catenin positive feedback loop, wherein the β-catenin degradation, which is typically induced by ubiquitin-proteosome pathway, was inhibited by Trop2/β-catenin complex. BD-mediated destabilization of β-catenin diminished nuclear translocation, which in turn decreased Trop2 transcription, reversed the epithelial-mesenchymal transition (EMT) process, and restrained the extracellular matrix (ECM) remodeling, thereby collectively impeding cancer metastasis. Furthermore, BD's efficacy in hampering lung metastatic colonization and its favorable impact on extending survival were corroborated using a 4T1 experimental metastasis model. These findings offer new insights into the discovery of anti-Trop2 inhibitors, with additional structural investigations required to confirm their detailed binding sites and MOAs.
Trop2-targeted imaging
Trop2 overexpression is linked to a poor prognosis in a variety of solid tumors and can also predict response to different Trop2-targeted therapies [43, 48, 88]. Consequently, accurate assessment of Trop2 status is critical for guiding clinical decision-making regarding treatment strategies. However, unlike other therapeutic targets, there are no standard methods for Trop2 testing when selecting patients for clinical trials, which may hinder achieving the best clinical outcomes. Below, we delve into the evolving Trop2-targeted imaging strategies that show promise for cancer diagnosis, treatment monitoring, and theranostic applications.
Sharkey and colleagues developed a novel bispecific antibody, named TF12, capable of binding to both Trop2 and histamine-succinyl-glycine (HSG) hapten [89]. The moderate internalization and prolonged retention of TF12 on the surface of Trop2-expressing tumor cells rendered it suitable for pretargeted imaging and therapy in Trop2-positive tumors. As a result, Lutje et al. designed a pretargeted dual-modality immuno-SPECT and near-infrared fluorescence (NIRF) imaging, for image-guided surgery in prostate cancer using TF12 together with the dual-labeled di-HSG peptide (RDC018) [90]. RDC018 was an IMP288 analog that contains a NIRF IRDye800CW moiety and a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) chelate, enabling radiolabeling with 111In. In prostate cancer metastases mouse models pre-targeted with the TF12, there was notable Trop2-specific uptake of 111In-RDC018, resulting in favorable signal-to-background ratios. The TF12-based pre-targeted radio-immunoimaging strategy, when combined with 68Ga di-HSG peptide IMP288 (68Ga-IMP288), achieved rapid, highly sensitive, and specific imaging in prostate cancer models [91]. Van Rij and colleagues reported that the combination of TF12 and 177Lu-IMP288 further constituted an effective pre-targeted radio-immunotherapy (PRIT) system, which substantially prolonged survival in mouse models with PC3 xenografts [92].
Beyond pre-targeted strategy, direct targeting approaches using labeled antibodies have also exhibited promising theranostic potential for Trop2-positive solid tumors. Chen and colleagues synthesized a radiolabeled agent, 89Zr-DFO-AF650, by conjugating an anti-Trop2 antibody AF650 with desferrioxamine (DFO) [93]. This agent demonstrated specific, rapid, and sustained accumulation in Trop2-positive tumor models, specifically BxPC-3. Notably, 89Zr-DFO-AF650 was effective in distinguishing primary tumors in the orthotopic BxPC-3 cancer model via immune-PET. The therapeutic antibody hIMB1636 was conjugated with p-SCN-Bn-NOTA (NOTA) and DOTA-NHS-ester (DOTA), enabling radiolabeling with 64Cu and 177Lu, respectively. In vivo evaluations indicated that 64Cu-labeled hIMB1636 could noninvasively assess Trop2 expression levels using positron emission tomography (PET) imaging, and 177Lu-labeled hIMB1636 significantly suppressed tumor growth in Trop2-overexpressing T3M-4 mouse models [94].
In addition to traditional mAbs, nanobodies have been used to develop tracers for Trop2-targeted molecular imaging. Huang and colleagues constructed two nanobody-based immune-PET probes (68Ga-NOTA-RTD98 and 68Ga-NOTA-RTD01) and assessed their diagnostic abilities in preclinical pancreatic and gastric cancer models. Immuno-PET with 68Ga-NOTA-RTD98 effectively delineated subcutaneous tumors in both cell-derived pancreatic cancer models and patient-derived gastric cancer models, demonstrating its superiority over imaging with 18F-FDG or the non-specific probe. Another probe, 68Ga-NOTA-RTD01, featuring improved pharmacokinetics targeting Trop2, was further developed and demonstrated advantageous diagnostic capabilities in preclinical pancreatic cancer models [95]. Subsequently, Huang and colleagues designed an optimal 68Ga-labelled nanobody tracer, 68Ga-NOTA-T4. Preclinical studies involving tumor-bearing mouse models and beagle dogs revealed good pharmacokinetics and diagnostic potential of the tracer. Moreover, in a pilot clinical trial involving patients with solid tumors, 68Ga-NOTA-T4 immuno-PET noninvasively and accurately visualized varying Trop2 expression in primary and metastatic tumors [96].
These findings underscore the potential of mAb-based or nanobody-based molecular imaging in Trop2-positive solid tumors. Although mAb-based imaging offers prolonged imaging capabilities and established clinical applicability with effective effector functions, its efficiency may be compromised by poor tumor penetration, higher production costs, and potential immunogenicity. In contrast, nanobody-based imaging provides excellent tumor penetration, rapid imaging capabilities, cost-effective production, and reduced immunogenicity, though it is limited by a lack of natural effector functions and shorter systemic circulation times. For optimal imaging and treatment of Trop2-positive solid tumors, the choice between mAb-based and nanobody-based strategies likely depends on the tumor types, desired imaging windows, and required pharmacokinetics. Wu and his team recently identified a multicyclic peptide binder to Trop2 using a phage-displayed peptide library. The cysteine-framework peptide binder exhibited a nanomolar affinity for the Trop2 antigen and demonstrated specific binding to Trop2 on the cell surface [97]. Given the advantages of peptides—namely their size and penetration properties, ease of synthesis and modification, versatility, customization, and lower immunogenicity—peptide-based imaging or therapeutic agents are promising for diagnosing and treating Trop2-positive tumors.
Overall, these findings highlight the role of Trop2-mediated imaging in tumor detection and monitoring of treatment responses. Advancing these Trop2-targeted imaging strategies through clinical validation and translational research could enhance the stratification and management of patients with Trop2-positive tumors.
Conclusions and Future Directions
Trop2 has emerged as a promising target for cancer therapy due to its overexpression in cancer cells. Advances in ADC-based anti-Trop2 therapies have significantly transformed the treatment landscape for a subset of patients with Trop2-positive solid tumors. However, challenges such as systemic toxicity and drug resistance persist. The growing body of knowledge regarding Trop2-mediated oncogenic mechanisms and advances in structural biology have spurred the development of next-generation therapies. Numerous emerging Trop2-targeted modalities, such as bispecific antibodies/ADCs and CAR-T cell therapies, are undergoing active investigation in the early clinical phase, with some showing the potential to revolutionize the care of Trop2-positive cancers. In this review, we provide a summary of the components and underlying rationale of innovative Trop2-targeted therapeutics. Importantly, we examine the varied MOAs and limitations of these therapies. To fully realize the therapeutic potential of these Trop2-targeted strategies, future research endeavors ought to concentrate on pivotal areas.
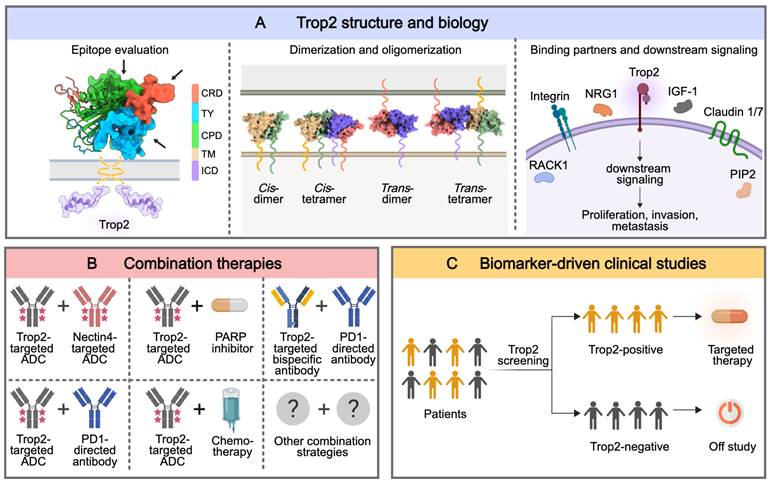
Firstly, both basic scientists and clinicians must undertake an in-depth analysis of the oncogenic mechanisms associated with Trop2 overexpression in tumor tissues (Figure 5A). As an intricate signal transducer, Trop2 is known for its complex oligomerization and interactions with other proteins. Recent advances in structural biology, such as the crystallographic determination of Trop2's dimeric structure, have shed light on aspects of its oligomerization [45, 46]. However, the relationship between Trop2's oligomeric assembly and its biological functions is not yet fully understood. The structural basis underlying interactions between Trop2 and its partners remains to be fully explained. Notably, Trop2 is not only expressed on tumor cells but also on some nonmalignant tissues, which can lead to inevitable on-target off-tumor toxicities. To enhance the tumor specificity of targeted therapies, identifying tumor-specific structural variants of Trop2 seems to be a viable way [36]. In this context, concentrated efforts to evaluate Trop2 epitopes, investigate its interaction dynamics, and understand its biology would lead to the development of anti-Trop2 therapies with greater precision and clinical effectiveness.
The second consideration is exploring the potential combination strategies for therapy (Figure 5B). Owing to the heterogeneity and individual specificity of tumors, relying on a single agent or therapy is often sufficient for better outcomes. Preliminary data from preclinical and early clinical studies have shown that Trop2-direct ADCs, when combined with chemotherapeutic agents, small molecule inhibitors, immunotherapy, or anti-angiogenic therapy, can lead to synergistic antitumor effects in drug-resistant tumors [43, 88, 98]. However, it should be noted that the superimposed toxicity from drug-drug interaction may elevate the risk of treatment failure. Therefore, finding the optimal combination therapies with a favorable response and manageable toxicity remains a significant challenge.
The third and final facet of future research involves establishing the standard methods of Trop2 testing, which has been overlooked for a long time during the development of Trop2-targeted therapies (Figure 5C). Indeed, there is no formal cut-off for 'Trop2 positivity'. Current Trop2-targeted therapies tend to be more effective in inhibiting tumors that exhibit medium to high levels of Trop2 expression. Therefore, patient stratification based on Trop2 status is expected to aid patient selection and further personalized medicine.
Future directions of Trop2-targeted therapies for the treatment of Trop2-positive tumors. (A) A deeper understanding of the structure and mechanisms of Trop2. A proposed composite model of full-length Trop2 dimer based on the structures of individual modules. Schematics for possible dimerization and tetramerization states of Trop2 on the cell surface. PDB identifier 7E5N for cis-dimer and cis-tetramer, PDB identifier 7E5M for trans-dimer and trans-tetramer. A general view of Trop2 interaction partners and downstream signaling pathways. NRG1, neuregulin 1; IGF-1, insulin-like growth factor 1; RACK1, receptor for activated C kinase 1; PIP2, phosphatidylinositol 4,5-bisphosphate. (B) Discovery of optimal combination strategies against Trop2-positive tumors. The figure shows representative examples of potential combination strategies with Trop2-targeted agents in preclinical or early clinical stages. PARP, poly (ADP-ribose) polymerase; PD1, programmed cell death protein 1. (C) Establishment of a standard method for Trop2 testing is necessary for the biomarker-driven clinical studies that can evaluate the efficacy of potential Trop2-targeted therapeutic agents. The image was created with MedPeer.cn.
Abbreviations
Trop2: trophoblast cell surface antigen 2; ADC: antibody-drug conjugate; TNBC: triple-negative breast cancer; MOA: mechanism of action; EGP-1: epithelial glycoprotein-1; M1S1: membrane component chromosome 1 surface marker 1; mBC: metastatic breast cancer; mUC: metastatic urothelial cancer; SG: Sacituzumab govitecan; FIC: first-in-class; DAR: drug-to-antibody ratio; FDA: Food and Drug Administration; HER2: human epidermal growth factor receptor 2; HR: hormone receptor; ORR: objective response rate; DAD: Double Antibody Drug Conjugate; EV: enfortumab vedotin; AEs: SG-related adverse events; OS: overall survival; NSCLC: non-small cell lung cancer; mAb: monoclonal antibody; PDX: patient-derived xenograft; BC: breast cancer; FIH: first-in-human; PFS: progression-free survival; cORR: confirmed ORR; AGAs: actionable genomic alterations; ILD: interstitial lung disease; IRRs: infusion-related reactions; OSEs: ocular surface events; CDX: cell-derived xenograft; PK-PD: pharmacokinetic-pharmacodynamic; DoR: duration of response; TKI: tyrosine kinase inhibitor; EGFR: epidermal growth factor receptor; DCR: disease control rate; DLT: dose-limiting toxicity; MMAE: monomethyl auristatin E; PDAC: pancreatic ductal adenocarcinoma; TI: therapeutic index; PD-1: programmed cell death 1; TRAEs: treatment-related adverse events; ECD: extracellular domain; CPD: cysteine-poor domain; ADAM10: ADAM metallopeptidase domain 10; MAPK: mitogen-activated protein kinase; CDC: complement-dependent cytotoxicity; CRD: cysteine-rich domain; ADCC: antibody-dependent cellular cytotoxicity; CLIPS: chemically linked peptides on scaffolds; CBIS: Cell‑Based Immunization and Screening; Nb: nanobody; TY: thyroglobulin type-1; NHP: non-human primate; BiTEs: bispecific T cell engagers; INFα: interferon-α; TME: tumor microenvironment; CDR: complementarity-determining region; CDH-17: cadherin-17; GC: gastric cancer; HER3: human epidermal growth factor receptor 3; PI3K: phosphatidylinositide-3 kinase; CDE: China's Center for Drug Evaluation; PTK7: Protein tyrosine kinase-7; HSA: human serum albumin; CARs: chimeric antigen receptors; PBMCs: peripheral blood mononuclear cells; NK cells: natural killer cells; VLPs: virus-like particles; SIV: simian immunodeficiency virus; NKT cells: natural killer T cells; TILs: tumor-infiltrating lymphocytes; Treg cells: regulatory T cells; MDSCs: myeloid-derived suppressor cells; IL-10: Interleukin 10; TGF-β: transforming growth factor-β; HIV: human immunodeficiency virus; DCs: dendritic cells; ODEX: oxidized dextran; BD: Bruceine D; CMC: cell membrane chromatography; CETSA: cellular thermal shift assay; ICD: intracellular domain; EMT: epithelial-mesenchymal transition; ECM: extracellular matrix; HSG: histamine-succinyl-glycine; NIRF: near-infrared fluorescence; DOTA: 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; PRIT: pretargeted radio-immunotherapy; DFO: desferrioxamine; PET: positron emission tomography.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province Grants ZR2022QH201 (Xinlin Liu) and the National Natural Science Foundation of China Grants 32300788 (Xinlin Liu).
Author contributions
Conceptualizing structure: M.Z. and S.S.Y. Preparing figures and/or tables: J.Y.X.L., L.S. and D.M.X. Writing—original draft: X.L.L. Writing—review and editing: M.Z., L.N.M. and T.L. Supervision: S.S.Y. and Y.Y.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A. 1981;78:5147-50
2. McDougall AR, Tolcos M, Hooper SB, Cole TJ, Wallace MJ. Trop2: from development to disease. Dev Dyn. 2015;244:99-109
3. Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84-105
4. Liao S, Wang B, Zeng R, Bao H, Chen X, Dixit R. et al. Recent advances in trophoblast cell-surface antigen 2 targeted therapy for solid tumors. Drug Dev Res. 2021;82:1096-110
5. Stepan LP, Trueblood ES, Hale K, Babcook J, Borges L, Sutherland CL. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J Histochem Cytochem. 2011;59:701-10
6. Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V. et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32:222-33
7. Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, Simeone P. et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9:e96993
8. Tsuchikama K, Anami Y, Ha SYY, Yamazaki CM. Exploring the next generation of antibody-drug conjugates. Nat Rev Clin Oncol. 2024;21:203-23
9. Liu X, Deng J, Yuan Y, Chen W, Sun W, Wang Y. et al. Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol Ther. 2022;239:108296
10. Weiss J, Glode A, Messersmith WA, Diamond J. Sacituzumab govitecan: breakthrough targeted therapy for triple-negative breast cancer. Expert Rev Anticancer Ther. 2019;19:673-9
11. Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011;17:3157-69
12. Kopp A, Hofsess S, Cardillo TM, Govindan SV, Donnell J, Thurber GM. Antibody-drug conjugate sacituzumab govitecan drives efficient tissue penetration and rapid intracellular drug release. Mol Cancer Ther. 2023;22:102-11
13. Bardia A, Mayer IA, Diamond JR, Moroose RL, Isakoff SJ, Starodub AN. et al. Efficacy and safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol. 2017;35:2141-8
14. Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR. et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741-51
15. Tagawa ST, Faltas BM, Lam ET, Saylor PJ, Bardia A, Hajdenberg J. et al. Sacituzumab govitecan (IMMU-132) in patients with previously treated metastatic urothelial cancer (mUC): Results from a phase I/II study. J Clin Oncol. 2019;37:354
16. Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M. et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529-41
17. Kevin K, Mafalda O, Tiffany AT, Sara MT, Delphine L, Kevin P. et al. Outcomes in patients (pts) aged ≥65 years in the phase 3 ASCENT study of sacituzumab govitecan (SG) in metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2021;39:1011
18. Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Fléchon A. et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39:2474-85
19. Hope SR, Aditya B, Frederik M, Javier C, Peter S, Delphine L. et al. Primary results from TROPiCS-02: A randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician's choice (TPC) in patients (Pts) with hormone receptor-positive/HER2-negative (HR+/HER2-) advanced breast cancer. J Clin Oncol. 2022;40:LBA1001-LBA
20. Rugo HS, Bardia A, Marme F, Cortes J, Schmid P, Loirat D. et al. Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2022;40:3365-76
21. Liu W, Du Q, Guo Z, Ye X, Liu J. Post-marketing safety surveillance of sacituzumab govitecan: an observational, pharmacovigilance study leveraging FAERS database. Front Pharmacol. 2023;14:1283247
22. Coates JT, Sun S, Leshchiner I, Thimmiah N, Martin EE, McLoughlin D. et al. Parallel genomic alterations of antigen and payload targets mediate polyclonal acquired clinical resistance to sacituzumab govitecan in triple-negative breast cancer. Cancer Discov. 2021;11:2436-45
23. Okajima D, Yasuda S, Maejima T, Karibe T, Sakurai K, Aida T. et al. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20:2329-40
24. Shimizu T, Sands J, Yoh K, Spira A, Garon EB, Kitazono S. et al. First-in-human, phase I dose-escalation and dose-expansion study of trophoblast cell-surface antigen 2-directed antibody-drug conjugate datopotamab deruxtecan in non-small-cell lung cancer: TROPION-PanTumor01. J Clin Oncol. 2023;41:4678-87
25. Ahn MJ, Lisberg A, Paz-Ares L, Cornelissen R, Girard N, Pons-Tostivint E. et al. LBA12 Datopotamab deruxtecan (Dato-DXd) vs docetaxel in previously treated advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC): Results of the randomized phase III study TROPION-Lung01. Ann Oncol. 2023;34:S1305-S6
26. Goto Y, Su W-C, Levy BP, Rixe O, Yang T-Y, Tolcher AW. et al. TROPION-Lung02: Datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). J Clin Oncol. 2023;41:9004
27. Paz-Ares L, Ahn MJ, Lisberg AE, Kitazono S, Cho BC, Blumenschein G. et al. 1314MO TROPION-Lung05: Datopotamab deruxtecan (Dato-DXd) in previously treated non-small cell lung cancer (NSCLC) with actionable genomic alterations (AGAs). Ann Oncol. 2023;34:S755-S6
28. Garon E, Johnson M, Lisberg A, Spira A, Yamamoto N, Heist R. et al. MA03.02 TROPION-PanTumor01: Updated results from the NSCLC cohort of the phase 1 study of datopotamab deruxtecan in solid tumors. J Thorac Oncol. 2021;16:S892-S3
29. Cheng Y, Yuan X, Tian Q, Huang X, Chen Y, Pu Y. et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front Oncol. 2022;12:951589
30. Fang W, Cheng Y, Chen Z, Wang W, Yin Y, Li Y. et al. SKB264 (TROP2-ADC) for the treatment of patients with advanced NSCLC: Efficacy and safety data from a phase 2 study. J Clin Oncol. 2023;41:9114
31. Strop P, Tran TT, Dorywalska M, Delaria K, Dushin R, Wong OK. et al. RN927C, a site-specific Trop-2 antibody-drug conjugate (ADC) with enhanced stability, is highly efficacious in preclinical solid tumor models. Mol Cancer Ther. 2016;15:2698-708
32. King GT, Eaton KD, Beagle BR, Zopf CJ, Wong GY, Krupka HI. et al. A phase 1, dose-escalation study of PF-06664178, an anti-Trop-2/Aur0101 antibody-drug conjugate in patients with advanced or metastatic solid tumors. Invest New Drugs. 2018;36:836-47
33. He N, Yang C, Yang Y, Xue Z, Xu J, Zhao L. et al. Abstract LB030: SHR-A1921, a novel TROP-2 ADC with an optimized design and well-balanced profile between efficacy and safety. Cancer Res. 2023;83:LB030-LB
34. Wang J, Wu L, Song Z, Li X, Liu C, Liu T. et al. Abstract CT181: A first-in-human (FIH) phase 1 study of SHR-A1921, a TROP-2 targeted antibody-drug conjugate (ADC), in patients with advanced solid tumors. Cancer Res. 2023;83:CT181-CT
35. Ma F, Qiu F, Tong Z, Wang J, Tan Y, Bai R. et al. Preliminary results from a first-in-human study of ESG401, a trophoblast cell-surface antigen 2 (TROP2) antibody drug conjugate (ADC), in patients with locally advanced/metastatic solid tumors. J Clin Oncol. 2023;41:1100
36. Guerra E, Trerotola M, Relli V, Lattanzio R, Tripaldi R, Ceci M. et al. 3D-informed targeting of the Trop-2 signal-activation site drives selective cancer vulnerability. Mol Cancer Ther. 2023;22:790-804
37. Kim H, Guerra E, Baek E, Jeong Y, You H, Yu B. et al. Abstract 328: LCB84, a TROP2-targeted ADC, for treatment of solid tumors that express TROP-2 using the hu2G10 tumor-selective anti-TROP2 monoclonal antibody, a proprietary site-directed conjugation technology and plasma-stable tumor-selective linker chemistry. Cancer Res. 2022;82:328
38. Liu X, Deng J, Zhang R, Xing J, Wu Y, Chen W. et al. The clinical development of antibody-drug conjugates for non-small cell lung cancer therapy. Frontiers in Immunology. 2023;14:1335252
39. Son S, Shin S, Rao NV, Um W, Jeon J, Ko H. et al. Anti-Trop2 antibody-conjugated bioreducible nanoparticles for targeted triple negative breast cancer therapy. Int J Biol Macromol. 2018;110:406-15
40. Jia L, Fu Y, Zhang N, Liu Y, Su L, Wang H. et al. Directional conjugation of Trop2 antibody to black phosphorus nanosheets for phototherapy in orthotopic gastric carcinoma. Nanomedicine. 2023;51:102687
41. Liu D, Cardillo TM, Wang Y, Rossi EA, Goldenberg DM, Chang CH. Trop-2-targeting tetrakis-ranpirnase has potent antitumor activity against triple-negative breast cancer. Mol Cancer. 2014;13:53
42. Parisi C, Mahjoubi L, Gazzah A, Barlesi F. TROP-2 directed antibody-drug conjugates (ADCs): The revolution of smart drug delivery in advanced non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2023;118:102572
43. Qiu S, Zhang J, Wang Z, Lan H, Hou J, Zhang N. et al. Targeting Trop-2 in cancer: Recent research progress and clinical application. Biochim Biophys Acta Rev Cancer. 2023;1878:188902
44. Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA. et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32:746-56
45. Pavsic M. Trop2 forms a stable dimer with significant structural differences within the membrane-distal region as compared to EpCAM. Int J Mol Sci. 2021;22:10640
46. Sun M, Zhang H, Jiang M, Chai Y, Qi J, Gao GF. et al. Structural insights into the cis and trans assembly of human trophoblast cell surface antigen 2. iScience. 2021;24:103190
47. Guerra E, Relli V, Ceci M, Tripaldi R, Simeone P, Aloisi AL. et al. Trop-2, Na+/K+ ATPase, CD9, PKCα, cofilin assemble a membrane signaling super-complex that drives colorectal cancer growth and invasion. Oncogene. 2022;41:1795-808
48. Liu X, Li J, Deng J, Zhao J, Zhao G, Zhang T. et al. Targeting Trop2 in solid tumors: a look into structures and novel epitopes. Front Immunol. 2023;14:1332489
49. Stein R, Chen S, Sharkey RM, Goldenberg DM. Murine monoclonal antibodies raised against human non-small cell carcinoma of the lung: specificity and tumor targeting. Cancer Res. 1990;50:1330-6
50. Stein R, Basu A, Goldenberg DM, Lloyd KO, Mattes MJ. Characterization of cluster 13: the epithelial/carcinoma antigen recognized by MAb RS7. Int J Cancer Suppl. 1994;8:98-102
51. Shih LB, Xuan H, Aninipot R, Stein R, Goldenberg DM. In vitro and in vivo reactivity of an internalizing antibody, RS7, with human breast cancer. Cancer Res. 1995;55:5857s-63s
52. Trerotola M, Guerra E, Ali Z, Aloisi AL, Ceci M, Simeone P. et al. Trop-2 cleavage by ADAM10 is an activator switch for cancer growth and metastasis. Neoplasia. 2021;23:415-28
53. Alberti S, Miotti S, Stella M, Klein CE, Fornaro M, Menard S. et al. Biochemical characterization of Trop-2, a cell surface molecule expressed by human carcinomas: formal proof that the monoclonal antibodies T16 and MOv-16 recognize Trop-2. Hybridoma. 1992;11:539-45
54. Ikeda M, Yamaguchi M, Kato K, Nakamura K, Shiina S, Ichikawa-Ando T. et al. Pr1E11, a novel anti-TROP-2 antibody isolated by adenovirus-based antibody screening, recognizes a unique epitope. Biochem Biophys Res Commun. 2015;458:877-82
55. Guerra E, Alberti S. The anti-Trop-2 antibody-drug conjugate Sacituzumab Govitecan-effectiveness, pitfalls and promises. Ann Transl Med. 2022;10:501
56. Guerra E, Trerotola M, Relli V, Lattanzio R, Tripaldi R, Vacca G. et al. Trop-2 induces ADAM10-mediated cleavage of E-cadherin and drives EMT-less metastasis in colon cancer. Neoplasia. 2021;23:898-911
57. Alberti S, Trerotola M, Guerra E. Abstract 340: The Hu2G10 tumor-selective anti-Trop-2 monoclonal antibody targets the cleaved-activated Trop-2 and shows therapeutic efficacy against multiple human cancers. Cancer Res. 2022;82:340
58. Guerra E, Trerotola M, Relli V, Lattanzio R, Ceci M, Boujnah K. et al. The 2EF Antibody Targets a Unique N-Terminal Epitope of Trop-2 and Enhances the In Vivo Activity of the Cancer-Selective 2G10 Antibody. Cancers (Basel). 2023;15:3721
59. Guerra E, Trerotola M, Relli V, Lattanzio R, Boujnah K, Travali N. et al. Phylogenetic conservation of Trop-2 across species-rodent and primate genomics model anti-Trop-2 therapy for pre-clinical benchmarks. Front Genet. 2023;14:1297367
60. Truong A, Feng N, Sayegh D, Mak B, O'Reilly K, Fung S-W. et al. AR47A6.4.2, a functional naked monoclonal antibody targeting Trop-2, demonstrates in vivo efficacy in human pancreatic, colon, breast and prostate cancer models. Mol Cancer Ther. 2007;6:PR-12 -PR-
61. Truong A, Feng N, Sayegh D, Mak B, Hahn S, Pereira D. et al. AR47A6.4.2, a naked monoclonal antibody targeting Trop-2, exhibits anti-tumor efficacy in multiple human cancer models as a monotherapeutic agent and demonstrates efficacy in combination therapy. Cancer Res. 2008;68:3990
62. Masahiro I, Kazunori K, Miki Y, Hirofumi H, Kazuyasu N, Yoshiyuki S. Cell Surface Antibody Retention Influences <em>In Vivo</em> Antitumor Activity Mediated by Antibody-dependent Cellular Cytotoxicity. Anticancer Res. 2016;36:5937
63. Zhou DD, Sun LP, Yu Q, Zhai XT, Zhang LW, Gao RJ. et al. Elucidating the development, characterization, and antitumor potential of a novel humanized antibody against Trop2. Int J Biol Macromol. 2023;253:127105
64. Sun LP, Bai WQ, Zhou DD, Wu XF, Zhang LW, Cui AL. et al. hIMB1636-MMAE, a novel TROP2-targeting antibody-drug conjugate exerting potent antitumor efficacy in pancreatic cancer. J Med Chem. 2023;66:14700-15
65. Tanaka T, Ohishi T, Asano T, Takei J, Nanamiya R, Hosono H. et al. An anti-TROP2 monoclonal antibody TrMab-6 exerts antitumor activity in breast cancer mouse xenograft models. Oncol Rep. 2021;46:132
66. Lin H, Zhang H, Wang J, Lu M, Zheng F, Wang C. et al. A novel human Fab antibody for Trop2 inhibits breast cancer growth in vitro and in vivo. Int J Cancer. 2014;134:1239-49
67. Liu J, Yang D, Yin Z, Gao M, Tong H, Su Y. et al. A novel human monoclonal Trop2-IgG antibody inhibits ovarian cancer growth in vitro and in vivo. Biochem Biophys Res Commun. 2019;512:276-82
68. Hu Y, Wang Y, Lin J, Wu S, Lv H, Ji X. et al. Identification and characterization of specific nanobodies against Trop-2 for tumor targeting. Int J Mol Sci. 2022;23:7942
69. Rossi EA, Rossi DL, Cardillo TM, Chang CH, Goldenberg DM. Redirected T-cell killing of solid cancers targeted with an anti-CD3/Trop-2-bispecific antibody is enhanced in combination with interferon-α. Mol Cancer Ther. 2014;13:2341-51
70. Chang CH, Wang Y, Li R, Rossi DL, Liu D, Rossi EA. et al. Combination Therapy with Bispecific Antibodies and PD-1 Blockade Enhances the Antitumor Potency of T Cells. Cancer Res. 2017;77:5384-94
71. Liu H, Bai L, Huang L, Ning N, Li L, Li Y. et al. Bispecific antibody targeting TROP2xCD3 suppresses tumor growth of triple negative breast cancer. J Immunother Cancer. 2021;9:e003468
72. Wang D, Zhang L, Wang B, Zhao L, Deng L, Xu W. et al. Construction of a novel TROP2/CD3 bispecific antibody with potent antitumor activity and reduced induction of Th1 cytokines. Protein Expr Purif. 2023;205:106242
73. O D, Chow K, Iyer D, Wong M, Wong TK, Wong DA. et al. An engineered Trop2/CD3 bispecific antibody for treatment of gastric, pancreatic, and colorectal cancers. J Clin Oncol. 2023;41:e14521-e
74. Rocha SS, Lin R, Dayao MR, Banzon RR, Thothathri S, Wright KJ. et al. Abstract 2928: TROP2 ProTriTAC™, a protease-activated T cell engager prodrug targeting TROP2 for the treatment of solid tumors. Cancer Res. 2023;83:2928
75. Li Z, Shang C, Guan X, An G, Guo Y, Zhang E. et al. Abstract LB215: A first-in-class anti-TROP2/EGFR bispecific antibody-drug conjugate, DM001, exhibits potent anti-tumor efficacy. Cancer Res. 2023;83:LB215-LB
76. Shang C, An G, Guo Y, Zhang E, Lin Q, Yang Y. Abstract 2977: A first-in-class anti-HER2/TROP2 bispecific antibody-drug conjugate (YH012) exhibits potent anti-tumor efficacy. Cancer Res. 2023;83:2977 -
77. Geuijen CAW, De Nardis C, Maussang D, Rovers E, Gallenne T, Hendriks LJA. et al. Unbiased Combinatorial Screening Identifies a Bispecific IgG1 that Potently Inhibits HER3 Signaling via HER2-Guided Ligand Blockade. Cancer Cell. 2018;33:922-36.e10
78. Cui NP, Qiao S, Jiang S, Hu JL, Wang TT, Liu WW. et al. Protein tyrosine kinase 7 regulates EGFR/Akt signaling pathway and correlates with malignant progression in triple-negative breast cancer. Front Oncol. 2021;11:699889
79. Yao S, Shang C, An G, Zhang E, Lin Q, Yang Y. Abstract LB216: Discovery of BCG033, a novel anti-PTK7 x TROP2 bispecific antibody-drug conjugate with promising efficacy against triple-negative breast cancer. Cancer Res. 2023;83:LB216-LB
80. Xu C, Zhu M, Wang Q, Cui J, Huang Y, Huang X. et al. TROP2-directed nanobody-drug conjugate elicited potent antitumor effect in pancreatic cancer. J Nanobiotechnology. 2023;21:410
81. Zhao W, Jia L, Zhang M, Huang X, Qian P, Tang Q. et al. The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am J Cancer Res. 2019;9:1846-56
82. Poorebrahim M, Quiros-Fernandez I, Marmé F, Burdach SE, Cid-Arregui A. A costimulatory chimeric antigen receptor targeting TROP2 enhances the cytotoxicity of NK cells expressing a T cell receptor reactive to human papillomavirus type 16 E7. Cancer Lett. 2023;566:216242
83. Schiller JT, Lowy DR, Frazer IH, Finn OJ, Vilar E, Lyerly HK. et al. Cancer vaccines. Cancer Cell. 2022;40:559-64
84. Cubas R, Zhang S, Li M, Chen C, Yao Q. Chimeric Trop2 virus-like particles: a potential immunotherapeutic approach against pancreatic cancer. J Immunother Cancer. 2011;34:251-63
85. Xi W, Ke D, Min L, Lin W, Jiahui Z, Fang L. et al. Incorporation of CD40 ligand enhances the immunogenicity of tumor-associated calcium signal transducer 2 virus-like particles against lung cancer. Int J Mol Med. 2018;41:3671-9
86. Liu T, Si X, Liu L, Ma S, Huang Z, Zhang Y. et al. Injectable Nano-in-Gel Vaccine for Spatial and Temporal Control of Vaccine Kinetics and Breast Cancer Postsurgical Therapy. ACS Nano. 2024;18:3087-100
87. Tang W, Hu Y, Tu K, Gong Z, Zhu M, Yang T. et al. Targeting Trop2 by Bruceine D suppresses breast cancer metastasis by blocking Trop2/β-catenin positive feedback loop. J Adv Res. 2024;58:193-210
88. Hsu EC, Rice MA, Bermudez A, Marques FJG, Aslan M, Liu S. et al. Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc Natl Acad Sci U S A. 2020;117:2032-42
89. Sharkey RM, van Rij CM, Karacay H, Rossi EA, Frielink C, Regino C. et al. A new Tri-Fab bispecific antibody for pretargeting Trop-2-expressing epithelial cancers. J Nucl Med. 2012;53:1625-32
90. Lutje S, Rijpkema M, Goldenberg DM, van Rij CM, Sharkey RM, McBride WJ. et al. Pretargeted dual-modality immuno-SPECT and near-infrared fluorescence imaging for image-guided surgery of prostate cancer. Cancer Res. 2014;74:6216-23
91. van Rij CM, Frielink C, Goldenberg DM, Sharkey RM, Franssen GM, Lütje S. et al. Pretargeted immunoPET of prostate cancer with an anti-TROP-2 x anti-HSG bispecific antibody in mice with PC3 xenografts. Mol Imaging Biol. 2015;17:94-101
92. van Rij CM, Frielink C, Goldenberg DM, Sharkey RM, Lutje S, McBride WJ. et al. Pretargeted radioimmunotherapy of prostate cancer with an anti-TROP-2xanti-HSG bispecific antibody and a (177)Lu-labeled peptide. Cancer Biother Radiopharm. 2014;29:323-9
93. Chen W, Li M, Younis MH, Barnhart TE, Jiang D, Sun T. et al. ImmunoPET of trophoblast cell-surface antigen 2 (Trop-2) expression in pancreatic cancer. Eur J Nucl Med Mol Imaging. 2022;49:861-70
94. Li C, Liu J, Yang X, Yang Q, Huang W, Zhang M. et al. Theranostic application of (64)Cu/(177)Lu-labeled anti-Trop2 monoclonal antibody in pancreatic cancer tumor models. Eur J Nucl Med Mol Imaging. 2022;50:168-83
95. Huang W, Liang C, Zhang Y, Zhang D, An S, Wu Q. et al. ImmunoPET imaging of Trop2 expression in solid tumors with nanobody tracers. Eur J Nucl Med Mol Imaging. 2024;51:380-94
96. Huang W, Zhang Y, Cao M, Wu Y, Jiao F, Chu Z. et al. ImmunoPET imaging of Trop2 in patients with solid tumours. EMBO Mol Med. 2024;16:1143-61
97. Li J, Liu H, Xiao S, Fan S, Cheng X, Wu C. De novo discovery of cysteine frameworks for developing multicyclic peptide libraries for ligand discovery. J Am Chem Soc. 2023;145:28264-75
98. Cardillo TM, Sharkey RM, Rossi DL, Arrojo R, Mostafa AA, Goldenberg DM. Synthetic lethality exploitation by an anti-Trop-2-SN-38 antibody-drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2-wild-type triple-negative breast cancer. Clin Cancer Res. 2017;23:3405-15
99. Zhang Y, Wan W, Zhuo S, Chen L, Li G, Zhao S. et al. Abstract 2644: BL-M02D1, a novel Trop2-targeting ADC, demonstrates robust anti-tumor efficacy in preclinical evaluation. Cancer Res. 2023;83:2644
100. Ueno NT, Gao J, Liu L, Shi R, Liu S, Tang L. et al. Abstract CT248: First in human trial of DB1305 in patients with advanced malignant solid tumors. Cancer Res. 2023;83:CT248-CT
101. Mei X, Tang W, Zhou X, Qi X, Mai S, Zhong Z. et al. Abstract P4-01-32: BAT8008, a novel Trop-2 ADC with strong bystander effect, for the treatment of Trop-2 positive cancer. Cancer Res. 2023;83:P4-01 -32-P4-01-32
102. Tang W, Huang X, Ou Z, Yan H, Gan J, Dong Q. et al. Abstract P6-20-16: BAT8003, a potent anti-Trop-2 antibody-drug conjugate, for the treatment of triple negative breast cancer. Cancer Res. 2019;79:P6-20 -16-P6-20-16
Author contact
![]() Corresponding authors: yss26edu.cn (S. Yan); zhangmiaoedu.cn (M. Zhang).
Corresponding authors: yss26edu.cn (S. Yan); zhangmiaoedu.cn (M. Zhang).
 Global reach, higher impact
Global reach, higher impact