13.3
Impact Factor
Theranostics 2024; 14(9):3548-3564. doi:10.7150/thno.95619 This issue Cite
Review
Metronomic chemotherapy in cancer treatment: new wine in an old bottle
State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, China.
#These authors contributed equally.
Received 2024-2-23; Accepted 2024-5-26; Published 2024-6-1
Abstract
Over the past two decades, metronomic chemotherapy has gained considerable attention and has demonstrated remarkable success in the treatment of cancer. Through chronic administration and low-dose regimens, metronomic chemotherapy is associated with fewer adverse events but still effectively induces disease control. The identification of its antiangiogenic properties, direct impact on cancer cells, immunomodulatory effects on the tumour microenvironment, and metabolic reprogramming ability has established the intrinsic multitargeted nature of this therapeutic approach. Recently, the utilization of metronomic chemotherapy has evolved from salvage treatment for metastatic disease to adjuvant maintenance therapy for high-risk cancer patients, which has been prompted by the success of several substantial phase III trials. In this review, we delve into the mechanisms underlying the antitumour effects of metronomic chemotherapy and provide insights into potential combinations with other therapies for the treatment of various malignancies. Additionally, we discuss health-economic advantages and candidates for the utilization of this treatment option.
Introduction
In the continuously evolving field of modern oncology, a diverse range of novel treatment modalities are capturing the attention of clinical scientists. Notably, targeted therapy, immunotherapy, hormonal therapy, and other advanced treatment modalities have achieved remarkable success [1-5], have profoundly reshaped the therapeutic anticancer paradigm and have seemingly reduced the prominence of chemotherapy as the predominant systemic approach to cancer treatment. Nevertheless, these emerging anticancer therapies, in numerous completed and ongoing clinical trials, continue to rely on combination regimens with chemotherapy, potentially underestimating that chemotherapy alone may affect patient health in positive and negative ways based on dose, schedule, and mechanism of action [6-8]. Conventional chemotherapy follows the maximum tolerated dose (MTD) paradigm and is associated with significant adverse effects, such as high systemic toxicity [9, 10]. This is especially true if the drug is not targeted, which leads to patient deterioration and delayed tumour growth, as resistance to further chemotherapeutic dosages is established [11]. However, metronomic chemotherapy (MCT) emphasizes the administration of low-dose and more frequent delivery of cytotoxic drugs to elicit a prolonged anticancer effect while concurrently minimizing toxicity [12, 13].
Over the past two decades, the implementation of MCT has not only demonstrated favourable outcomes in various randomized clinical trials within palliative settings [14, 15] but has also yielded progression-free survival benefits in patients with metastatic solid cancers when used either as a maintenance treatment or in combination with other therapies [16-19]. Recently, the scope of MCT indications has been further extended as an adjuvant treatment for high-risk patients with locoregionally advanced breast cancer and nasopharyngeal carcinoma [20, 21]. Additionally, numerous studies have revealed additional mechanisms of action of MCT, including its impact on the regulation of the immune tumour microenvironment (TME), direct tumour cell death and metabolic reprogramming, which has broadened our understanding of the antitumour activity of MCT beyond its originally perceived antiangiogenic mechanisms [22-24]. This review presents an overview of the advancements in MCT and its mechanisms in cancer treatment. Furthermore, we summarize the clinical significance of MCT and its synergistic effects with other therapies. In the era of precision medicine, we want to emphasize that the “old bottles” of “MCT” regimens are constantly being filled with “new wine” and should be put in front of the “older bottles” of MTD already on the shelf.
Evolving concept of MCT
Sixty years ago, Skipper, Schabel and Wilcox were the first to introduce theoretical concepts for the optimal design of chemotherapies based on the log-kill effect of several cytotoxic drugs (Figure 1) [25]. Additionally, their findings indicated that a large-dose/short-time schedule yielded better results than a frequent low-dose schedule with a similar total dose [26]. Consequently, conventional cytotoxic drugs were administered in single doses or as short courses of therapy at the highest possible doses that did not result in life-threatening levels of toxicity [27]. This approach, known as the 'maximum tolerated dose', gained prominence and became the dominant chemotherapy schedule. In the 1970s, Norton and Simon re-examined the Skipper-Schabel-Wilcox log-kill hypothesis and proposed that these chemotherapeutic drugs exhibit activity specifically against actively proliferating cells (Figure 1) [28]. Moreover, the log-kill effect theory fails to explain the limited antitumour effects of chemotherapy on both small tumours and very large tumours [9]. Consequently, the Norton-Simon model was developed to address this limitation, which led to the implementation of densified high-dose chemotherapy schedules in clinical practice [29, 30].
Historical development and breakthroughs of MCT in cancer treatment. MCT: Metronomic chemotherapy; MTD: Maximum tolerated dose; CTLs: Cytotoxic T lymphocytes; NK: Natural killer; TME: Tumour microenvironment; ICB: Immune checkpoint blocker; HIF-1α: Hypoxia-inducible factor 1; TNBC: Triple-negative breast cancer.
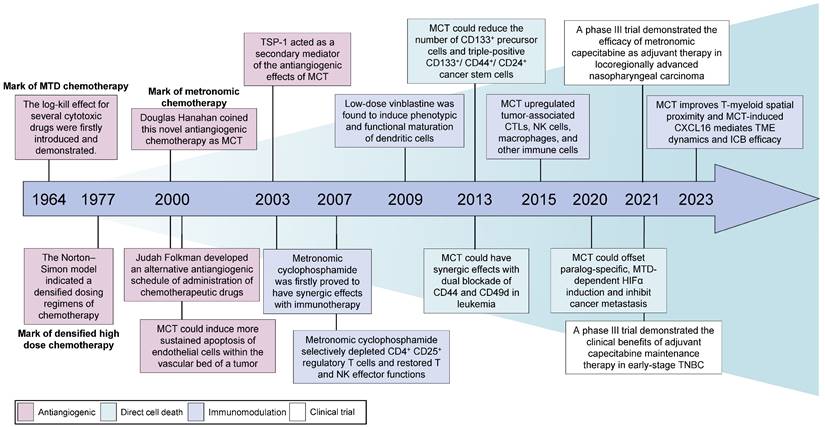
During the initial phases of chemotherapy, physicians suggested shifting the therapeutic goal from complete eradication of the tumour to long-term management of the disease [31]. Several clinical precedents aimed for better disease control and revealed the potential antitumour effectiveness of MCT. Kakolyris et al. observed that a subset of patients with non-small cell lung cancer (NSCLC), metastatic breast cancer or ovarian cancer exhibited resistance to conventional chemotherapy but showed positive responses when the same drugs were administered orally at low doses but at a relatively high frequency [32]. This novel approach, which involves the frequent administration of low-dose cytotoxic drugs, emerged in the year 2000 as a departure from the conventional MTD chemotherapy paradigm [33, 34]. In 2000, Judah Folkman and Timothy Browder developed an alternative antiangiogenic schedule for the administration of cyclophosphamide [33]. Douglas Hanahan described the concept of this less toxic and continuous chemotherapy and originally coined the term 'MCT' [34, 35]. Although no universal definition of MCT has been accepted, MCT is defined as the minimum biologically effective dose of a chemotherapeutic agent, which still induces antitumour activity, given as a continuous dosing regimen without prolonged drug-free breaks [36]. Other alternative terminologies of MCT include low-dose antiangiogenic chemotherapy, low-dose maintenance chemotherapy and metronomic scheduling of chemotherapy [13].
MCT was initially utilized as a single-drug treatment [37, 38]. However, as a deeper understanding of its mechanisms has emerged, clinicians have begun to frequently combine MCT with nonchemotherapeutic drugs, such as antiangiogenic drugs and targeted therapies [39-41]. Although MCT was originally defined as an antiangiogenic anticancer strategy [27, 42], additional mechanisms, including immunomodulation, elimination of cancer stem cells, and metabolic reprogramming, have since been revealed [43-45].
Mechanisms and rationale of MCT
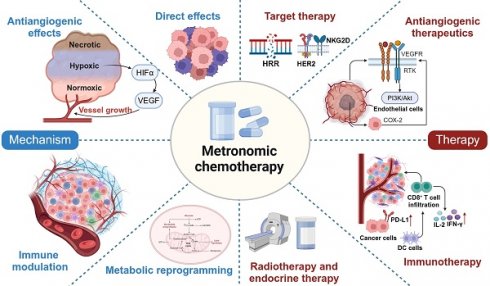
Antiangiogenic effects on endothelial cells
The mechanisms of MCT were originally attributed to its antiangiogenic effects on dividing endothelial cells (Figure 2A) [46]. Alternatively, conventional chemotherapy-induced cell death in endothelial cells could be circumvented by the secretion of endothelial cell survival factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and angiopoietin 1 [47]. However, when chemotherapy is administered more frequently without extended breaks, as in MCT, the damaged endothelium has significantly fewer opportunities to undergo repair. This leads to the irreversible accumulation of antiangiogenic effects [27]. Another study also demonstrated the antiangiogenic effects of metronomic 5-fluorouracil plus vinorelbine in triple-negative breast cancer (TNBC) through the disruption of FAK/VEGFR/VEGF signalling [48]. In addition, several studies have indicated that thrombospondin 1 (TSP1) acts as a mediator of the effects of MCT and that its expression is closely correlated with antitumour effects [49, 50]. Specifically, TSP1 primarily binds to CD36 receptors, which induces apoptosis of endothelial cells [51]. The utilization of MCT consisting of low-dose cytotoxic drugs leads to fewer side effects, such as anaemia and myelosuppression [45, 52]. This low-toxicity therapy offers the advantage of reducing the need for biopharmaceutical recombinant erythropoietin and recombinant methionyl granulocyte colony-stimulating factor (GCSF) compared with conventional chemotherapy [53]. These cytokines typically promote the mobilization of marrow progenitor cells into the peripheral circulation, which may lead to worse outcomes [54]. Furthermore, Bertolini et al. revealed that MCT has promising effects in preventing circulating endothelial progenitor (CEP) cell mobilization and inhibiting tumour growth, whereas MTD chemotherapy has been found to exert the opposite effects [55, 56].
In addition to the involvement of VEGF and the VEGF receptor family in tumour angiogenesis, the Notch signalling pathway is another key stimulator of vascular growth and tumour progression [57]. As a receptor of the Notch signalling pathway, NOTCH-1 expression was previously reported to correlate with specific subtypes of breast cancer and chemoresistance [58, 59]. Ilari et al. analysed the modulation of the expression of oncogenes and the cancer stemness-associated gene NOTCH-1 after metronomic therapy in patients with advanced TNBC [60]. The immunoreactivity of NOTCH-1 shifted from prevalent at the time of diagnosis of metastatic TNBC to decreased membrane expression at relapse [60]. As membranous NOTCH is cleaved, Notch intracellular domain (NICD) can translocate to the nucleus or remain in the cytoplasm to inhibit several oncogenic pathways, such as the c-MYC and AKT pathways [60].
Hypoxia, or low oxygen levels, is a common microenvironmental characteristic in many solid tumours [61]. Hypoxia is closely linked to disease progression and poor survival rates due to increased potential for metastatic spread and resistance to cancer therapies. This challenging aspect of the TME contributes to the aggressiveness and treatment resistance of solid tumours, which indicates its importance as a target for novel therapeutic strategies, including MCT. The combination of low-dose chemotherapy, such as doxorubicin, with a limited number of antiangiogenic drugs can overcome resistance in tumours that exhibit enhanced HIF-1 activation, which leads to elevated VEGF-A levels and subsequent hypoxia [62]. Consistently, metronomic cyclophosphamide has been found to offset HIF-1α induction and limit hypoxia in colon cancers [63]. This approach effectively decreases HIF-1α, reduces VEGF expression, and inhibits angiogenesis, thereby rectifying the oxygen imbalance [64]. These findings provide a compelling rationale for the combination of MCT and antiangiogenic drugs, as this treatment offers a potential synergistic approach to inhibit cancer growth and angiogenesis.
Mechanisms of Action of MCT. MCT could induce antitumour effects via A Antiangiogenic effects on endothelial cells; B Direct effects on cancer cells; C Immunomodulation of microenvironment; D Metabolic reprogramming. MCT: Metronomic chemotherapy; TSP1: Thrombospondin 1; VEGF: Vascular endothelial cell growth factor; bFGF: basic fibroblast growth factor; HIF-1α: Hypoxia-inducible factor 1; EMT: Epithelial-mesenchymal transition; SA-β-Gal: Senescence-associated-beta-galactosidase; NK: Natural killer; OXPHOS: Oxidative phosphorylation.
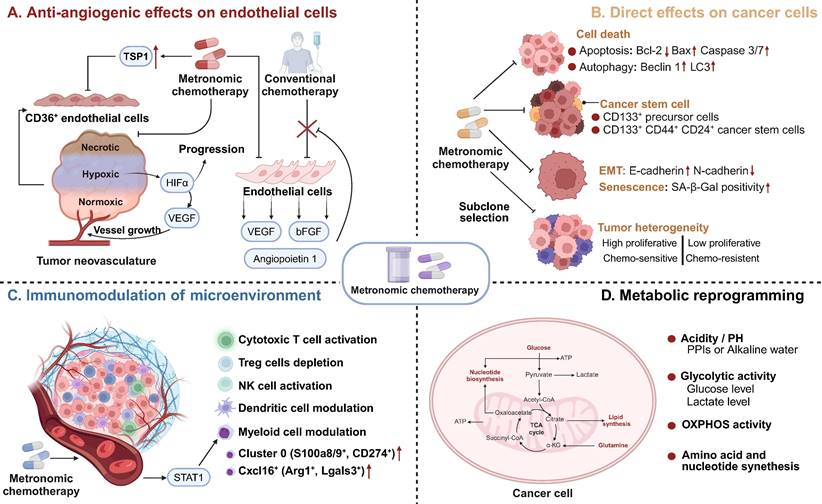
Direct effects on cancer cells
As a novel schedule for chemotherapeutic agents, MCT affects cancer cells through various mechanisms, including the induction of cell death, direct reduction of cancer stem cells, inhibition of epithelial-mesenchymal transition (EMT), and promotion of senescence and subclone selection (Figure 2B) [43, 63]. Numerous studies have indicated that MCT directly induces cancer cell death, including apoptosis and autophagy-dependent cell death [65-68]. Compared with drugs administered alone, metronomic vinorelbine combined with Endostar exhibited significantly increased antitumour activity and induced apoptosis in Lewis lung carcinoma by downregulating Bcl-2 expression and upregulating the expression of Bax and caspase 3/7 [69]. Interestingly, Bruni et al. observed a shift from caspase-dependent to caspase-3-independent apoptosis when the dose of etoposide was decreased in the treatment of acute myeloid leukaemia (AML) [66]. Similarly, another study revealed that metronomic temozolomide caused the accumulation of cytotoxicity through apoptosis, cellular senescence, and DNA damage to inhibit glioblastoma cells [70]. In addition to its effect on apoptosis, MCT also exerts its antitumour effects through autophagy [71]. The metronomic use of the podophyllotoxin derivative etoposide induced autophagy in non-Hodgkin's lymphoma through increased expression of Atg5, Beclin1, and LC3 [71]. Analysis of the neoadjuvant trial JBCRG-07 indicated that metronomic cyclophosphamide plus letrozole increased the expression of the autophagy-related markers Beclin 1 and LC3 in hormonal receptor (HR)-positive breast cancer tissues [67].
Vives et al. reported that metronomic cyclophosphamide could reduce the number of CD133+ precursor cells and triple-positive CD133+/CD44+/CD24+ cancer stem cells in orthotopic models of human pancreatic adenocarcinoma [72] (Figure 1). Similar targeting of drug-resistant CD44+ and CD133+ prostate cancer cells was also observed in vivo after treatment with a combined metronomic regimen of docetaxel and the metabolic blocker fenofibrate [73]. Interestingly, in mice with CD44+CD49d+ lymphoma, only leukaemia cell apoptosis was observed after treatment with a combination of anti-CD44, anti-CD49d, and low-dose cisplatin, which disrupted apoptotic resistance to antibodies and drove apoptosis during chemotherapy [74] (Figure 1). These findings demonstrate that MCT can target CD44+ cancer stem cells and exert synergistic effects with CD44 blockade.
In addition, the metronomic application of several chemotherapeutics has been found to inhibit the EMT process in cancer cells [75, 76]. Single-cell RNA-seq and RNA-seq analysis have indicated that metronomic topotecan treatment induced the downregulation of EMT markers, including CD55 and HAS3, in metastatic castration-resistant prostate cancer [76]. A preclinical study revealed that metronomic cordycepin upregulated E-cadherin and downregulated N-cadherin protein expression in a human oral squamous cell carcinoma xenograft model, which suggests the EMT process was inhibited [75].
Senescent cells are characterized by cell cycle arrest, flattened cell bodies, and high levels of senescence biomarkers such as senescence-associated-beta-galactosidase (SA-β-Gal), p16 and p21 [77, 78]. Metronomic topotecan impedes tumour growth by inducing cell cycle arrest, p21WAF/CIP1 upregulation and DNA damage, but favourable NFKB1/p50 activation does not occur [79]. Another study indicated that a significant increase in the number of senescent cells (characterized by SA-β-gal activity) was observed after metronomic 5-fluorouracil and vinorelbine was applied in TNBC [80].
Another mechanism by which MCT exerts direct effects on cancer cells is subclone selection. Tumours acquire more genetic mutations as they grow, which leads to greater heterogeneity during tumour progression [81]. Consequently, the bulk tumour might include a diverse collection of subclones harbouring distinct molecular signatures with differential levels of treatment sensitivity. These subclones, comprising both sensitive and resistant variants, compete for space and resources within the TME. Phenotypic resistance to chemotherapy in cancer cells is attributed to the proliferation of chemoresistant cancer cells after chemosensitive cancer cells are eliminated [31, 82]. Compared with MTD-based chemotherapy, MCT can restrain the proliferation of resistant subclones and hinder 'subclonal switching signals' to prevent dormant subclones from becoming the dominant subclones that can lead to tumour progression and recurrence [83, 84]. In a transgenic mouse model of cancer, additional use of metronomic cyclophosphamide increased endothelial cell apoptosis and improved survival outcomes [83].
Immunomodulation of the immune microenvironment
Accumulating evidence suggests that chemotherapeutic agents can modulate the immune microenvironment of tumours, which is crucial for the long-term control of cancer [85, 86]. The immunological effects of chemotherapeutic drugs are extremely diverse due to variations in their mechanisms and dosing schedules [87]. A key goal of MCT is to facilitate immunostimulation and promote the “cold to hot” transition of the microenvironment [88]. Previous studies have indicated that MCT can promote the induction of immunogenic cell death (ICD) and increase the susceptibility of tumour cells to immune effectors [89, 90]. ICD refers to a specific form of cancer cell death that is induced by certain chemotherapeutic drugs [91]. This process is triggered by the release of damage-associated molecular patterns (DAMPs) from dying tumour cells, which leads to the activation of tumour-specific immune responses [91]. Metronomic oxaliplatin was found to upregulate the expression of ICD markers, including calreticulin and high mobility group box-1 (HMGB1), both in vivo and in vitro [90]. Consistently, metronomic cyclophosphamide was shown to trigger immunogenic cell death, as indicated by elevated calreticulin, adenosine triphosphate (ATP) release, and HMGB1 secretion [92].
Metronomic dosing with specific chemotherapies has shown certain desirable immune responses, such as the activation of cytotoxic T cells and natural killer (NK) cells, preferential depletion of regulatory T (Treg) cells, and enhancement of antigen presentation through the activation of dendritic cells (DCs) (Figure 2C) [22, 93-95]. Despite numerous studies that have investigated the immunomodulatory effects of MCT, limited research has provided a comprehensive understanding of the precise impact of MCT in a high-resolution context [22]. Recently, Bhavana and colleagues systemically described the dynamic changes in the immune microenvironment after metronomic doxorubicin and cyclophosphamide at the single-cell level and analysed the impact on myeloid cells [22] (Figure 1). They observed a highly heterogeneous transcriptome status of tumour-associated myeloid cells after MCT, which was indicative of a balance in the immune stimulatory and immunosuppressive environments in the TME. Specifically, chemotherapeutics were reported to induce a refreshed reconstitution of immune populations [96]. Low-dose cyclophosphamide and paclitaxel have been shown to enhance NK cell effector functions and CD8+ T-cell activation in cancer patients [95, 97]. Phenotypic and functional maturation of DCs could be induced by low-dose noncytotoxic vinblastine [33]. Furthermore, MCT plays an important role in the elimination and inhibition of immunosuppressive cell populations [96]. Previous studies have indicated that metronomic cyclophosphamide and gemcitabine suppress the infiltration and function of Tregs [95, 98].
In addition to the immunostimulatory function of MCT, some chemotherapeutic agents can increase the sensitivity of tumour cells to immune effectors such as cytotoxic T lymphocytes (CTLs) and NK cells [99, 100]. Ramakrishnan et al. reported that common chemotherapeutic drugs could sensitize tumour cells to CTLs by increasing the permeability of tumour cells to granzyme B via the upregulation of mannose-6-phosphate receptors [99]. Moreover, metronomic gemcitabine was found to promote the expression of MICA/B on the cell surface, which enhances innate immunity against tumour cells [101].
Metabolic reprogramming
Metabolic reprogramming is regarded as a hallmark of carcinogenesis and cancer progression [102]. However, growing evidence indicates that metabolic pathways and metabolites could function as regulators in signalling and in interactions and modulation between the immune microenvironment and cancer cells [103, 104]. An integrated analysis of metabolomics and PCR array data revealed that MCT consisting of certain agents inhibits tumour growth via metabolic reprogramming in cancer cells, including the inhibition of glycolysis, amino acid metabolism and nucleotide synthesis-associated metabolic pathways (Figure 2D) [105]. MCT has been shown to increase Hif-1a, Aldoa, and Pgk1 expression, which implies an upregulation of glycolysis in colon cancer [106]. Moreover, in one study, metronomic doxorubicin significantly increased the concentrations of amino acids, especially alanine, aspartate, and glutamine, while the MTD of doxorubicin decreased the concentrations of these amino acids [105]. Furthermore, metronomic doxorubicin slightly and broadly upregulated the expression of key enzymes involved in nucleotide metabolism, such as carbamoyl phosphate synthetase II, CTP synthetase 1, phosphoribosyl pyrophosphate aminotransferase, and IMP dehydrogenase 1 [105].
OXPHOS activity was previously reported to correlate with the efficacy of chemotherapeutics [107]. Oresta et al. indicated that the chemotherapeutic drug mitomycin C-induced ICD relies on the metabolic reprogramming of tumour cells towards increased oxidative phosphorylation (OXPHOS) activity [108]. This process leads to increased mitochondrial permeability and the release of mitochondrial DNA into the cytoplasm, which subsequently activates the inflammasome to efficiently secrete interleukin-1β and promote DC maturation [108, 109]. Consistently, metformin-induced disruptions in mitochondrial respiration were found to enhance the antitumour effects of metronomic cyclophosphamide in patients with neuroblastoma [107]. Bondarenko et al. demonstrated that drug-sensitive NSCLC subclones mostly relied on aerobic glycolysis, while drug-resistant clones relied on OXPHOS [109]. A similar finding was demonstrated in AML, in which chemotherapy-resistant AML cells exhibited increased mitochondrial mass and retained active polarized mitochondria, indicative of OXPHOS [110].
Furthermore, the anaerobic metabolism of cancer cells can lead to an increase in hydrogen generation, resulting in tumour acidity [111]. The highly acidic microenvironment can quickly protonate and neutralize weak bases from chemotherapeutics, which triggers chemoresistance [112]. However, patient alkalization through the use of proton-pump inhibitors (PPIs) or water alkalizers has been shown to be well tolerated and to enhance the tumour response to MCT agents [113].
Application of MCT in oncology clinics
Since the inception of MCT in 2000, its clinical application has progressively expanded and has been accompanied by sustained growth in designed clinical trials. MCT has been applied to a range of cancer types, including breast cancer, NSCLC, gastrointestinal cancer, gynaecological cancer, and nasopharyngeal cancer [20, 114-117]. Additionally, it has been utilized in cancers with comparatively lower incidence rates, such as oral cancer and nervous system tumours [118, 119]. Therefore, the clinical application of MCT is extensive. MCT agents can not only be used in combination with various types of therapeutics, but this approach also offers low-toxicity and effective treatment regimens for elderly patients who are not suitable for intensive therapies [120].
MCT plays dual roles not only as a final salvage treatment for advanced tumours but also as a widely adopted maintenance therapy due to the convenience and minimal toxicity of the agents used [121]. The primary agents used in MCT are mainly cyclophosphamide, methotrexate, vinorelbine, tegafur-uracil and capecitabine.
MCT as salvage treatment
The predominant agents for single-agent metronomic therapy are capecitabine and vinorelbine. In the context of advanced breast cancer, a phase III clinical trial that compared the efficacy of capecitabine MCT (1,000 mg twice daily for 14 of 21 days) with classical cyclophosphamide, methotrexate, and fluorouracil (CMF) therapy as a first-line treatment confirmed the equivalence of single-agent metronomic therapy to capecitabine in terms of efficacy and also demonstrated superior tolerability [122]. Notably, capecitabine monotherapy has yielded favourable outcomes even in patients with advanced disease. In a single-arm phase II clinical trial conducted by Fedele, late-stage breast cancer patients who received capecitabine monotherapy (1,500 mg once per day) achieved a clinical benefit rate (CBR) of 62%, along with a median time to treatment progression (mTTP) of 7 months [52]. Consistently, metronomic capecitabine is a well-tolerated and effective therapeutic option for patients with liver cancer and those with gastrointestinal tumours [123-125].
Moreover, metronomic oral vinorelbine (mVNR) is regarded as a well-tolerated and effective therapeutic capable of achieving long-term disease control and stability in metastatic diseases [126, 127]. When conventional chemotherapy is not feasible for elderly patients, the use of mVNR, which is less toxic, can serve as a rescue treatment, and an impressive CBR of 72% has been demonstrated in elderly patients with advanced NSCLC [128]. Similarly, Addeo et al. observed good tolerability and promising results (with an objective response rate (ORR) of 38%) in a cohort of 34 patients with metastatic breast cancer (median age, 74 years) treated with the mVNR regimen (70 mg/m2, fractionated on days 1, 3, and 5, for 3 weeks on and 1 week off, every 4 weeks) [127].
The combination of cyclophosphamide and methotrexate (CM) constituted one of the earliest dual-drug metronomic regimens employed in oncology practice. In 2002, Colleoni et al. demonstrated that metronomic oral CM (cyclophosphamide 50 mg/day and methotrexate 2.5 mg twice per day on days 1 and 2 every week) was efficacious (with a CBR of 31.7%) and had low toxicity in the context of advanced breast cancer [129]. Hussein et al. revealed the efficacy and decreased toxicity of metronomic oral CM regimens (cyclophosphamide 50 mg/day and methotrexate 2.5 mg twice per day on days 1 and 2 every week) in advanced breast cancer [130]. While limited clinical studies have explored the application of metronomic CM in patients with advanced glioma (cyclophosphamide 100 mg daily and methotrexate 5 mg twice weekly) and prostate cancer (cyclophosphamide 50 mg/d and MTX 2.4 mg twice per week), the findings have not been encouraging [131, 132].
Additional dual-drug metronomic regimens, including cyclophosphamide with capecitabine (CX) and vinorelbine with capecitabine (NX), have demonstrated promising outcomes in clinical investigations. In a phase II study, CX (capecitabine 828 mg/m2 twice daily with cyclophosphamide 33 mg/m2 twice daily, days 1-14 every 3 weeks) was employed as a salvage treatment for human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer, which resulted in a median progression-free survival (PFS) of 12.3 months and a CBR of 57.8% [133]. The NX regimen has been established as an effective treatment for breast cancer, boasting a median time to progression (mTTP) of 10.5 months, an ORR of 33%, and a CBR of 67% [134].
In recent years, the three-drug combination metronomic regimen of vinorelbine and cyclophosphamide plus capecitabine (VEX) has achieved exceptional results in clinical trials. In a phase 2 clinical study, VEX (cyclophosphamide 50 mg daily, capecitabine 500 mg three times daily and vinorelbine 30 or 40 mg) achieved mTTP values of 25.1 and 11.2 months in untreated and treated breast cancer patients, respectively [135]. In the subsequent randomized controlled study METEORA-II, VEX (cyclophosphamide 50 mg daily, capecitabine 500 mg three times daily and vinorelbine 40 three times per week) extended the time to treatment failure (TTF) by 2.5 months and the PFS by 4.2 months compared with weekly intravenous paclitaxel [17].
MCT as a maintenance treatment
In addition to salvage treatment, MCT could be used as a maintenance treatment strategy, including maintenance following adjuvant chemotherapy or as maintenance therapy after salvage treatment (Table 1). Retrospective data have illustrated that adjuvant chemotherapy consisting of metronomic tegafur-uracil (2 capsules twice daily) could significantly improve the 5-year OS rate (71.6% vs. 28.7%, p<0.001) in patients with high-risk nasopharyngeal carcinoma after radiotherapy [136]. In recent years, several high-impact, randomized, controlled, phase 3 trials have achieved impressive success with adjuvant treatment according to the MCT strategy [20, 21]. Chen et al. conducted a phase 3 trial with 675 patients and reported that the addition of the metronomic adjuvant capecitabine (650 mg/m2 body surface area twice daily for 1 year) to chemoradiotherapy significantly improved failure-free survival (85.3% vs. 75.7%, p=0.002) in patients with high-risk locoregionally advanced nasopharyngeal carcinoma [20] (Figure 1). In another well-known trial with substantial cohorts, the SYSUCC001 study demonstrated that patients with early-stage triple-negative breast cancer could achieve a significant improvement in 5-year disease-free survival (DFS) from 1 year of treatment with metronomic capecitabine (650 mg/m2 twice a day) after adjuvant chemotherapy (82.8% vs. 73.0%, p=0.03) [21] (Figure 1). Moreover, maintenance chemotherapy with tegafur-uracil significantly enhanced both DFS and OS among patients with nasopharyngeal cancer [137]. The option of first-line XELOX (500 mg bid daily) or FOLFOX (400 mg daily) followed by metronomic capecitabine maintenance is also considered for patients with colorectal cancer [138, 139].
MCT combined with other therapies
Combination with antiangiogenic agents
As mentioned above, MCT has been regarded as a drug scheduling scheme that is associated with lower toxicity and better tolerance, which makes it highly compatible for combination with other treatment modalities in clinical settings. Numerous ongoing clinical trials have aimed to determine the most effective synergistic therapies that can be combined with MCT to enhance antitumour effects and prolong the survival of cancer patients. Browder et al. demonstrated the potential effectiveness of combining MCT with endothelial cell-specific angiogenesis inhibitors, such as anti-VEGF receptor and COX-2 inhibitors, to achieve promising outcomes [33, 140].
The rationale for the adoption of this strategy was based on several critical considerations (Figure 3A). Specifically, VEGF-receptor tyrosine kinases are preferentially expressed by endothelial cells within the actively growing neovasculature of a tumour, while COX-2 is expressed in both invasive and in situ cancer cells [141, 142]. In one study, VEGF inhibited the apoptosis of endothelial cells within newly formed vessels by activating the PI3K-AKT pro-survival signalling pathway [143, 144]. Furthermore, COX-2 has been found to upregulate the expression of the proangiogenic growth factor VEGF and to promote the inhibition of endothelial cell apoptosis through the stimulation of Bcl-2 or Akt activation [145].
In addition, it has been observed that conventional chemotherapy with docetaxel induces the expression of VEGF along with other antiapoptotic effectors [47]. However, the elevated levels of VEGF promoted by conventional chemotherapy contribute to the development of multidrug resistance to chemotherapeutics [143, 146]. Therefore, the combination of MCT with antiangiogenic drugs could enhance the proapoptotic effects of chemotherapeutic agents on proliferating endothelial cells. Numerous studies have demonstrated that VEGF inhibitors or COX-2 inhibitors combined with MCT may be associated with survival benefits in patients with various cancers [147-149]. In a single-arm prospective clinical trial, the combination of the VEGF inhibitor bevacizumab and metronomic capecitabine and cyclophosphamide was found to be effective in advanced breast cancer [148]. Similarly, a phase II trial demonstrated the effectiveness of bevacizumab combined with metronomic cyclophosphamide in patients with recurrent ovarian cancer [149]. However, in a phase III randomized trial, patients with head and neck cancer who received the adjuvant metronomic methotrexate and celecoxib (a COX-2 inhibitor) failed to experience improvements in PFS or overall survival (OS) [147].
Combination with immunotherapy
Due to its diverse range of immunomodulatory activities, MCT has emerged as an ideal candidate for combination therapy with immunotherapy, including drugs that function in immune checkpoint blockade (ICB) and immune vaccination [19, 150].
Metronomic chemotherapy could be employed as maintenance treatment in various types of cancer.
| Patient population/ disease setting | Type of study | N | Treatment regimen | Efficacy |
|---|---|---|---|---|
| Pretreated nasopharyngeal carcinoma | Retrospective | 625 | Tegafur-uracil | 5-year OS: 71.6% |
| Triple negative breast cancer | Phase III | 158 | Cyclophosphamide with methotrexate | mPFS: 28m |
| Advanced ovarian carcinoma | Clinial trial | 60 | Cyclophosphamide with methotrexate | mPFS: 18m |
| Metastatic colorectal cancer | Clinial trial | 233 | Capecitabine | PFS: 66.7% |
| Colon cancer | Retrospective | 132 | Tegafur-uracil | 5-year OS: 86.8% |
| Primary hepatic carcinoma | Clinial trial | 114 | Tegafur | PFS:16.25m; PFS%: 83.3% |
| Advanced oral cancer | Retrospective | 356 | Tegafur-uracil | 5-year OS: 65%; 5-year DFS: 57%; 5-year DSS: 74% |
| Stage IV nasopharyngeal carcinoma | Retrospective | 70 | Tegafur-uracil | 5-year OS: 91.89% |
| Metastatic colorectal cancer | Clinial trial | 48 | Capecitabine | mPFS: 5.66m; mOS: 23.82m |
| Locoregionally advanced nasopharyngeal carcinoma | Phase III | 675 | Capecitabine | 3-year failure-free survival: 85.3% |
| Triple negative breast cancer | Retrospective | 223 | Cyclophosphamide with capecitabine / Cyclophosphamide with methotrexate | 5-year DFS: 64.5%; 10-year DFS: 59.8%; 5-year OS: 71.2%; 10-year OS: 67.1% |
| Stage II colorectal cancer | Retrospective | 233 | Tegafur-uracil | 5-year DFS: 81.39% |
| Locally advanced head and neck squamous cell carcinoma | Retrospective | 240 | Tegafur-Uracil | OS was not reached |
| Early-stage triple-negative breast cancer | Phase III | 443 | Capecitabine | 5-year DFS: 82.8%; 5-year DSS:85.8%; 5-year OS: 85.5% |
| Pretreated nasopharyngeal carcinoma | Retrospective | 98 | Tegafur-uracil | mPFS:24.7m; mOS:36m |
Therapeutic strategies combined with metronomic chemotherapy in clinic. A Combined with antiangiogenesis; B Combined with immunotherapy; C Combined with target therapy; D Combined with radiotherapy and endocrine therapy. MCT: Metronomic chemotherapy; GF: Growth factor; VEGF: Vascular endothelial cell growth factor; PARP: Poly(ADP‐ribose) polymerase; HRR: Homology-dependent recombination repair; PD-L1: Programmed Death Ligand 1; DC: Dendritic cells; IFN-γ: Interferon-gamma; IL-2: Interleukin 2; ER: Estrogen receptor; pERα: phosphorylated form of ER alpha; HER2: Human epidermal growth factor receptor 2; NK: Natural killer; ADCC: Antibody-dependent cellular cytotoxicity.
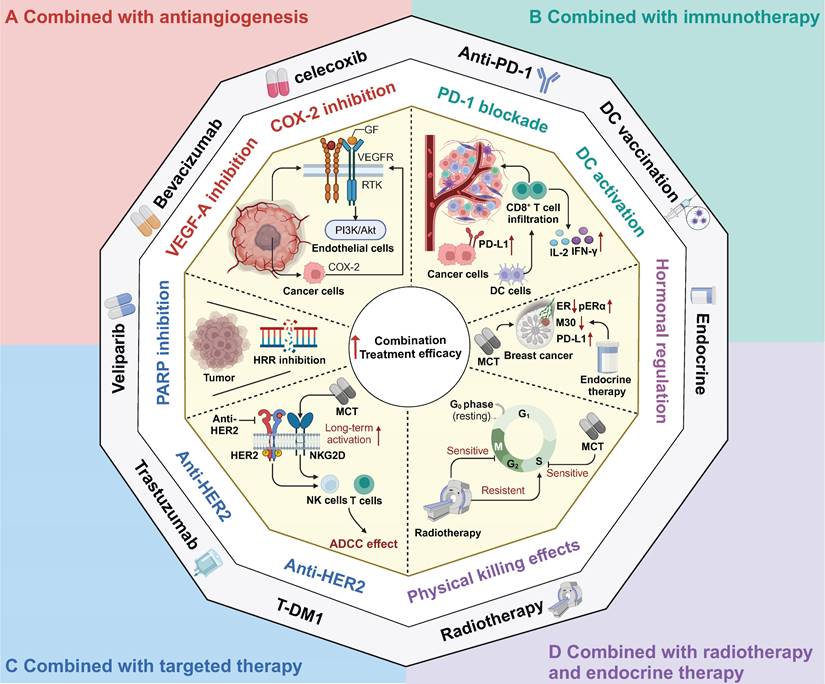
In recent years, ICB therapy has led to an antitumour immune response and has achieved great progress in various types of cancer, such as melanoma, NSCLC, and haematological malignancies [151-153]. Despite its promising success in several cancer types, ICB therapy has not achieved satisfactory results in solid tumours such as breast cancer, colorectal cancer and hepatocellular carcinoma [154-156]. Hence, to facilitate the transformation from a "cold" to a "hot" TME and to enhance the response rate to ICB, the combination of immunotherapy with conventional chemotherapy has been extensively explored and has yielded promising results, as demonstrated in trials such as Keynote-522, Keynote-048, and IMpower 130 [6, 157, 158]. Furthermore, the combination of MCT with immunotherapy has also shown synergistic effects in various types of cancer [159-161]. MCT has immunostimulatory effects on both immune cells and cancer cells, which provides a rationale for the combination strategy of MCT and immunotherapy (Figure 3B). Khan and colleagues found that metronomic cyclophosphamide could increase the general immune response (and also upregulated PD-L1 expression) in preclinical models of breast cancer [159]. Similarly, Zhou et al. confirmed this finding in their study on lung cancer, where they observed that low-dose carboplatin could facilitate the "cold-to-hot" transition of the TME, which resulted in increased infiltration of CD8+ T cells and increased PD-L1 expression [162]. Furthermore, the combination of MCT with an anti-PD-1 agent demonstrated a substantial antitumour effect on squamous cell lung carcinoma [162]. This effect was attributed to the increase in activated type I macrophages, DCs, and cytotoxic CD8+ T cells, as well as the preservation of intestinal gut microbiota diversity [162]. In addition to ICB, MCT promoted the proliferation and infiltration of CD8+ T cells to sensitize patients with mesothelioma and metastatic melanoma to DC-based vaccines [163, 164].
In addition to the combination of chemotherapy and immunotherapy as a dual treatment approach, chemotherapy can also be synergistically combined with other treatment strategies to augment sensitivity to immunotherapy [22, 165]. For instance, researchers have shown that MCT-induced treatment sensitizes TNBC to ICB treatment by chemically inhibiting STAT1 signalling [22]. Similarly, in small cell lung cancer, the combination of metronomic gemcitabine with a checkpoint kinase 1 inhibitor has been found to enhance the efficacy of ICB therapy. This enhancement is achieved through activation of CD8+ cytotoxic T cells, DCs, and M1 macrophages, along with the downregulation of immunosuppressive M2 macrophages and myeloid-derived suppressor cells (MDSCs) [165].
Based on the rationale for the immunomodulatory effects of MCT, several clinical trials have further explored the combination of MCT with immunotherapy strategies in multiple cancer types [19, 117, 166]. A phase III randomized trial demonstrated that the addition of low-dose nivolumab to MCT significantly increased the one-year OS from 16.3% to 43.4% [19]. In another phase II study, Zsiros et al. reported that the combination of pembrolizumab with bevacizumab and metronomic cyclophosphamide achieved an impressive ORR of 47.5% and a median PFS of 10.0 months in patients with recurrent ovarian cancer [117]. Moreover, a phase II trial revealed that when metronomic cyclophosphamide and a COX-2 inhibitor were given in conjunction with a DC vaccine, 57% of patients had achieved stable disease at the first evaluation [164]. Another preclinical study demonstrated that MCT (taxanes and alkylating agents) plus a vaccine (a multipeptide cocktail including hepatitis C virus and tumour antigen TERT epitopes) enhanced the specific T-cell response and improved antitumour effects [167].
Combination with targeted therapy
MCT combined with targeted therapy has shown considerable promise in multiple preclinical studies and clinical trials [168-171] (Figure 3C). Poly(ADP‐ribose) polymerase (PARP) inhibitors have been demonstrated to inhibit homologous recombination repair (HRR) and exhibit an immunomodulatory function [172]. Kummar et al. conducted a phase I study, which provided evidence that the combination of veliparib and metronomic cyclophosphamide was well tolerated and exhibited encouraging activity in patients with BRCA mutations and refractory solid tumours and lymphoma [172]. In another phase I study, this same combination treatment demonstrated consistent antitumour effectiveness in metastatic HER2-positive breast cancer [173].
Traditional anti-HER2 drugs, such as trastuzumab, primarily exert their antitumour effects through antibody-dependent cellular cytotoxicity (ADCC) [3, 174]. Extensive research has demonstrated that trastuzumab can activate NK cells and T cells, enhancing the effects of ADCC and inhibiting HER2-positive breast cancer [175, 176]. To further enhance therapeutic efficacy, chemotherapy can elevate interferon-gamma (IFN-γ) and interleukin 2 (IL-2) levels, which consequently activates cytotoxic T cells and NK cells [177]. Currently, the combination of dual anti-HER2 targeted therapy with chemotherapy has become the prevailing approach in the neoadjuvant and adjuvant treatment of breast cancer [178, 179]. However, limited attention has been given to exploring the combination of MCT and anti-HER2 targeted therapy. A preclinical study revealed that MCT combined with VEGF inhibition demonstrated antitumour effects on human breast cancer xenografts with acquired resistance to trastuzumab [180].
Orlando et al. conducted a phase II trial to show the clinical efficacy of the combination of metronomic cyclophosphamide and capecitabine with trastuzumab as a first-line therapy for HER2-positive breast cancer [181]. Consistently, in a phase II randomized trial, the addition of metronomic oral cyclophosphamide to trastuzumab plus pertuzumab resulted in a significant increase in the median PFS of 7 months compared with dual HER2 blockade alone in patients with HER2-positive metastatic breast cancer [18]. Additionally, a phase I trial of T-DM1 combined with metronomic temozolomide showed potential activity in the secondary prevention of HER2+ brain metastases [170].
Radiotherapy combined with endocrine therapy
MCT regimens have also been investigated in combination with radiotherapy and endocrine therapy [182, 183] (Figure 3D). Chemoradiotherapy can be regarded as a form of "accelerated" radiotherapy, where radiation therapy is combined with anti-S phase radiosensitizing chemotherapy [184]. Numerous studies have demonstrated that induction chemotherapy can improve the efficacy of radiotherapy in cancer patients [185-187]. Furthermore, the combination of MCT and radiotherapy has shown potential for the development of treatment regimens with improved tolerability and increased response rates. For instance, a phase II trial evaluated the efficacy of metronomic vinorelbine combined with temozolomide and radiotherapy in breast cancer patients with previously untreated brain metastasis [188]. Overall, 52% of patients (19/36) achieved either complete response or partial response after treatment with these combination regimens. In a recent clinical trial that evaluated the efficacy of bevacizumab, etoposide, and cisplatin in combination with whole-brain radiotherapy for previously untreated brain metastases in breast cancer patients, a comparative ORR of 52.6% was observed [189]. Another retrospective study reported the PFS benefits of adding metronomic cyclophosphamide to radiotherapy versus radiotherapy alone in patients with NSCLC, although the response rates were not significantly different between these two groups [190].
Endocrine treatment with a selective oestrogen receptor modulator has been recommended as the first-line treatment for breast cancer patients who are positive for hormone receptors [191]. In addition, endocrine therapy could be considered for patients with low-grade ovarian cancers and serous borderline ovarian tumours [192]. MCT, when used in combination with endocrine therapy, has been extensively investigated in both preclinical studies and clinical trials [67, 193-195]. Adamo et al. conducted a study to evaluate the effectiveness of oral metronomic vinorelbine in combination with endocrine therapy, specifically in HR-positive HER2-negative breast cancer [195]. Another preclinical study indicated that the combination of metronomic 5-FU and exemestane exerted excellent tumour suppressive effects in gastric cancer [196].
Exploratory analysis of this combination strategy revealed an interesting observation: that this approach could induce an increase in PD-L1 expression and a decrease in oestrogen receptor (ER)-related gene expression [195]. Furthermore, the phosphorylated form of ER alpha (pERα) was identified as an independent factor that affects the sensitivity of patients to letrozole plus metronomic cyclophosphamide therapy [197]. Furthermore, in the clinical trial JBCRG-07, the addition of metronomic cyclophosphamide was shown to improve the therapeutic effects of letrozole in patients with HR-positive breast cancer [193]. In response to metronomic chemoendocrine therapy, the levels of the apoptosis-related marker M30 decreased [67].
A multicentre phase II trial investigated the effectiveness of neoadjuvant letrozole plus low-dose cyclophosphamide in early-stage ER-positive breast cancer [193]. This therapeutic combination demonstrated a favourable clinical response among the enrolled ER-positive breast cancer patients, with a clinical response rate of 67.5% [193]. Similarly, in another randomized phase II trial, the combination of neoadjuvant letrozole and metronomic cyclophosphamide showed superior clinical efficacy compared with letrozole alone in early-stage breast cancer (disease response: 82.7% vs. 73.2%) [197]. Beyond early-stage breast cancer, a phase II trial explored the use of metronomic capecitabine in combination with aromatase inhibitors in advanced breast cancer patients [198]. This treatment regimen exhibited a significant ORR of 70.5% and achieved a PFS of 16.2 months when used to treat HR-positive advanced breast cancer patients who were treated with second-line therapy or beyond [198].
Health-economic benefits are derived from MCT. The progress achieved in cancer treatment in high-income countries is evident, as the average 5-year net survival in these countries is approximately 12 times greater than that in low-income countries [199]. Despite that 75.1% of cancer-related deaths occur in low- and middle-income countries (LMICs), their proportion of the economic burden of cancer was comparatively lower at 49.5% [200]. In addition, authors from the Harvard School of Public Health estimated that cancer treatment costs needed to increase the survival of patients with eleven cancer types will increase by 6.9% between 2020 and 2023 [201]. The disparities in medical resources and health outcomes and the increase in financial burdens underscore the need for oncologists and governments to prioritize the health-economic considerations of anticancer therapies. In the realm of cancer treatment options, MCT regimens are remarkable due to their cost-effectiveness and convenient administration [202]. The reasons for this are that metronomic therapies are often associated with lower direct costs due to their inclusion of affordable generic drugs, many of which have been available for a long time [202]. Moreover, the oral formulation of these metronomic drugs eliminates the requirement for expensive hospital stays, intravenous injections, and the use of central venous access, which results in additional cost savings [202]. Finally, compared with MTD chemotherapy, MCT usually does not expose patients to a greater risk of infections or additional nutritional problems, which potentially decreases the need for monitoring, supportive care and fees for adverse effects [203].
For instance, several studies have compared the cost-effectiveness of MCT regimens to that of alternative chemotherapy regimens [204, 205]. The use of metronomic capecitabine as an adjuvant chemotherapy has been shown to be a cost-effective treatment strategy in patients with locoregionally advanced nasopharyngeal carcinoma [204]. In this cost-effectiveness analysis, the use of the adjuvant metronomic capecitabine resulted in an incremental cost-effective ratio (ICER) of $9,669.99 per quality-adjusted life year in China, which is significantly lower than the value of the WTP and even lower than the one-time per capita gross domestic product in China [204]. Another comparative pharmacoeconomic assessment demonstrated that metronomic cyclophosphamide-methotrexate represents a notably cost-effective approach in the treatment of metastatic breast cancer [206].
In addition to improvement in survival rates, the assessment of treatment options and their influence on changes in health-related quality of life (HRQoL) holds significant importance, as it can provide valuable insights for health care practitioners. Several studies have investigated the correlation between the use of MCT regimens and their impact on HRQoL [207-211]. Dal Lago et al. indicated that additional metronomic cyclophosphamide had no significant impact on HRQoL in older/frail patients with HER2-positive metastatic breast cancer [208]. Moreover, metronomic methotrexate and celecoxib were found to significantly improve the pain QLQ-C30 score compared with cisplatin chemotherapy in patients with squamous cell carcinoma of the head and neck [211]. Collectively, these findings substantiate the health-economic benefits of MCT regimens across a range of clinical contexts, especially in LMICs.
Conclusion
After more than two decades since its inception, MCT has garnered increasing interest and remarkable success in cancer treatment. Recent discoveries of novel mechanisms across multiple dimensions of this approach, coupled with its combination with various therapies, have breathed new life into this traditional strategy. MCT should not be perceived merely as antagonistic; rather, MCT regimens can directly affect cancer cells, modulate the immune microenvironment, and induce metabolic reprogramming. Emphasizing the potential of combination approaches could expand the spectrum of MCT applications in clinical settings. Notably, when combined with antiangiogenic agents, immunotherapy, targeted therapy, endocrine therapy, and radiotherapy, MCT exerts promising synergistic effects against various cancers. In addition, compared with conventional chemotherapy, MCT offers the advantages of cost-effectiveness and reduced toxicity. Recently, the utilization of MCT has evolved from salvage treatment for metastatic disease to adjuvant maintenance therapy for high-risk cancer patients, which has been prompted by the success of several substantial phase III trials. The potential of MCT holds promise for its application in elderly and frail patients and in individuals with financial constraints.
Abbreviations
ADCC: Antibody-Dependent Cellular Cytotoxicity
AML: Acute Myeloid Leukemia
bFGF: basic Fibroblast Growth Factor
CBR: Clinical Benefit Rate
CEPs: Circulating Endothelial Progenitor Cells
CM: Cyclophosphamide and Methotrexate
CMF: Cyclophosphamide, Methotrexate, and Fluorouracil
CX: Cyclophosphamide with Capecitabine
CTLs: Cytotoxic T Lymphocytes
DC: Dendritic Cell
DFS: Disease-Free Survival
DSS: Disease Specific Survival
ER: Estrogen Receptor
GCSF: Granulocyte Colony-Stimulating Factor
GF: Growth Factor
HER2: Human Epidermal Growth Factor Receptor 2
HIF-1α: Hypoxia-Inducible Factor 1
HRQoL: Health-Related Quality of Life
HRR: Homology-Dependent Recombination Repair
ICB: Immune Checkpoint Blockers
ICD: Immunogenic Cell Death
IFN-γ: Interferon-Gamma
IL-2: Interleukin 2
mTTP: Median Time to Progression
MCT: Metronomic Chemotherapy
mOS: Median Overall Survival
mPFS: Median Progression-Free Survival
mVNR: Metronomic Vinorelbine
MDSCs: Myeloid-Derived Suppressor Cells
MTD: Maximum Tolerated Dose
NICD: Notch Intracellular Domain
NK: Natural Killer
NSCLC: Non-Small-Cell Lung Cancer
ORR: Objective Response Rate
OS: Overall Survival
OXPHOS: Oxidative Phosphorylation
PARP: Poly(ADP‐Ribose) Polymerase
PD-L1: Programmed Death Ligand 1
pERα: Phosphorylated Form of ER Alpha
PFS: Progression-Free Survival
PPI: Proton-Pump Inhibitors
SA-β-Gal: Senescence-associated-beta-galactosidase
Treg: Regulatory T Cells
TNBC: Triple-Negative Breast Cancer
TME: Tumour Microenvironment
TSP1: Thrombospondin 1
TTF: Time to Treatment Failure
VEX: Vinorelbine, Cyclophosphamide Plus Capecitabine
VEGF: Vascular Endothelial Cell Growth Factor
Acknowledgements
Figure 2 and 3 were created with Biorender.com.
Funding
This work was supported by the grants from the Sun Yat-sen University Clinical Research 5010 Program (2017011), Guangdong Basic and Applied Basic Research Foundation (2021B1515230010). The funders had no role in the data collection and decision to publish or preparation of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381:e071674
2. Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 2022;19:37-50
3. Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33-48
4. Desai K, McManus J, Sharifi N. Hormonal Therapy for Prostate Cancer. Endocr Rev. 2021;42:354-73
5. Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21:141-62
6. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S. et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. 2022;386:556-67
7. Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A. et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791-800
8. Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7-12
9. Benzekry S, Pasquier E, Barbolosi D, Lacarelle B, Barlési F, André N. et al. Metronomic reloaded: Theoretical models bringing chemotherapy into the era of precision medicine. Semin Cancer Biol. 2015;35:53-61
10. Wong HH, Halford S. Dose-limiting toxicity and maximum tolerated dose: still fit for purpose? Lancet Oncol. 2015;16:1287-8
11. Beumer JH, Chu E, Salamone SJ. All Optimal Dosing Roads Lead to Therapeutic Drug Monitoring-Why Take the Slow Lane. JAMA Oncol. 2022;8:1733-5
12. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv Pharm Bull. 2017;7:339-48
13. Andre N, Carre M, Pasquier E. Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11:413-31
14. Penel N, Clisant S, Dansin E, Desauw C, Dégardin M, Mortier L. et al. Megestrol acetate versus metronomic cyclophosphamide in patients having exhausted all effective therapies under standard care. Br J Cancer. 2010;102:1207-12
15. Chen Y, Fan W, Tsai C, Liu S, Shih J, Chou T. et al. A phase II randomized trial of gefitinib alone or with tegafur/uracil treatment in patients with pulmonary adenocarcinoma who had failed previous chemotherapy. J Thorac Oncol. 2011;6:1110-6
16. Simkens L, van Tinteren H, May A, ten Tije A, Creemers G, Loosveld O. et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385:1843-52
17. Munzone E, Regan M, Cinieri S, Montagna E, Orlando L, Shi R. et al. Efficacy of Metronomic Oral Vinorelbine, Cyclophosphamide, and Capecitabine vs Weekly Intravenous Paclitaxel in Patients With Estrogen Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: Final Results From the Phase 2 METEORA-II Randomized Clinical Trial. JAMA Oncol. 2023
18. Wildiers H, Tryfonidis K, Dal Lago L, Vuylsteke P, Curigliano G, Waters S. et al. Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): an open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group. Lancet Oncol. 2018;19:323-36
19. Patil VM, Noronha V, Menon N, Rai R, Bhattacharjee A, Singh A. et al. Low-Dose Immunotherapy in Head and Neck Cancer: A Randomized Study. J Clin Oncol. 2023;41:222-32
20. Chen Y, Liu X, Zhou Q, Yang K, Jin F, Zhu X. et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. 2021;398:303-13
21. Wang X, Wang S, Huang H, Cai L, Zhao L, Peng R. et al. Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA. 2021;325:50-8
22. Palakurthi B, Fross SR, Guldner IH, Aleksandrovic E, Liu X, Martino AK. et al. Targeting CXCL16 and STAT1 augments immune checkpoint blockade therapy in triple-negative breast cancer. Nat Commun. 2023;14:2109
23. Cazzaniga ME, Cordani N, Capici S, Cogliati V, Riva F, Cerrito MG. Metronomic Chemotherapy. Cancers (Basel). 2021 13
24. Munzone E, Colleoni M. Clinical overview of metronomic chemotherapy in breast cancer. Nat Rev Clin Oncol. 2015;12:631-44
25. Skipper HE, Schabel FM Jr, Wilcox WS. Experimental evaluation of potential anticancer agents. XIII. On the criteria and kinetics associated with "curability" of experimental leukemia. Cancer Chemother Rep. 1964;35:1-111
26. Skipper HE. The effects of chemotherapy on the kinetics of leukemic cell behavior. Cancer Res. 1965;25:1544-50
27. Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423-36
28. Norton L, Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep. 1977;61:1307-17
29. Norton L, Simon R. The Norton-Simon hypothesis revisited. Cancer Treat Rep. 1986;70:163-9
30. Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ. et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431-9
31. Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459:508-9
32. Kakolyris S, Samonis G, Koukourakis M, Vlachonicolis I, Chalkiadakis G, Kalbakis K. et al. Treatment of non-small-cell lung cancer with prolonged oral etoposide. Am J Clin Oncol. 1998;21:505-8
33. Browder T, Butterfield C, Kräling B, Shi B, Marshall B, O'Reilly M. et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878-86
34. Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ. et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15-24
35. Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045-7
36. Klement GL, Kamen BA. Nontoxic, fiscally responsible, future of oncology: could it be beginning in the Third World? J Pediatr Hematol Oncol. 2011;33:1-3
37. Regazzoni S, Pesce G, Marini G, Cavalli F, Goldhirsch A. Low-dose continuous intravenous infusion of 5-fluorouracil for metastatic breast cancer. Ann Oncol. 1996;7:807-13
38. Blumenreich MS, Sheth SP, Miller CL, Farnsley ES, Kellihan MJ, Joseph UG. et al. Inefficacy of low-dose continuous oral etoposide in non-small cell lung cancer. Am J Clin Oncol. 1994;17:163-5
39. Hafner C, Reichle A, Vogt T. New indications for established drugs: combined tumor-stroma-targeted cancer therapy with PPARgamma agonists, COX-2 inhibitors, mTOR antagonists and metronomic chemotherapy. Curr Cancer Drug Targets. 2005;5:393-419
40. Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/ metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol. 2002;13:12-5
41. Spieth K, Kaufmann R, Gille J. Metronomic oral low-dose treosulfan chemotherapy combined with cyclooxygenase-2 inhibitor in pretreated advanced melanoma: a pilot study. Cancer Chemother Pharmacol. 2003;52:377-82
42. Montagna E, Cancello G, Dellapasqua S, Munzone E, Colleoni M. Metronomic therapy and breast cancer: a systematic review. Cancer Treat Rev. 2014;40:942-50
43. Mpekris F, Baish JW, Stylianopoulos T, Jain RK. Role of vascular normalization in benefit from metronomic chemotherapy. Proc Natl Acad Sci U S A. 2017;114:1994-9
44. Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30:219-35
45. Fares J, El Tomb P, Khalil L, Atwani R, Moukadem H, Awada A. et al. Metronomic chemotherapy for patients with metastatic breast cancer: Review of effectiveness and potential use during pandemics. Cancer Treat Rev. 2020;89:102066
46. Kerbel RS, Emmenegger U, Man S, Munoz R, Bertolini F, Shared Y. Metronomic Antiangiogenic Chemotherapy: Questions and Answers. In: Marmé D, Fusenig N, editors. Tumor Angiogenesis: Basic Mechanisms and Cancer Therapy. Berlin, Heidelberg: Springer Berlin Heidelberg. 2008 p. 593-607
47. Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J. et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369-72
48. Scagliotti A, Capizzi L, Cazzaniga ME, Ilari A, De Giorgi M, Cordani N. et al. Co-targeting triple-negative breast cancer cells and endothelial cells by metronomic chemotherapy inhibits cell regrowth and migration via downregulation of the FAK/VEGFR2/VEGF axis and autophagy/apoptosis activation. Front Oncol. 2022;12:998274
49. Longhi E, Carminati L, Carlessi E, Belotti D, Taraboletti G. Thrombospondin-1 in drug activity and tumor response to therapies. Semin Cell Dev Biol. 2023
50. Shi H, Jiang J, Ji J, Shi M, Cai Q, Chen X. et al. Anti-angiogenesis participates in antitumor effects of metronomic capecitabine on colon cancer. Cancer Lett. 2014;349:128-35
51. Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41-8
52. Fedele P, Marino A, Orlando L, Schiavone P, Nacci A, Sponziello F. et al. Efficacy and safety of low-dose metronomic chemotherapy with capecitabine in heavily pretreated patients with metastatic breast cancer. Eur J Cancer. 2012;48:24-9
53. Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I. et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164-70
54. undefined u, undefined u, undefined u, undefined u. G-CSF-primed bone marrow as a source of stem cells for allografting: revisiting the concept. Bone Marrow Transplant. 2015 50
55. Kim JY, Kim YM. Tumor endothelial cells as a potential target of metronomic chemotherapy. Arch Pharm Res. 2019;42:1-13
56. Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y. et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342-6
57. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039-49
58. Xiao YS, Zeng D, Liang YK, Wu Y, Li MF, Qi YZ. et al. Major vault protein is a direct target of Notch1 signaling and contributes to chemoresistance in triple-negative breast cancer cells. Cancer Lett. 2019;440-441:156-67
59. Giuli MV, Giuliani E, Screpanti I, Bellavia D, Checquolo S. Notch Signaling Activation as a Hallmark for Triple-Negative Breast Cancer Subtype. J Oncol. 2019;2019:8707053
60. Ilari A, Cogliati V, Sherif N, Grassilli E, Ramazzotti D, Cordani N. et al. Differential Expression of NOTCH-1 and Its Molecular Targets in Response to Metronomic Followed by Conventional Therapy in a Patient with Advanced Triple-Negative Breast Cancer. Biomedicines. 2024 12
61. Schito L, Semenza GL. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer. 2016;2:758-70
62. Kim YJ, Lee HJ, Kim TM, Eisinger-Mathason TS, Zhang AY, Schmidt B. et al. Overcoming evasive resistance from vascular endothelial growth factor a inhibition in sarcomas by genetic or pharmacologic targeting of hypoxia-inducible factor 1α. Int J Cancer. 2013;132:29-41
63. Schito L, Rey S, Xu P, Man S, Cruz-Muñoz W, Kerbel R. Metronomic chemotherapy offsets HIFα induction upon maximum-tolerated dose in metastatic cancers. EMBO Mol Med. 2020;12:e11416
64. Cao Y, Eble JM, Moon E, Yuan H, Weitzel DH, Landon CD. et al. Tumor cells upregulate normoxic HIF-1α in response to doxorubicin. Cancer Res. 2013;73:6230-42
65. Rebecca A P, Guillermo N A-P, Yvonne G L, Ashley N D, Sunila P, Heather J D. et al. Dual Metronomic Chemotherapy with Nab-Paclitaxel and Topotecan Has Potent Antiangiogenic Activity in Ovarian Cancer. Mol Cancer Ther. 2015 14
66. Bruni E, Reichle A, Scimeca M, Bonanno E, Ghibelli L. Lowering Etoposide Doses Shifts Cell Demise From Caspase-Dependent to Differentiation and Caspase-3-Independent Apoptosis via DNA Damage Response, Inducing AML Culture Extinction. Front Pharmacol. 2018;9:1307
67. Ueno T, Masuda N, Kamigaki S, Morimoto T, Saji S, Imoto S. et al. Differential Involvement of Autophagy and Apoptosis in Response to Chemoendocrine and Endocrine Therapy in Breast Cancer: JBCRG-07TR. Int J Mol Sci. 2019 20
68. Rosero G, Pattarone G, Peñaherera A, Pilz J, Bödecker J, Perez M. et al. Metronomic doses and drug schematic combination response tested within chambered coverslips for the treatment of breast cancer cells (JIMT-1). PLoS One. 2022;17:e0274911
69. Qin R-S, Zhang Z-H, Zhu N-P, Chen F, Guo Q, Hu H-W. et al. Enhanced antitumor and anti-angiogenic effects of metronomic Vinorelbine combined with Endostar on Lewis lung carcinoma. BMC Cancer. 2018;18:967
70. Beltzig L, Stratenwerth B, Kaina B. Accumulation of Temozolomide-Induced Apoptosis, Senescence and DNA Damage by Metronomic Dose Schedule: A Proof-of-Principle Study with Glioblastoma Cells. Cancers (Basel). 2021 13
71. Wu K, Sun XQ, Wang CQ, Gao TX, Sun P, Wang Y. et al. Metronomic combination chemotherapy using everolimus and etoposide for the treatment of non-Hodgkin lymphoma. Cancer Med. 2019;8:4688-98
72. Vives M, Ginesta MM, Gracova K, Graupera M, Casanovas O, Capella G. et al. Metronomic chemotherapy following the maximum tolerated dose is an effective anti-tumour therapy affecting angiogenesis, tumour dissemination and cancer stem cells. Int J Cancer. 2013;133:2464-72
73. Wróbel T, Luty M, Catapano J, Karnas E, Szczygieł M, Piwowarczyk K. et al. CD44 cells determine fenofibrate-induced microevolution of drug-resistance in prostate cancer cell populations. Stem Cells. 2020;38:1544-56
74. Singh V, Erb U, Zöller M. Cooperativity of CD44 and CD49d in leukemia cell homing, migration, and survival offers a means for therapeutic attack. J Immunol. 2013;191:5304-16
75. Su NW, Wu SH, Chi CW, Liu CJ, Tsai TH, Chen YJ. Metronomic Cordycepin Therapy Prolongs Survival of Oral Cancer-Bearing Mice and Inhibits Epithelial-Mesenchymal Transition. Molecules. 2017;22:629
76. Mitra Ghosh T, Mazumder S, Davis J, Yadav J, Akinpelu A, Alnaim A. et al. Metronomic Administration of Topotecan Alone and in Combination with Docetaxel Inhibits Epithelial-mesenchymal Transition in Aggressive Variant Prostate Cancers. Cancer Res Commun. 2023;3:1286-311
77. Huang W, Hickson L, Eirin A, Kirkland J, Lerman L. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol. 2022;18:611-27
78. López-Otín C, Pietrocola F, Roiz-Valle D, Galluzzi L, Kroemer G. Meta-hallmarks of aging and cancer. Cell Metab. 2023;35:12-35
79. Taschner-Mandl S, Schwarz M, Blaha J, Kauer M, Kromp F, Frank N. et al. Metronomic topotecan impedes tumor growth of MYCN-amplified neuroblastoma cells in vitro and in vivo by therapy induced senescence. Oncotarget. 2016;7:3571-86
80. Cerrito MG, De Giorgi M, Pelizzoni D, Bonomo SM, Digiacomo N, Scagliotti A. et al. Metronomic combination of Vinorelbine and 5Fluorouracil is able to inhibit triple-negative breast cancer cells. Results from the proof-of-concept VICTOR-0 study. Oncotarget. 2018;9:27448-59
81. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81-94
82. Kumar N, Cramer GM, Dahaj SAZ, Sundaram B, Celli JP, Kulkarni RV. Stochastic modeling of phenotypic switching and chemoresistance in cancer cell populations. Sci Rep. 2019;9:10845
83. Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose "chemo-switch" regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939-52
84. Li SC, Lee KL, Luo J. Control dominating subclones for managing cancer progression and posttreatment recurrence by subclonal switchboard signal: implication for new therapies. Stem Cells Dev. 2012;21:503-6
85. Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19:776-800
86. Wu HL, Gong Y, Ji P, Xie YF, Jiang YZ, Liu GY. Targeting nucleotide metabolism: a promising approach to enhance cancer immunotherapy. J Hematol Oncol. 2022;15:45
87. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215-33
88. Zhang J, Huang D, Saw PE, Song E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol. 2022;43:523-45
89. Muraro E, Vinante L, Fratta E, Bearz A, Höfler D, Steffan A. et al. Metronomic Chemotherapy: Anti-Tumor Pathways and Combination with Immune Checkpoint Inhibitors. Cancers (Basel). 2023 15
90. Choi JU, Maharjan R, Pangeni R, Jha SK, Lee NK, Kweon S. et al. Modulating tumor immunity by metronomic dosing of oxaliplatin incorporated in multiple oral nanoemulsion. J Control Release. 2020;322:13-30
91. Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14:2994-3006
92. Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P. et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212-23
93. Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F. et al. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol. 2011;41:2217-28
94. Wu J, Waxman D. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8 T-cell responses and immune memory. Oncoimmunology. 2015;4:e1005521
95. Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J. et al. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133:98-107
96. Serrano-Del Valle A, Naval J, Anel A, Marzo I. Novel Forms of Immunomodulation for Cancer Therapy. Trends Cancer. 2020;6:518-32
97. Chang CL, Hsu YT, Wu CC, Lai YZ, Wang C, Yang YC. et al. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res. 2013;73:119-27
98. Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J. et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2012;61:353-62
99. Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S. et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111-24
100. Okimoto T, Kotani H, Iida Y, Koyanagi A, Tanino R, Tsubata Y. et al. Pemetrexed sensitizes human lung cancer cells to cytotoxic immune cells. Cancer Sci. 2020;111:1910-20
101. Miyashita T, Miki K, Kamigaki T, Makino I, Nakagawara H, Tajima H. et al. Low-dose gemcitabine induces major histocompatibility complex class I-related chain A/B expression and enhances an antitumor innate immune response in pancreatic cancer. Clin Exp Med. 2017;17:19-31
102. Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27-47
103. Shang M, Yang H, Yang R, Chen T, Fu Y, Li Y. et al. The folate cycle enzyme MTHFD2 induces cancer immune evasion through PD-L1 up-regulation. Nat Commun. 2021;12:1940
104. Wei JL, Wu SY, Yang YS, Xiao Y, Jin X, Xu XE. et al. GCH1 induces immunosuppression through metabolic reprogramming and IDO1 upregulation in triple-negative breast cancer. J Immunother Cancer. 2021;9:e002383
105. Shao C, Lu W, Wan N, Wu M, Bao Q, Tian Y. et al. Integrative Omics Analysis Revealed that Metabolic Intervention Combined with Metronomic Chemotherapy Selectively Kills Cancer Cells. J Proteome Res. 2019;18:2643-53
106. Mundo AI, Muhammad A, Balza K, Nelson CE, Muldoon TJ. Longitudinal examination of perfusion and angiogenesis markers in primary colorectal tumors shows distinct signatures for metronomic and maximum-tolerated dose strategies. Neoplasia. 2022;32:100825
107. Catalano L, Aminzadeh-Gohari S, Weber DD, Poupardin R, Stefan VE, Smiles WJ. et al. Triple Therapy with Metformin, Ketogenic Diet, and Metronomic Cyclophosphamide Reduced Tumor Growth in MYCN-Amplified Neuroblastoma Xenografts. Metabolites. 2023;13:910
108. Oresta B, Pozzi C, Braga D, Hurle R, Lazzeri M, Colombo P. et al. Mitochondrial metabolic reprogramming controls the induction of immunogenic cell death and efficacy of chemotherapy in bladder cancer. Sci Transl Med. 2021 13
109. Bondarenko M, Le Grand M, Shaked Y, Raviv Z, Chapuisat G, Carrere C. et al. Metronomic Chemotherapy Modulates Clonal Interactions to Prevent Drug Resistance in Non-Small Cell Lung Cancer. Cancers (Basel). 2021 13
110. Farge T, Saland E, de Toni F, Aroua N, Hosseini M, Perry R. et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017;7:716-35
111. Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930-42
112. Taylor S, Spugnini E, Assaraf Y, Azzarito T, Rauch C, Fais S. Microenvironment acidity as a major determinant of tumor chemoresistance: Proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Updat. 2015;23:69-78
113. Spugnini EP, Buglioni S, Carocci F, Francesco M, Vincenzi B, Fanciulli M. et al. High dose lansoprazole combined with metronomic chemotherapy: a phase I/II study in companion animals with spontaneously occurring tumors. J Transl Med. 2014;12:225
114. Nasr KE, Osman MA, Elkady MS, Ellithy MA. Metronomic methotrexate and cyclophosphamide after carboplatin included adjuvant chemotherapy in triple negative breast cancer: a phase III study. Ann Transl Med. 2015;3:284
115. Camerini A, Morabito A, Montanino A, Bernabé R, Grossi F, Ramlau R. et al. Metronomic oral vinorelbine in previously untreated advanced non-small-cell lung cancer patients unfit for platinum-based chemotherapy: results of the randomized phase II Tempo Lung trial. ESMO Open. 2021;6:100051
116. Hagman H, Frödin JE, Berglund Å, Sundberg J, Vestermark LW, Albertsson M. et al. A randomized study of KRAS-guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first-line induction treatment of metastatic colorectal cancer: the Nordic ACT2 trial. Ann Oncol. 2016;27:140-7
117. Zsiros E, Lynam S, Attwood K, Wang C, Chilakapati S, Gomez E. et al. Efficacy and Safety of Pembrolizumab in Combination With Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2021;7:78-85
118. Hsieh MY, Chen G, Chang DC, Chien SY, Chen MK. The Impact of Metronomic Adjuvant Chemotherapy in Patients with Advanced Oral Cancer. Ann Surg Oncol. 2018;25:2091-7
119. Peters KB, Lipp ES, Miller E, Herndon JE 2nd, McSherry F, Desjardins A. et al. Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J Neurooncol. 2018;137:349-56
120. Simsek C, Esin E, Yalcin S. Metronomic Chemotherapy: A Systematic Review of the Literature and Clinical Experience. J Oncol. 2019;2019:5483791
121. Cazzaniga ME, Capici S, Cordani N, Cogliati V, Pepe FF, Riva F. et al. Metronomic Chemotherapy for Metastatic Breast Cancer Treatment: Clinical and Preclinical Data between Lights and Shadows. J Clin Med. 2022 11
122. Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B. et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29:4498-504
123. Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L. et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist. 2013;18:1256-7
124. Romiti A, Onesti CE, Roberto M, Barucca V, Tomao S, D'Antonio C. et al. Continuous, low-dose capecitabine for patients with recurrent colorectal cancer. Med Oncol. 2015;32:54
125. Pelizzaro F, Sammarco A, Dadduzio V, Pastorelli D, Giovanis P, Soldà C. et al. Capecitabine in advanced hepatocellular carcinoma: A multicenter experience. Dig Liver Dis. 2019;51:1713-9
126. Camerini A, Banna GL, Cinieri S, Pezzuto A, Mencoboni M, Rosetti F. et al. Metronomic oral vinorelbine for the treatment of advanced non-small cell lung cancer: a multicenter international retrospective analysis. Clin Transl Oncol. 2019;21:790-5
127. Addeo R, Sgambato A, Cennamo G, Montella L, Faiola V, Abbruzzese A. et al. Low-dose metronomic oral administration of vinorelbine in the first-line treatment of elderly patients with metastatic breast cancer. Clin Breast Cancer. 2010;10:301-6
128. Estevinho F, Gomes R, Hasmucrai D, Barata F. Metronomic oral vinorelbine in a real-world population of advanced non-small cell lung cancer patients. Pulmonology. 2022;28:368-75
129. Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nolè F. et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73-80
130. Hussein M, Gaafar R, Abdel-Warith A, Ahmed W, Allahloubi N, Salem S. et al. Efficacy and Toxicity of Metronomic Chemotherapy in Metastatic Breast Cancer: Egyptian Experience. Clin Breast Cancer. 2017;17:618-28
131. Herrlinger U, Rieger J, Steinbach JP, Nägele T, Dichgans J, Weller M. UKT-04 trial of continuous metronomic low-dose chemotherapy with methotrexate and cyclophosphamide for recurrent glioblastoma. J Neurooncol. 2005;71:295-9
132. Gebbia V, Serretta V, Borsellino N, Valerio MR. Salvage therapy with oral metronomic cyclophosphamide and methotrexate for castration-refractory metastatic adenocarcinoma of the prostate resistant to docetaxel. Urology. 2011;78:1125-30
133. Yoshimoto M, Takao S, Hirata M, Okamoto Y, Yamashita S, Kawaguchi Y. et al. Metronomic oral combination chemotherapy with capecitabine and cyclophosphamide: a phase II study in patients with HER2-negative metastatic breast cancer. Cancer Chemother Pharmacol. 2012;70:331-8
134. Cazzaniga ME, Torri V, Riva F, Porcu L, Cicchiello F, Capici S. et al. Efficacy and safety of vinorelbine-capecitabine oral metronomic combination in elderly metastatic breast cancer patients: VICTOR-1 study. Tumori. 2017;103:e4-e8
135. Montagna E, Palazzo A, Maisonneuve P, Cancello G, Iorfida M, Sciandivasci A. et al. Safety and efficacy study of metronomic vinorelbine, cyclophosphamide plus capecitabine in metastatic breast cancer: A phase II trial. Cancer Lett. 2017;400:276-81
136. Twu CW, Wang WY, Chen CC, Liang KL, Jiang RS, Wu CT. et al. Metronomic adjuvant chemotherapy improves treatment outcome in nasopharyngeal carcinoma patients with postradiation persistently detectable plasma Epstein-Barr virus deoxyribonucleic acid. Int J Radiat Oncol Biol Phys. 2014;89:21-9
137. Twu CW, Lin PJ, Tsou HH, Liu YC, Jiang RS, Liang KL. et al. Maintenance metronomic chemotherapy for metastatic/recurrent nasopharyngeal carcinoma. Head Neck. 2022;44:1453-61
138. Geng R, Wang G, Qiu L, Liu B, Yang F, Zhang J. et al. Metronomic capecitabine as maintenance treatment after first line induction with XELOX for metastatic colorectal cancer patients. Medicine (Baltimore). 2020;99:e23719
139. Huang WY, Ho CL, Lee CC, Hsiao CW, Wu CC, Jao SW. et al. Oral tegafur-uracil as metronomic therapy following intravenous FOLFOX for stage III colon cancer. PLoS One. 2017;12:e0174280
140. Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T. et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006-11
141. Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676-81
142. Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024-8
143. Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci U S A. 2002;99:4349-54
144. Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V. et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336-43
145. Gately S. The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 2000;19:19-27
146. Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM. et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374-8
147. Patil V, Noronha V, Menon N, Mathrudev V, Bhattacharjee A, Nawale K. et al. Metronomic adjuvant chemotherapy evaluation in locally advanced head and neck cancers post radical chemoradiation - a randomised trial. Lancet Reg Health Southeast Asia. 2023;12:100162
148. Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R. et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899-905
149. Garcia A, Hirte H, Fleming G, Yang D, Tsao-Wei D, Roman L. et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76-82
150. Cox M, Lapenta C, Santini S. Advances and perspectives of dendritic cell-based active immunotherapies in follicular lymphoma. Cancer immunology, immunotherapy: CII. 2020;69:913-25
151. Huang A, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022;23:660-70
152. Mountzios G, Remon J, Hendriks L, García-Campelo R, Rolfo C, Van Schil P. et al. Immune-checkpoint inhibition for resectable non-small-cell lung cancer - opportunities and challenges. Nat Rev Clin Oncol. 2023;20:664-77
153. Salik B, Smyth MJ, Nakamura K. Targeting immune checkpoints in hematological malignancies. J Hematol Oncol. 2020;13:111
154. Liao W, Overman M, Boutin A, Shang X, Zhao D, Dey P. et al. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell. 2019;35:559-72.e7
155. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-502
156. Kwa MJ, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer. 2018;124:2086-103
157. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915-28
158. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ. et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924-37
159. Khan KA, Ponce de Léon JL, Benguigui M, Xu P, Chow A, Cruz-Muñoz W. et al. Immunostimulatory and anti-tumor metronomic cyclophosphamide regimens assessed in primary orthotopic and metastatic murine breast cancer. NPJ Breast Cancer. 2020;6:29
160. He X, Du Y, Wang Z, Wang X, Duan J, Wan R. et al. Upfront dose-reduced chemotherapy synergizes with immunotherapy to optimize chemoimmunotherapy in squamous cell lung carcinoma. J Immunother Cancer. 2020;8:e000807
161. Petrizzo A, Mauriello A, Luciano A, Rea D, Barbieri A, Arra C. et al. Inhibition of tumor growth by cancer vaccine combined with metronomic chemotherapy and anti-PD-1 in a pre-clinical setting. Oncotarget. 2018;9:3576-89
162. Zhou L, Xu Q, Huang L, Jin J, Zuo X, Zhang Q. et al. Low-dose carboplatin reprograms tumor immune microenvironment through STING signaling pathway and synergizes with PD-1 inhibitors in lung cancer. Cancer Lett. 2021;500:163-71
163. Noordam L, Kaijen M, Bezemer K, Cornelissen R, Maat L, Hoogsteden H. et al. Low-dose cyclophosphamide depletes circulating naïve and activated regulatory T cells in malignant pleural mesothelioma patients synergistically treated with dendritic cell-based immunotherapy. Oncoimmunology. 2018;7:e1474318
164. Ellebaek E, Engell-Noerregaard L, Iversen T, Froesig T, Munir S, Hadrup S. et al. Metastatic melanoma patients treated with dendritic cell vaccination, Interleukin-2 and metronomic cyclophosphamide: results from a phase II trial. Cancer immunology, immunotherapy: CII. 2012;61:1791-804
165. Sen T, Della Corte CM, Milutinovic S, Cardnell RJ, Diao L, Ramkumar K. et al. Combination Treatment of the Oral CHK1 Inhibitor, SRA737, and Low-Dose Gemcitabine Enhances the Effect of Programmed Death Ligand 1 Blockade by Modulating the Immune Microenvironment in SCLC. J Thorac Oncol. 2019;14:2152-63
166. Le Cesne A, Marec-Berard P, Blay J, Gaspar N, Bertucci F, Penel N. et al. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: results from the PEMBROSARC study. Eur J Cancer. 2019;119:151-7
167. Tagliamonte M, Petrizzo A, Napolitano M, Luciano A, Arra C, Maiolino P. et al. Novel metronomic chemotherapy and cancer vaccine combinatorial strategy for hepatocellular carcinoma in a mouse model. Cancer Immunol Immunother. 2015;64:1305-14
168. Kummar S, Ji J, Morgan R, Lenz H, Puhalla S, Belani C. et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res. 2012;18:1726-34
169. Ghonim M, Ibba S, Tarhuni A, Errami Y, Luu H, Dean M. et al. Targeting PARP-1 with metronomic therapy modulates MDSC suppressive function and enhances anti-PD-1 immunotherapy in colon cancer. J Immunother Cancer. 2021;9:e001643
170. Jenkins S, Zhang W, Steinberg S, Nousome D, Houston N, Wu X. et al. Phase I Study and Cell-Free DNA Analysis of T-DM1 and Metronomic Temozolomide for Secondary Prevention of HER2-Positive Breast Cancer Brain Metastases. Clin Cancer Res. 2023;29:1450-9
171. Wildiers H, Meyskens T, Marréaud S, Lago L, Vuylsteke P, Curigliano G. et al. Long term outcome data from the EORTC 75111-10114 ETF/BCG randomized phase II study: Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer, followed by T-DM1 after progression. Breast (Edinburgh, Scotland). 2022;64:100-11
172. Padella A, Ghelli Luserna Di Rora A, Marconi G, Ghetti M, Martinelli G, Simonetti G. Targeting PARP proteins in acute leukemia: DNA damage response inhibition and therapeutic strategies. J Hematol Oncol. 2022;15:10
173. Anampa J, Chen A, Wright J, Patel M, Pellegrino C, Fehn K. et al. Phase I Trial of Veliparib, a Poly ADP Ribose Polymerase Inhibitor, Plus Metronomic Cyclophosphamide in Metastatic HER2-negative Breast Cancer. Clin Breast Cancer. 2018;18:e135-e42
174. Musolino A, Gradishar WJ, Rugo HS, Nordstrom JL, Rock EP, Arnaldez F. et al. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J Immunother Cancer. 2022;10:e003171
175. Li F, Liu S. Focusing on NK cells and ADCC: A promising immunotherapy approach in targeted therapy for HER2-positive breast cancer. Front Immunol. 2022;13:1083462
176. Ramamoorthi G, Kodumudi K, Snyder C, Grover P, Zhang H, Greene MI. et al. Intratumoral delivery of dendritic cells plus anti-HER2 therapy triggers both robust systemic antitumor immunity and complete regression in HER2 mammary carcinoma. J Immunother Cancer. 2022;10:e004841
177. Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21-7
178. van Ramshorst M, van der Voort A, van Werkhoven E, Mandjes I, Kemper I, Dezentjé V. et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1630-40
179. von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G. et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377:122-31
180. du Manoir J, Francia G, Man S, Mossoba M, Medin J, Viloria-Petit A. et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab in human breast cancer xenografts. Clin Cancer Res. 2006;12:904-16
181. Orlando L, Lorusso V, Giotta F, Di Maio M, Schiavone P, Fedele P. et al. Metronomic oral chemotherapy with cyclophosphamide plus capecitabine combined with trastuzumab (HEX) as first line therapy of HER-2 positive advanced breast cancer: A phase II trial of the Gruppo Oncologico Italia Meridionale (GOIM). Breast (Edinburgh, Scotland). 2020;53:18-22
182. Nicolas A, Carré M, Pasquier E. Metronomics: Intrinsic Anakoinosis Modulator? Front Pharmacol. 2018;9:689
183. Aurilio G, Munzone E, Botteri E, Sciandivasci A, Adamoli L, Minchella I. et al. Oral metronomic cyclophosphamide and methotrexate plus fulvestrant in advanced breast cancer patients: a mono-institutional case-cohort report. Breast J. 2012;18:470-4
184. Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol. 2004;22:2214-32
185. Liu ZC, Zeng KH, Gu ZB, Chen RP, Luo YJ, Tang LQ. et al. Comparison of induction chemotherapy combined with concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in Lymph-Node-Stage III nasopharyngeal carcinoma based on propensity score-matching. Radiother Oncol. 2023;178:109421
186. Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer G, Fietkau R. et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022;8:e215445
187. Huang H, Feng Y, Wan T, Zhang Y, Cao X, Huang Y. et al. Effectiveness of Sequential Chemoradiation vs Concurrent Chemoradiation or Radiation Alone in Adjuvant Treatment After Hysterectomy for Cervical Cancer: The STARS Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7:361-9
188. Addeo R, Sperlongano P, Montella L, Vincenzi B, Carraturo M, Iodice P. et al. Protracted low dose of oral vinorelbine and temozolomide with whole-brain radiotherapy in the treatment for breast cancer patients with brain metastases. Cancer Chemother Pharmacol. 2012;70:603-9
189. Tom Wei-Wu C, Ming-Shen D, Ling-Ming T, Shin-Cheh C, Tsu-Yi C, Ta-Chung C. et al. Whole-Brain Radiotherapy Alone vs Preceded by Bevacizumab, Etoposide, and Cisplatin for Untreated Brain Metastases From Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2023;10:325-34
190. Revannasiddaiah S, Joshi S, Pandey K, Rastogi M, Sharma M, Gupta M. The results with the addition of metronomic cyclophosphamide to palliative radiotherapy for the treatment of non-small cell lung carcinoma. Ann Transl Med. 2015;3:305
191. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397:1750-69
192. Tang M, O'Connell RL, Amant F, Beale P, McNally O, Sjoquist KM. et al. PARAGON: A Phase II study of anastrozole in patients with estrogen receptor-positive recurrent/metastatic low-grade ovarian cancers and serous borderline ovarian tumors. Gynecol Oncol. 2019;154:531-8
193. Ueno T, Masuda N, Kamigaki S, Morimoto T, Akiyama F, Kurosumi M. et al. A multicenter phase II trial of neoadjuvant letrozole plus low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer (JBCRG-07): therapeutic efficacy and clinical implications of circulating endothelial cells. Cancer Med. 2018;7:2442-51
194. Sato N, Masuda N, Morimoto T, Ueno T, Kanbayashi C, Kaneko K. et al. Neoadjuvant endocrine therapy with exemestane followed by response-guided combination therapy with low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer: A multicenter, open-label, phase II study. Cancer Med. 2018;7:3044-56
195. Adamo B, Bellet M, Paré L, Pascual T, Vidal M, Pérez Fidalgo JA. et al. Oral metronomic vinorelbine combined with endocrine therapy in hormone receptor-positive HER2-negative breast cancer: SOLTI-1501 VENTANA window of opportunity trial. Breast Cancer Res. 2019;21:108
196. Yang J, Chang N, Wu D, Cheng W, Chung W, Chang W. et al. Preclinical evaluation of exemestane as a novel chemotherapy for gastric cancer. J Cell Mol Med. 2019;23:7417-26
197. Generali D, Buffa F, Berruti A, Brizzi M, Campo L, Bonardi S. et al. Phosphorylated ERalpha, HIF-1alpha, and MAPK signaling as predictors of primary endocrine treatment response and resistance in patients with breast cancer. J Clin Oncol. 2009;27:227-34
198. Li J, Zuo W, Ivanova D, Jia X, Lei L, Liu G. Metronomic capecitabine combined with aromatase inhibitors for new chemoendocrine treatment of advanced breast cancer: a phase II clinical trial. Breast Cancer Res Treat. 2019;173:407-15
199. Ward Z, Scott A, Hricak H, Abdel-Wahab M, Paez D, Lette M. et al. Estimating the impact of treatment and imaging modalities on 5-year net survival of 11 cancers in 200 countries: a simulation-based analysis. Lancet Oncol. 2020;21:1077-88
200. Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L. et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023;9:465-72
201. Ward Z, Scott A, Hricak H, Atun R. Global costs, health benefits, and economic benefits of scaling up treatment and imaging modalities for survival of 11 cancers: a simulation-based analysis. Lancet Oncol. 2021;22:341-50
202. André N, Banavali S, Snihur Y, Pasquier E. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol. 2013;14:e239-48
203. Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455-65
204. She L, Tian K, Han J, Zuo W, Wang Z, Zhang N. Cost-effectiveness analysis of metronomic capecitabine as adjuvant chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Front Oncol. 2022;12:904372
205. Sharma A, Kataria B, Biswas B, Bakhshi S, Pushpam D. Oral metronomic chemotherapy is a cost effective alternative to pazopanib in advanced soft tissue sarcoma. J Oncol Pharm Pract. 2022;28:560-8
206. Bocci G, Tuccori M, Emmenegger U, Liguori V, Falcone A, Kerbel RS. et al. Cyclophosphamide-methotrexate 'metronomic' chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol. 2005;16:1243-52
207. Pramanik R, Agarwala S, Sreenivas V, Dhawan D, Bakhshi S. Quality of life in paediatric solid tumours: a randomised study of metronomic chemotherapy versus placebo. BMJ Support Palliat Care. 2023;13:234-7
208. Dal Lago L, Uwimana AL, Coens C, Vuylsteke P, Curigliano G, Brouwers B. et al. Health-related quality of life in older patients with HER2+ metastatic breast cancer: Comparing pertuzumab plus trastuzumab with or without metronomic chemotherapy in a randomised open-label phase II clinical trial. J Geriatr Oncol. 2022;13:582-93
209. Patil V, Joshi A, Noronha V, Bhattacharjee A, Dhumal S, Chandrakanth MV. et al. Quality of life and quality-adjusted time without toxicity in palliatively treated head-and-neck cancer patients. South Asian J Cancer. 2018;7:249-53
210. Perroud HA, Alasino CM, Rico MJ, Queralt F, Pezzotto SM, Rozados VR. et al. Quality of life in patients with metastatic breast cancer treated with metronomic chemotherapy. Future Oncol. 2016;12:1233-42
211. Noronha V, Joshi A, Marfatia S, Patil V, Juvekar S, Arya S. et al. Health-related quality of life in patients with metastatic, relapsed, or inoperable squamous cell carcinoma of the head and neck in India. Support Care Cancer. 2016;24:1595-602
Author contact
![]() Corresponding author: Prof Shu-sen Wang, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, China. Telephone number: +86 139 2616 8469; E-mail: wangshsorg.cn.
Corresponding author: Prof Shu-sen Wang, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, China. Telephone number: +86 139 2616 8469; E-mail: wangshsorg.cn.
 Global reach, higher impact
Global reach, higher impact