13.3
Impact Factor
Theranostics 2024; 14(6):2490-2525. doi:10.7150/thno.91394 This issue Cite
Review
Nanotechnology in inflammation: cutting-edge advances in diagnostics, therapeutics and theranostics
1. Department of Pharmaceutics, Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang 110016, P. R. China.
2. Joint International Research Laboratory of Intelligent Drug Delivery Systems, Ministry of Education, Shenyang Pharmaceutical University, Shenyang 110016, P.R. China.
Received 2023-10-22; Accepted 2024-2-14; Published 2024-4-8
Abstract
Inflammatory dysregulation is intimately associated with the occurrence and progression of many life-threatening diseases. Accurate detection and timely therapeutic intervention on inflammatory dysregulation are crucial for the effective therapy of inflammation-associated diseases. However, the clinical outcomes of inflammation-involved disorders are still unsatisfactory. Therefore, there is an urgent need to develop innovative anti-inflammatory strategies by integrating emerging technological innovations with traditional therapeutics. Biomedical nanotechnology is one of the promising fields that can potentially transform the diagnosis and treatment of inflammation. In this review, we outline recent advances in biomedical nanotechnology for the diagnosis and treatment of inflammation, with special attention paid to nanosensors and nanoprobes for precise diagnosis of inflammation-related diseases, emerging anti-inflammatory nanotherapeutics, as well as nanotheranostics and combined anti-inflammatory applications. Moreover, the prospects and challenges for clinical translation of nanoprobes and anti-inflammatory nanomedicines are highlighted.
Keywords: inflammation, biomedical nanotechnology, precise diagnosis, anti-inflammatory nanotherapeutics, nanotheranostics
Introduction
Inflammation is generally considered as a defense mechanism to protect the body from external stimuli and invasions [1]. However, long-term inflammatory reactions can lead to the dysfunction of cells, tissues, organs and living systems, and also increase the risks of chronic diseases [2]. Therefore, timely identification and expeditious intervention are essential for the effective management of chronic inflammatory disorders [3]. Recent advancements in nanoparticle drug delivery systems (nano-DDSs) offer the potential to revolutionize both diagnostic and therapeutic approaches to inflammation intervention [3]. Nanosensors and nanoprobes can facilitate the detection, monitoring and imaging of inflammatory lesions [3]. Moreover, elaborately engineered nano-DDSs can endow anti-inflammatory nanomedicines with a multitude of benefits, including improving unfavorable physicochemical properties, prolonging the systemic circulation time and reducing off-target drug toxicity [4]. In recent decades, considerable endeavors have also been made to achieve the synchronized co-delivery of two or more probes and/or therapeutic agents within a nano-DDS for theranostics and combination therapy of inflammatory diseases [5].
Inflammation-related diseases
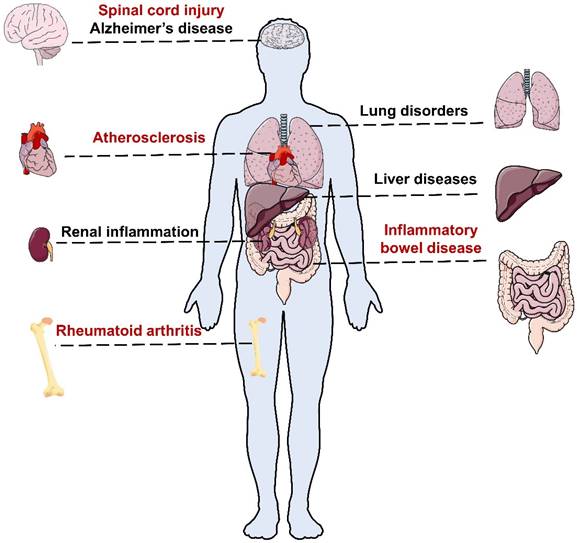
Acute inflammation is usually accompanied by redness, swelling, fever, pain, dysfunction and other clinical symptoms with pathological changes, whereas chronic inflammation is often asymptomatic in early stages, posing challenges for early detection [2]. Chronic inflammation can affect different organs of the body and cause various types of inflammatory diseases (Figure 1). Without proper treatment, chronic inflammation can result in severe diseases such as atherosclerotic cardiovascular disease (ASCVD) [6], diabetes [7], degenerative diseases [8] and even cancer [9].
Rheumatoid arthritis (RA) is a chronic and complex autoimmune disease that affects about 0.5-1.0% of the population [10]. According to the Global Burden of Disease (GBD) study, 17.6 million people suffered from RA worldwide in 2020, and this number is projected to rise to 31.7 million people by 2050 [10]. RA usually begins gradually with pain and swelling in polyarticular joints [10]. Without adequate treatment, RA can cause serious complications including cardiovascular (CV), pulmonary, gastrointestinal, and neurological diseases [11, 12].
Inflammatory bowel disease (IBD) includes Crohn's disease and ulcerative colitis [13]. According to the 2019 GBD study, about 4.9 million individuals worldwide had IBD and 35,600 died from it [13]. IBD often causes non-specific symptoms in the early stages such as abdominal pain, diarrhea and weight loss. However, long-term inflammation can result in serious and irreversible intestinal damage and increase the risk of colorectal cancer [13].
Atherosclerosis (AS) is a condition in which plaques build up inside the arteries, narrowing them and reducing blood flow to vital organs, which can lead to many heart diseases and strokes [14, 15]. Among them, ASCVD is the most common and deadly complication of AS [16]. The 2019 Global Burden of Disease (GBD) study estimated that AS affected 226.7 million people and caused 2.9 million deaths worldwide [16].
Lung disorders such as asthma, pneumonia and pulmonary fibrosis (PF) are also major causes of morbidity and mortality worldwide, affecting people of all ages. Asthma is a common chronic inflammatory disease that affects over 300 million people globally [17]. Pneumonia is a frequent and potentially fatal complication of Coronavirus Disease 2019 (COVID-19), which can cause fluid accumulation and inflammation in the lungs [18]. Notably, COVID-19-associated pneumonia often involves both lungs and may persist even after recovery from the infection [18].
In addition to the above disorders, inflammation can also affect other organs and tissues, such as the brain, liver and kidney. Neuroinflammation is a process of inflammation in the nervous tissue, which is involved in the development of Alzheimer's disease (AD), spinal cord injury (SCI) and other neurological disorders [19]. Liver and renal inflammation can also result in severe outcomes, such as liver failure, cirrhosis, kidney failure and chronic kidney disease, without proper treatment [20, 21].
Schematic representation of inflammatory-related diseases in different organs.
Cytokines and pathways of inflammation
Inflammation is a complex and dynamic process that involves various inflammatory cytokines [22]. Inflammatory cytokines can be classified into two categories: pro-inflammatory and anti-inflammatory. Pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukins (e.g., IL-1, IL-6, IL-12) and interferons (e.g., IFN-α, IFN-γ), promote inflammation and immune responses, while anti-inflammatory cytokines, such as interleukins (e.g., IL-4, IL-10, IL-13) and transforming growth factor beta (TGF-β), suppress inflammation and regulate immune tolerance [22]. The balance between pro-inflammatory and anti-inflammatory cytokines is essential for maintaining a healthy immune system and preventing inflammatory-related diseases [23]. Excessive pro-inflammatory cytokines can lead to chronic inflammation and tissue damage. Inflammatory cytokines can also activate different signaling pathways by binding to specific receptors on target cells, as shown below [22].
Oxidative stress results from an imbalance between the generation and removal of reactive oxygen species (ROS) in the body [24]. ROS are unstable molecules that can damage cellular structures and functions, such as DNA, membranes and proteins [24]. Oxidative stress can induce pro-inflammatory cytokines and contribute to various inflammatory diseases, such as diabetes, asthma and RA, by modulating and amplifying several molecular pathways, including nuclear factor kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs), which regulate the expression of genes involved in inflammation [24].
The NF-κB pathway plays a central role in inflammation, as it induces the transcription of pro-inflammatory genes, such as cytokines, chemokines, immunoreceptors, cell adhesion molecules and regulators of apoptosis [25]. Dysregulation of the NF-κB pathway may cause chronic inflammation disorders, such as autoimmune diseases, IBD, RA and cancer [25].
The Janus kinase (JAK)/activator of transcription (STAT) pathway is a key signaling pathway that transmits the effects of various cytokines and growth factors to the nucleus [26]. By regulating the expression of genes related to inflammation and immunity, this pathway is involved in various inflammatory diseases, such as RA, IBD and asthma [26].
Toll-like receptor (TLR) signaling is a process that enables the immune system to detect and respond to pathogens and damaged cells [27]. TLRs recognize specific molecular patterns in microbes or host cells and activate signaling pathways, leading to the production of inflammatory molecules such as cytokines, chemokines and interferons [27]. These molecules recruit and activate immune cells, which initiate inflammatory responses and tissue repair [27]. However, excessive or prolonged TLR signaling can also cause tissue damage and chronic inflammation, contributing to conditions such as infections, autoimmune disorders, allergies and cancer [27].
The MAPK pathway is also a key regulator of inflammation, as it governs the production and release of pro-inflammatory cytokines and chemokines, such as TNF-α [28]. In addition, the MAPK pathway modulates the activation of transcription factors, such as NF-κB [28]. Dysregulation of the MAPK pathway can lead to chronic inflammation and even cancer [28].
Current diagnosis for inflammation
Accurate diagnosis is an important prerequisite for effective treatment of diseases. Inflammation is a common indicator of many diseases, but it can vary in type and location. Therefore, different methods are needed to diagnose inflammation in different parts of the body. Some of the most common methods are blood tests and imaging tests.
Blood tests
Blood tests are the primary method to indicate inflammation, employing various markers that reflect the level and type of inflammatory response. Some of these markers, such as erythrocyte sedimentation rate (ESR) and white blood cell count (WBC), have been used for inflammation detection for over fifty years [29]. However, they are not highly specific and may be influenced by other factors [29]. In recent years, more sensitive and accurate serum markers have emerged and become widely available in clinical practice. For instance, C-reactive protein (CRP) is the foremost and widely used inflammatory marker, particularly effective at identifying infections, as it can increase up to 1000-fold from its basal plasma/serum levels within a 19-hour half-life [30]. Additionally, procalcitonin (PCT) levels in healthy individuals are usually below 0.05 μg/L, but they can surge dramatically within hours during severe inflammation [31]. Furthermore, IL-6, as an early-phase marker, is also valuable for monitoring early inflammation [29].
While expedient and straightforward, these tests do not pinpoint the precise cause or site of inflammation [32]. Moreover, their reliability can be influenced by other factors, such as medications, infections or chronic diseases [32]. Therefore, they are generally not sufficient and need to be interpreted along with imaging modalities.
Imaging modalities
Imaging modalities have been developed for years to diagnose inflammation-related diseases in preclinical and clinical studies. For instance, bioluminescence imaging of inflammation is facilitated by the reaction of luminol with myeloperoxidase (MPO) in inflamed regions [33]. Additionally, ultrasound (US) can measure plaque inflammation by evaluating enhanced permeability and neovascularization using non-targeted microbubbles. Furthermore, computed tomography (CT) is the most commonly used clinical imaging method among other imaging modalities and injectable iodinated compounds are employed as contrast agents [34]. Other imaging methods, such as radionuclide imaging (RI) and magnetic resonance imaging (MRI) usually utilize 18F fluorodeoxyglucose (FDG) and Gd3+ complex as contrast agents in the clinic, respectively [35, 36].
Despite widespread application, these imaging approaches still have certain shortcomings, including (i) Inferior penetration and sensitivity of bioluminescence imaging [37]; (ii) Limited sensitivity of US imaging in soft tissues [38]; (iii) Inadequate spatial resolution and safety concerns of CT and RI [39]; and (iv) Low detection resolution of MRI in some organs, such as liver, lung and gastrointestinal tract [40].
Current treatment for inflammation
Inflammatory diseases require not only precise diagnosis but also timely and effective drug therapy. A variety of drugs have been developed for inflammation treatment.
Chemical drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) are a group of medicines that can reduce pain, fever and inflammation [41]. There are many types of NSAIDs, such as aspirin, ibuprofen, naproxen, diclofenac, celecoxib and etoricoxib [42]. They work by blocking the enzymes that produce prostaglandins, which are chemicals that cause inflammation and pain in the body [42]. However, NSAIDs have several limitations that affect their clinical efficacy and safety. These include unfavorable physicochemical properties, low in vivo delivery efficiency and potential for off-target toxicity, which induce some risks and side effects, such as stomach ulcers, bleeding, high blood pressure, kidney problems and heart problems [42].
Corticosteroids including dexamethasone (Dex) are a class of synthetic drugs that can reduce inflammation and suppress the immune system [43]. They are used to treat various conditions, such as asthma, allergies, eczema, RA and autoimmune diseases [43]. However, long-term or high-dose use of corticosteroids can cause serious side effects that outweigh their benefits. These include weight gain, mood changes, high blood pressure, diabetes, osteoporosis and an increased risk of infections [43].
Gene drugs
Gene therapy is a novel approach to treat or prevent diseases by modifying genes within the body's cells [44]. It has potential applications for various inflammatory conditions, such as cystic fibrosis, arthritis and chronic pain [45]. However, gene therapy for inflammation faces several hurdles that need to be overcome before it can be widely used in the clinic. These include instability, immunogenicity and off-target toxicity of the gene delivery vectors, which can cause unwanted immune reactions, gene mutations or side effects [46]. Therefore, more research and clinical trials are needed to ensure the safety and efficacy of gene therapy for inflammation.
Protein and peptide drugs
Some protein drugs, such as infliximab, adalimumab and certolizumab, have been approved for marketing and can be used to treat inflammatory diseases, such as RA and IBD [47-49]. Peptide drugs, such as semaglutide (Rybelsus®) and octreotide (MYCAPSSA®), are also used for inflammation treatment, as they can regulate insulin secretion and treat diabetes [50]. However, protein and peptide drugs have some drawbacks, such as instability, easy degradation, immunogenicity, side effects and high cost [51, 52]. Therefore, a pressing need for developing effective drug delivery methods to enhance anti-inflammatory therapy.
Nanotechnology-driven diagnosis and treatment
Nanotechnology has been widely applied in biomedicine, especially for developing nano-DDSs to treat inflammation-related diseases [53-58]. The size, surface charge and shape of nanoparticles (NPs) are crucial factors that affect their interactions with biological systems [59]. Moreover, surface modification is also an important aspect in the design of NPs to enhance their targeting and circulation [59].
The size of NPs determines their interactions with various tissues and organs in the body [60]. Smaller NPs (< 10 nm) can cross the blood-brain barrier more easily than larger ones [60]. NPs (< 100 nm) tend to accumulate in the alveoli, while those > 200 nm are cleared by alveolar macrophages [60]. NPs (> 100 nm) are also prone to be captured by the reticuloendothelial system in the liver, spleen and lymph nodes [60]. Smaller NPs (6-100 nm) have a higher chance of penetrating the blood vessel wall and reaching inflammation sites than larger ones owing to their favorable contribution and permeability [61]. NPs (100-200 nm) may have lower efficiency in reaching inflammation sites, but they exhibit longer circulation and better selective retention at inflammatory sites [59]. Moreover, NPs < 6 nm may face renal excretion or clearance [60].
The surface charge of NPs has a significant impact on their behavior in biological environments. Cationic NPs are typically internalized more efficiently than neutral or anionic ones [62]. However, the high positive charge (> 15 mV) could increase the adsorption of plasma proteins, forming protein coronas that reduce their targeting and biocompatibility [62]. On the other hand, negatively charged NPs can accumulate more in serum, prolonging the retention time of the drug carrier due to lower charge-selective filtration [62].
The shape of NPs influences their cellular uptake [59]. Typically, spherical NPs have higher fluidity and stability, but are also easily cleared by phagocytic cells [59]. Non-spherical NPs, such as rod-shaped, sheet-shaped or star-shaped, have larger surface area and more functional groups, which enhance the drug loading and density of target ligands [59]. For instance, the cellular internalization of methylpolyethylene glycol-coated anisotropic gold NPs in RAW264.7 cells showed shape-dependent preferences for various endocytosis pathways [63]. The efficiency of cellular uptake increased from stars, to rods, to triangles [63].
Surface modification improves the in vivo circulation and targeting of NPs [64]. For instance, polyethylene glycolylation (PEGylation), especially with PEG molecular weight over 2000 Da, helps NPs evade clearance by macrophages, leading to longer circulation in the body [64]. In addition, some targeted ligands on NPs' surfaces can bind to specific receptors or molecules on the cell surface, resulting in selectively delivery of anti-inflammatory agents [65]. For example, mannose acts as a targeted ligand, binding to the mannose receptor expressed on activated macrophages and immune cells [65]. In a mouse model of RA, mannose-decorated poly(lactic-co-glycolic) acid (PLGA) NPs loaded with methotrexate (MTX), a potent anti-inflammatory drug, exhibited enhanced accumulation in inflamed joints and reduced arthritis severity compared to free MTX or non-targeted NPs [65].
Compared with conventional formulations, nano-DDSs have the following advantages based on the scale effect of nanostructures, including (i) Improving the pharmacokinetics of drugs by changing their physicochemical properties (e.g., water solubility, lipid solubility) and helping them cross physiological and pathological barriers, thus enhancing their bioavailability [66, 67]; (ii) Exploiting the unique physical, chemical, optical and biological properties of nanomaterials for biosensing and imaging, thus providing real-time monitoring and feedback of the disease status and treatment outcome [68]; (iii) Endowing nanomedicines with multiple functionalities by modifying the nanocarriers, prolonged circulation and intelligent drug release, thus enhancing the precision and efficiency of the therapy [69]; (iv) Co-delivering two or more probes and/or therapeutic agents for combined theranostics of inflammatory diseases, thus achieving synergistic effects and overcoming drug resistance [70].
Significance of this review
Recent reviews on nanotechnology applications for inflammation have highlighted key areas including diagnostics, therapeutics and theranostics. For instance, Tu et al. offer a comprehensive overview of biomaterials for inflammation control [43]. However, their review may not encompass all nanobiomaterials and lack detailed examples [43]. Similarly, Han et al. provide a novel perspective on metal-based NPs for inflammation control [71]. Nevertheless, their review only focuses on the specific metal-based NPs for treating inflammatory diseases [71]. Moreover, numerous studies have shown promising results in nanotechnology-based diagnosis and treatment of inflammation in recent years [54, 72-74]. Consequently, a systematic review is warranted to provide the latest developments in emerging nanotechnology-based approaches for diagnosing and managing chronic inflammation.
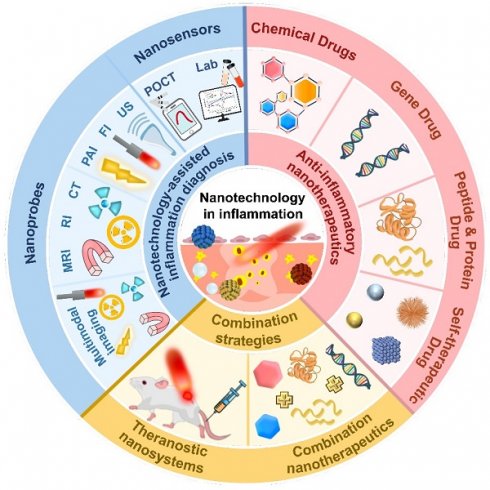
In this review, we aim to provide a timely overview of the latest developments in inflammation diagnosis and treatment (Figure 2), highlighting the advances and challenges of nanotechnology-based methods for inflammation diagnosis, therapeutics and theranostics. First, various nanotechnology-based diagnostic methods were pointed out via emphasizing their benefits, applications and prospects in inflammation diagnosis. Then, a large quantity of emerging anti-inflammatory nanotherapeutics were provided to elucidate the design principles of different nanocarriers and compare their advantages and disadvantages in anti-inflammatory therapy. Furthermore, biomedical nanotechnology-driven theranostics and combination therapy of inflammation were also discussed. Eventually, we shed light on the potential and hurdles pertaining to the clinical translatability of anti-inflammatory nanomedicines.
Nanotechnology-assisted inflammation diagnosis
Chronic inflammation is often asymptomatic in the early stage, but it can lead to serious diseases if left untreated [75]. Therefore, early inflammation detection is of importance for accurate confirmation of inflammatory lesions and timely disease intervention [76]. Nanotechnology-driven solutions are increasingly being applied in inflammation diagnosis [77]. Their high sensitivity and specificity enable the detection of biomarkers associated with inflammation at incredibly low concentrations [78]. Additionally, nanotechnologies not only improve imaging accuracy and sensitivity but offer real-time monitoring for assessing inflammation levels in the body [79]. Herein, we discuss nanobiosensors and imaging nanoprobes in diagnosing inflammation by comparing their advantages, disadvantages and clinical application prospects.
Nano-biosensing
Biosensors are analytical devices used to detect and measure biological molecules or substances within a sample. Biosensors typically consist of analytes, bioreceptors, signal transducers and display panels [80]. While traditional clinical detection methods such as indirect immunofluorescence (IIF), western blotting and enzyme-linked immunosorbent assay (ELISA) have been crucial in diagnostics, they often face challenges related to standardization and scalability. Nanotechnology-based biosensors have revolutionized diagnostics by leveraging nanomaterials and structures to detect biomarkers with incredible precision and sensitivity (Table 1) [81]. These biosensors utilize nanoscale components, such as NPs, nanowires or nanotubes, to detect specific biological molecules or signals, including autoantibodies, genetic markers, inflammatory factors and complements [81, 82].
Additionally, unlike complex, time-consuming hospital or lab tests, point-of-care testing (POCT) nanosensing offers user-friendly interfaces, rapid detection and cost-effective diagnosis, holding great promise in diagnosis of inflammatory-related diseases [83].
Nano-biosensors in lab
Recently, nanomaterials such as quantum dots (QDs), carbon dots (CDs) and gold NPs (AuNPs) have revolutionized biosensor technology by amplifing signals and improving biocompatibility due to their chemical, electrical, optical, mechanical or magnetic properties. These nanomaterials can further improve sensitivity and reduce side effects of biosensors, making them more suitable for inflammation diagnosis.
Graphic scheme of advancements in nano-delivery systems for diagnosing and treating inflammation.
Representative nanosensors for detection of inflammation in 2021-2023.
| Platform | Analyte | Transducer | Linear range | Detection limit | Disease | Year | Refs. |
|---|---|---|---|---|---|---|---|
| Platinum nanoclusters | H2O2 | Fluorescent and volumetric chip | 1-500 μM | / | IBD | 2022 | [84] |
| Colloidal quantum dots | MPO | Amperometric | 0.001-1 ng/ml | 31.6 fg/ml | / | 2023 | [85] |
| Ag2S quantum dots deposited Bi2S3 nanorods, | PCT | Electrochemical | 0.5-50 pg/ml | 0.18 pg/ml | / | 2023 | [86] |
| AuNPs | Lipoproteinassociated phospholipase A2 | Electrochemical | / | 0.21 ng/ml | AS | 2023 | [87] |
| Ionic liquid crystal, carbon nanotubes and Fe-Ni alloy nanoparticles | H2O2 | Electrochemical | 0.007-1000 μM | 0.971 nM | / | 2021 | [88] |
| Graphene quantum dots and AuNPs aggregate-embodied copolymer hydrogel | Cardiac troponin-I | Electrochemical | 1-1000 pg/ml | 0.1 pg/ml | Myocardial infarction | 2021 | [89] |
QDs are innovative fluorescent nanomaterials that enable the development of efficient biosensors with high sensitivity, selectivity, rapidity and simplicity [90]. Their unique optical and electronic properties, including high brightness, photostability, broad absorption spectrum, tunable emission spectrum and distinctive photoelectrochemical activity, contribute to their effectiveness [90]. When integrated into functionalized sensing systems, QDs can successfully detect various inflammation biomarkers, such as CRP [91], PCT [92] and TNF-α [93], showing great potential for diagnosing and monitoring inflammation. For instance, Cai et al. constructed a photoelectrochemical biosensor for ultra-sensitive “on-off” detection of inflammation biomarkers: TNF-α and methylase (MTase) [93]. The biosensor combines selenide (WSe2) nanoflowers, AgInS2 (AIS)/ZnS QDs and DNA nanostructures. The (AIS)/ZnS QDs, acting as excellent photosensitive materials, matched the energy level of WSe2 nanoflowers, boosting the photocurrent signal by 65 times compared to WSe2 nanoflowers alone. Additionally, (AIS)/ZnS QDs serve as signal amplifiers and specific recognition elements for the target molecules. Such a nanobiosensor showed a linear range of 0.1-1000 pg/ml for TNF-α and 0.01-100 U/ml for MTase, with a limit of detection (LOD) of 0.03 pg/ml for TNF-α and 0.003 U/ml for MTase, indicating high efficiency and sensitivity in inflammation detection [93]. In another study, Lv et al. systematically investigated the influence mechanism of metal ions on QD fluorescence signal amplification and discovered that Ca2+ could not only increase the fluorescence intensity of QDs, but facilitate the binding efficiency of antigen-antibody [94]. Compared with the common QD-fluorescence-linked immunosorbent assay (QD-FLISA), Ca2+-QD-FLISA showed ultra-high detection sensitivity of CRP, an inflammation biomarker, by 4 times, reaching 0.23 ng/ml. The ion-QD-FLISA method was then successfully extended to use other ions, including Mg2+, Ba2+, Fe2+ and Mn2+ as well as applied to detect other biomarkers such as serum amyloid A (SAA) and PCT. The amalgamation of metal ions with QDs presents a simple and effective approach for the early detection of inflammation [94].
CDs are versatile fluorescent nanomaterials prized for their adjustable luminescence and excellent biocompatibility in biosensing applications [95]. Immunomagnetic CDs are a class of carbon-based nanomaterials that integrate magnetic functionalities and specific antibodies [95]. They are valuable in analytical chemistry and biosensing due to their ability to recognize and isolate specific targets efficiently without complex sample preparation procedures [95]. For instance, Liu et al. employed a fluorescence amplification system using nanocapsules and magnetic CDs to detect PCT [95]. They proposed two different sensing strategies: the magnetic separation strategy and the homogeneous immunoassay strategy. The magnetic separation strategy used immunonanocapsules as sensors and immunomagnetic CDs as capture probes, achieving ultra-sensitive trace detection of PCT through normal immune reaction and magnetic separation, with a detection range of 1-1000 pg/ml and an LOD of 0.3 pg/ml. While the homogeneous immunoassay strategy utilized immunonanocapsules as energy transmitters and immunomagnetic CDs as sensors, achieving direct rapid detection of PCT through the principle of fluorescence resonance energy transfer (FRET), with a detection range of 0-100 ng/ml and an LOD of 0.41 ng/ml. In conclusion, the former strategy is suitable for complex samples and the later suitable for rapid detection. Both strategies exhibit stability and reliability in achieving accurate quantification of PCT, with better linear relationship and lower detection limit than the traditional chemiluminescence microparticle immunoassay (CMIA) method [95].
AuNPs are widely used for electrochemical signal amplification in immunosensors due to their large surface area, strong catalytic activity, biocompatibility, and ability to improve electron transfer rates and create an optimal microenvironment for capture antibodies [96]. For instance, Yola et al. reported a novel voltammetric immunosensor for the detection of TNF-α, using AuNPs as part of the sensor platform (AuNPs/S-MWCNTs) and the signal enhancer (bimetallic Ni/Cu-MOFs) [96]. The capture TNF-α antibody was attached to the sensor platform through amino-gold affinity to achieve immunoreaction. The proposed immunosensor showed high sensitivity, selectivity, stability and reproducibility and the detection time was less than 30 minutes without complex sample labeling or multiple washing steps, providing an effective method for the diagnosis and monitoring of TNF-α-related diseases [96]. In addition, Wang et al. introduced an electrochemiluminescent (ECL) immunosensor for highly sensitive detection of lipoproteinassociated phospholipase A2 (Lp-PLA2), an AS biomarker, via a multifunctional nanoplatform (AuNPs@CoFe PBA) consisting of CoFe prussian blue analogue (PBA) and AuNPs [87]. The AuNPs@CoFe PBA's exceptional peroxidase-like activity significantly amplified the ECL signal by approximately 29-fold owing to the synergistic effect between PBA and AuNPs. Additionally, the abundant AuNPs presented more active sites for immobilization of antibody proteins, enhancing the sensor response. Upon capturing Lp-PLA2, the sensor emitted a reduced ECL signal due to increased mass and electron transfer resistance. Under optimized conditions, the developed ECL immunosensor exhibited a wide linear detection range from 1 to 2200 ng/ml and an LOD of 0.21 ng/ml. [87].
Nano-biosensors in POCT
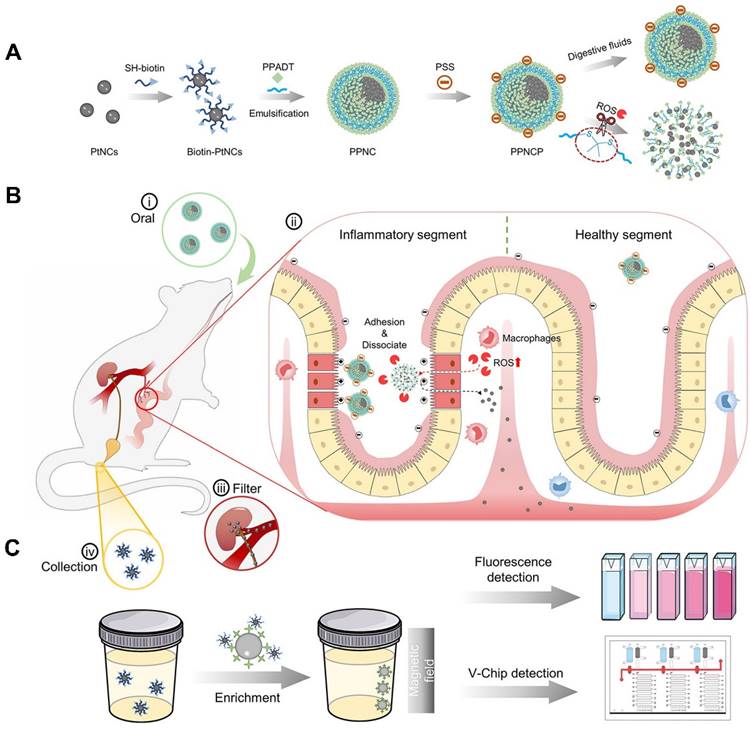
POCT is a diagnosis strategy that has been widely used for disease diagnosis and treatment monitoring outside the laboratory in recent years [97]. Unlike complex and time-consuming tests in hospitals and laboratories that often require specialized equipment and trained personnel, POCT shows many benefits such as convenient, efficient and cheap, especially for large-scale disease diagnostics [98]. The World Health Organization (WHO) has established the criteria for POCT sensing platforms, including sensitivity, specificity, user-friendliness and rapidity (delivering results within 30 minutes) [99]. Nanomaterials are suitable for enhancing signal transduction and amplifying sensor response to improve performance of biosensors, thus holding great promise in POCT [99]. As shown in Figure 3, Zhou et al. constructed a non-invasive nanosensor (PPNC@PSS) that could disassemble into ultrasmall platinum nanoclusters (PtNCs) within inflammatory microenvironments associated with IBD [84]. To form supernanoparticles (PPNC), poly (1,4-phenyleneacetone dimethylene thioketal) with ROS-responsiveness was employed to facilitate encapsulation of PtNCs, which was further modified with Poly-(styrenesulfonate) to form PPNC@PSS and targeted inflamed intestinal sites. Upon oral administration, PPNC@PSS responded to elevated ROS levels in inflamed intestines, releasing small PtNCs (2 nm) that were filtered into urine and then detected by simple and sensitive urine readers, such as fluorescence and volumetric bar-chart chips (V-Chip). Notably, nanosensor showed higher accuracy and sensitivity than conventional fCAL-based ELISA assays, due to the amplified signals from both urinary biological enrichment and PtNCs' catalytic activity. Such an adaptable nanosensor holds great promise for home-care applications to personalize the assessment of diseases and follow-up therapeutic efficacy [84].
Recently, a flexible amperometric immunosensor targeting MPO has been developed using a modified electrode with colloidal QDs (CQDs) [85]. CQDs have exceptional surface characteristics that enable them to bind directly and stably to protein surfaces, facilitating the conversion of antigen-antibody specific binding reactions into detectable electrical currents [85]. The portable nanobiosensor offered precise quantitative analysis of the MPO with high sensitivity (LOD = 31.6 fg/ml) and exhibited impressive stability during short-term storage at low temperatures [85].
Wearable biosensor devices can monitor various physiological parameters, such as blood pressure, glucose level and sweat composition, by attaching to the human body or clothing [100]. They offer a potential solution for diagnosis and monitoring due to their convenience, reusability and sensitivity [3]. For example, Wang et al. presented a flexible and regenerative biosensor using a graphene-Nafion composite membrane to detect cytokine storm biomarkers in undiluted biofluids linked to inflammation [101]. Among them, graphene as a two-dimensional (2D) nanomaterial, demonstrated excellent electrical conductivity, mechanical properties and biocompatibility, and could enhance the transmission and amplification of electrical signals. Additionally, Nafion was a fluorinated polymer, showing good ion exchange properties and anti-biofouling ability, and could protect the active molecules on the electrode surface. Then, the graphene-Nafion film was modified with specific aptamers for biomarkers. This nanobiosensor not only exhibited high consistency and sensitivity in detecting biomarkers (IFN-γ) with an LOD 740 fM in undiluted human sweat, but could withstand multiple regenerative cycles (up to 80) by simple washing and regeneration steps and 100 cyclic crumpling tests without mechanical failure. What's more, the detection data could be uploaded in real-time to the cloud or mobile devices via wireless communication technology, enabling remote monitoring and intervention for doctors and patients, thus enhancing medical efficiency and quality [101].
Nano-imaging
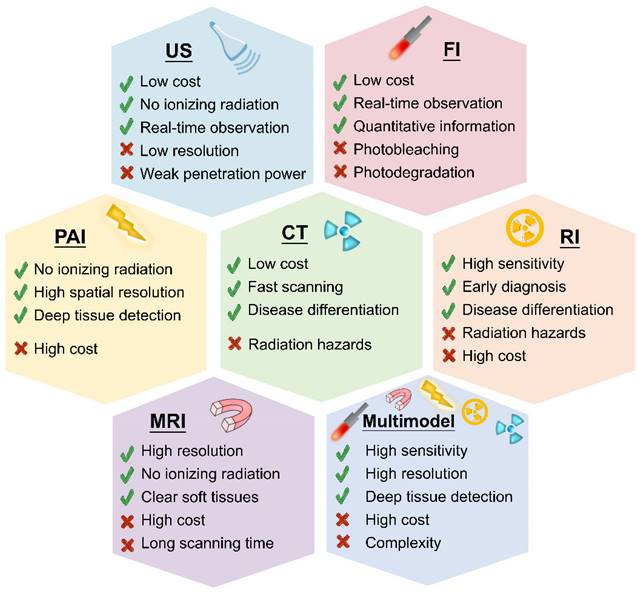
Several imaging methods are available for inflammation diagnosis, including US [102], FI [103], PAI [104], CT [105], RI [106], MRI [107] and multimodal imaging [108]. Figure 4 compares these diagnostic approaches, highlighting their major advantages and disadvantages.
An oral nanosensor for IBD monitoring. (A) The preparation of PPNCP nanosensor, which is composed of PtNCs encapsulated by a ROS-responsive polymer. (B) The release of ROS-sensitive PPNCP supernanoparticles in inflamed intestine. (C) The collection of biotin-PtNCs by magnetic enrichment and the detection of by fluorescence and V-Chip assay. Adapted with permission, from [84] Copyright 2021 American Chemical Society.
However, the diagnostic agents used in these methods still face some challenges, such as poor inflammation targeting, insufficient accumulation at inflammation sites, and rapid clearance by blood circulation, which affect the accuracy of inflammation diagnosis [109]. Therefore, there is a growing interest in combining nanotechnology and conventional imaging technology for inflammation detection, as nanotechnology can overcome some of these limitations and enhance the performance of imaging modalities.
US nanoprobes
US imaging is a widely used diagnostic modality for clinical applications, as it harnesses high-frequency sound waves that create echoes when interacting with tissues [110]. These echoes detected by ultrasonic sensors are meticulously analyzed for their amplitude and time, forming the basis for generating real-time detailed images [110]. To enhance US imaging, contrast agents are often introduced. However, traditional US contrast agents struggle with limited echogenicity and inadequate accumulation at target sites, resulting in reduced imaging resolution [111]. To overcome the challenge, nano-based US nanoprobes such as silica NPs and perfluorocarbon-based NPs are introduced [110, 112]. For instance, Chudal et al. conducted perfluorobutane nanodroplets (NDs) filled with a low-boiling point perfluorobutane as ultrasound nanoprobes to label inflammation-related macrophages [102]. The NDs were internalized by macrophages and vaporized into microbubbles by ultrasound, which showed higher contrast-to-noise ratio (CNR), resulting in enhanced US signal and sensitivity. The outcomes also demonstrated that the NDs did not affect the viability and function of the macrophages and that the vaporization could be achieved within the energy limits of a clinical ultrasound scanner. In vivo, NDs enabled tracking and labeling of macrophages in rats and achieved visualization of macrophage function [102].
However, US encounters hurdles in depicting bones and lungs owing to the challenges in US wave propagation [110]. Therefore, US is often employed with other imaging modalities to boost imaging resolution and diagnostic precision such as FI and PAI [110].
FI nanoprobes
FI is an innovative technique for inflammation detection, allowing visualization of dynamic processes and temporal changes in inflammatory sites via intravenous application of a fluorescence dye [113]. However, clinical organic dyes, such as indocyanine green (ICG) and methylene blue (MB) often suffer from limited in vivo stability, leading to potential issues such as impaired liver or kidney function [113, 114]. With the rapid development of nanotechnology, various nanotechnology-driven FI nanoprobes, including QDs-based, aggregation-induced emission (AIE)-based, “turn-on,” and ratiometric fluorescent nanoprobes, have been developed [77, 115]. These nanoprobes have demonstrated high optical stability, sensitivity, selectivity and reduced side effects, holding promising potential for accurate diagnosis of inflammation.
Schematic illustration of strengths and weaknesses of US, FI, PAI, RI, CT, MRI and multimodal imaging.
QDs have multiple advantages over traditional fluorescent dyes, including high quantum yield, photobleaching resistance, ease of surface modification and tunable emission ranges [90]. Leveraging the advances in QDs, QDs-based nanoprobes have been developed for the detection of inflammation [116]. For instance, Liu et al. successfully integrated elastase-specific peptides, CdSe/ZnS QDs and sulforhodamine B (Rh) into a nanosystem to develop a QDs-based nanoprobe (QDP) for detecting human neutrophil elastase, a common protease in pulmonary inflammation [116]. Compared with reported small-molecule and peptide probes, QDP possessed higher sensitivity and lower limit of detection (LOD: 7.15 pM), reduced environmental interference and improved in vivo HNE imaging, which helped in distinguishing patients with lung inflammation from healthy individuals [116]. Despite multiple advantages, QDs may induce oxidative stress and inflammatory-associated disorders due to the poor biocompatibility and cytotoxicity of heavy metal elements such as Cd and Pb [117]. Therefore, the development of emerging organic materials is essential to mitigate the potential toxicity associated with inorganic materials.
AIE-based nanosystems offer a promising platform with unique properties [118]. Unlike conventional organic fluorophores that are susceptible to fluorescence turn-off modes by aggregation-caused quenching (ACQ) effect, AIE NPs are highly fluorescent in the aggregated state due to restricted molecular motion, which leads to enhanced radiative decay rate and high photobleaching resistance [119]. Furthermore, AIE NPs exhibit high biocompatibility and stability, making them suitable for in vivo deep-biological imaging [118]. For specific peroxynitrite (ONOO-) detection, an inflammation-related species, Xie et al. fabricated an AIE-active luminogenic nanoprobe by encapsulating an AIE luminogen, namely tetraphenylethene-dimethylaminoboronic acid (TPE-DMAB), within a lipid-PEG matrix [120]. Unlike conventional organic fluorophores, TPE-DMAB demonstrated exceptional sensitivity towards ONOO- (LOD: 54 nM) and desirable chemical stability, resulting in fluorescence enhancement of up to 100-fold upon phenylboronic moiety cleavage by ONOO-. In vivo, the nanoprobe also facilitated the visualization of ONOO- in a lipopolysaccharide (LPS)-induced inflammation-bearing nude mouse model [120].
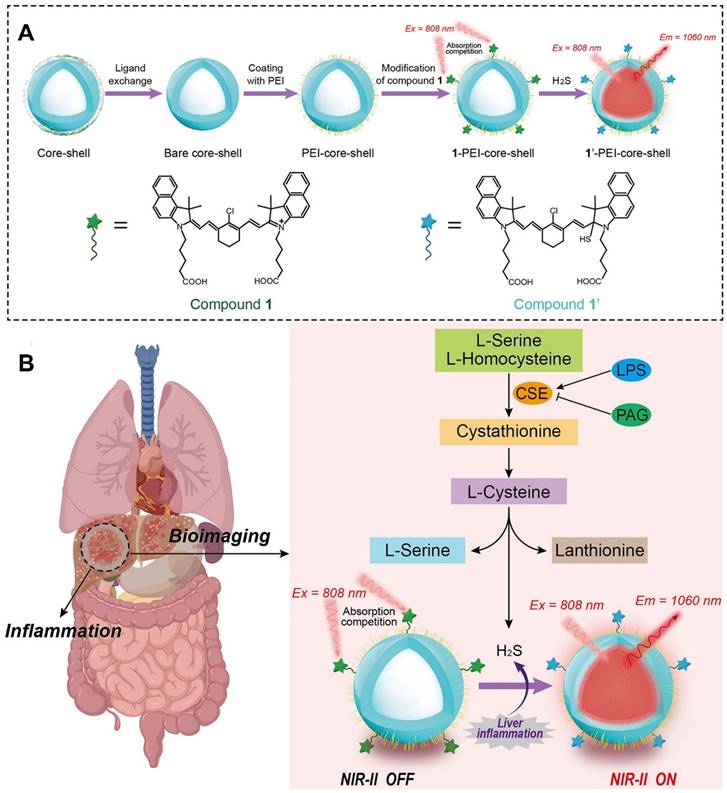
Advancements in “smart” fluorescent nanoprobes that switch between “on” and “off” states have garnered significant attentions [58, 121]. Unlike “always-on” fluorescent nanoprobes, “turn-on” fluorescent nanoprobes can be selectively activated by endogenous inflammatory triggers, such as endogenous hydrogen peroxide (H2O2) and hydrogen sulfide (H2S), enhancing both the sensitivity of biosensors and bioimaging resolution [122]. Furthermore, near-infrared-II (NIR-II, 900-1700 nm) fluorescent nanoprobes have demonstrated remarkable capabilities in terms of deep-tissue penetration and non-invasive imaging contrast enhancement [123-125]. Therefore, integrating both NIR-II fluorescent materials and “turn-on” fluorescent probes into a unified nanosystem might exhibit outstanding sensing capabilities. As shown in Figure 5, Liu et al. devised a “turn-on” NIR-II luminescent NPs (1-PEI-DCNPs) by attaching H2S-responsive chromophores onto the NaGdF4:2%Nd@NaGdF4 NPs via polyethyleneimine (PEI) linkers for visual monitoring of overproduced H2S in vivo [124]. By leveraging the absorption competition-induced emission (ACIE) mechanism, the fluorescence emission of 1-PEI-DCNPs at 808 nm was effectively quenched. Nevertheless, the presence of H2S within endogenous inflammatory sites could trigger a reaction that activated the fluorescence of 1-PEI-DCNPs at 1060 nm. Notably, given the remarkable penetrability and tissue contrast provided by the NIR-II window, 1-PEI-DCNPs could achieve accurate imaging of liver inflammation and real-time visualization of overproduced H2S in a LPS-induced liver inflammation mouse model [124].
Ratiometric FI nanoprobes enable accurate molecular detection and imaging by using signal ratios, overcoming limitations of non-ratiometric nanoprobes such as nanoprobe concentration and background interference [126]. Additionally, ratiometric nanoprobes are more accurate and reliable than non-ratiometric ones, providing more information about the analyte, including concentration, location and dynamics [126]. For instance, Pei et al. developed 3D-printed bioactive glass scaffolds (ErBG@IR808 scaffolds) by incorporating Erbium (Er)-doped NPs (ErNPs) and modifying them with HClO-responsive IR808 fluorophores, enabling real-time monitoring of early-stage inflammation [127]. Under 808 nm excitation, IR808 absorption quenched ErBG scaffold emission at 1525 nm via the ACIE mechanism. Increasing HClO reversed this quenching by oxidizing IR808, while the reference signal at 1525 nm remained stable under 980 nm excitation. Furthermore, the dual-excitation ratiometric ErBG@IR808 scaffolds showed a linear correlation with HOCl concentration (LOD: 0.42 μM) at 1525 nm, achieving the visualization of ongoing inflammation during bone repair in a mouse calvarial defect model [127].
The structure and imaging mechanism of a NIR-II fluorescent probe. (A) The synthesis route of 1-PEI-DCNPs. (B) The imaging mechanism of 1-PEI-DCNPs. 1-PEI-DCNPs exhibited responsiveness to LPS-induced liver inflammation and high sensitivity to H2S, with the ACIE effect completely suppressing their NIR-II luminescent signals at 1060 nm. In the presence of excessive H2S generated during LPS-induced liver inflammation, the nucleophilic addition between HS- and the benzpyrole group in compound 1 bleached the absorption of compound 1 at 808 nm. However, the presence of H2S within endogenous inflammatory sites activated the fluorescence of 1-PEI-DCNPs at 1060 nm, achieving a "turn-on" luminescence. Adapted with permission, from [124] Copyright 2021 American Chemical Society.
PAI nanoprobes
Despite the distinct advantages, FI has some defects such as poor tissue penetration and light scattering in biological tissues. By contrast, PAI based on both US and FI, possesses the inherent benefit of high spatial resolution, no ionizing radiation, deep tissue penetration (up to approximately 7 cm) and real-time imaging capabilities, attracting tremendous attention [128]. As reported, excessive oxidative stress levels in vivo are closely related to chronic inflammatory diseases [129, 130]. However, accurate detection of inflammation-related endogenous biomarkers remains a tough task due to the low substrate concentration in physiological environments (∼50 μM) and the limited tissue penetration depth of imaging agents [131]. To overcome these obstacles, Ma et al. investigated a biocompatible nanoplatform (PLCDP@PMH, 252.7 nm) to load the polymeric photoacoustic probe for AS theranostics [132]. The prepared PLCDP@PMH possessed ROS/matrix metalloproteinase (MMP) dual-responsive properties and targeted delivery capabilities. Furthermore, it was also capable of absorbing NIR light and generating robust photoacoustic signals, thus providing high-resolution images of inflamed blood vessels. In vitro, PLCDP@PMH demonstrated superior photoacoustic conversion efficiency even at a lower concentration of 5 μg/ml. In vivo, PLCDP@PMH facilitated highly contrasting photoacoustic imaging compared to normal tissue, suggesting promising clinical potential for noninvasive AS diagnosis [132].
In spite of significant advancements in PA nanoprobes, accurately measuring inflammation-related biomarkers remains challenging due to their dynamic changes and short lifespans [133, 134]. Hence, the design of highly sensitive PA nanoprobes is crucial for monitoring the complex inflammatory microenvironment. Compared to non-ratiometric PAI, ratiometric PAI demonstrates greater accuracy and sensitivity in monitoring excessive ROS in inflamed tissues [104, 135]. For example, Ye et al. successfully designed an H2O2-activated ratiometric nanoprobe (Au-Pd@Ag NR) for precise and reliable detection of H2O2, a biomarker of inflammation, according to the regular change in PA signals [136]. Au-Pd@Ag NR consisted of Pd-tipped gold nanorods with an Ag shell that can produce ratiometric PA signals and release Ag ions upon H2O2 exposure. Notably, the ratiometric PAI (PA1260/PA700) signals exhibited a direct correlation with H2O2 concentration, enabling reliable quantification of H2O2 levels at inflamed sites. Furthermore, the exceptional resolution and deep tissue penetration of Au-Pd@Ag NR enabled precise differentiation between inflamed regions and normal tissues. In vivo experiments revealed that Au-Pd@Ag NR could accurately quantify H2O2 levels in a mouse model of abdominal inflammation and a rabbit model of osteoarthritis (OA) [136].
CT nanoprobes
CT imaging is a technique that uses X-rays to scan the human body from different angles and then reconstructs the cross-sectional images of the body using a computer [137]. CT visualizes the human skeleton, organs and blood vessels, detailing their structure, size, density and enhancement, which is helpful for diagnosing and staging diseases [137]. Compared with MRI, CT scanning offers several benefits such as rapid image acquisition and relatively low costs [138]. Despite considerable advancements in CT contrast agents over time, they still have limitations. Specifically, traditional iodinated contrast agents suffer from inadequate targeting, short half-life and severe nephrotoxicity, leading to substantial side effects [139].
In recent years, nanoprobes, specifically AuNPs, have attracted significant interest. AuNPs are ideal CT nanoprobes, which can greatly increase CT contrast with minimal toxicity [140]. For example, Yu et al. devised a pH-sensitive gold nanotracer (CPP-PSD@Au) functionalized with a cell-penetrating peptide (CPP) and a sulfonamide-based polymer (PSD) to improve the cellular uptake and biocompatibility, leading to prolonged CT monitoring of mesenchymal stem cells (MSCs) in a murine model of idiopathic pulmonary fibrosis (IPF) [141]. CPP-PSD@Au exhibited divergent surface charges at pH 7.4 and 5.5, respectively. Upon internalization within endosomes, CPP-PSD@Au progressively aggregated in acidic environments due to PSD protonation and CPP dissociation, promoting substantial cellular retention and enabling long-term tracing of the transplanted stem cells. Employing the CT imaging technique in conjunction with CPP-PSD@Au, the transplanted MSCs were successfully monitored for up to 35 days post-transplantation into the IPF mouse lungs, thus yielding valuable insights into the in vivo migration process of MSCs. [141].
Although AuNPs have superior characteristics, such as easy preparation and robust CT contrast, their potential side effects and high costs present challenges for clinical translation [142]. With the K-edge of cerium at 40.4 keV, cerium oxide (CeO2) NPs have recently emerged as an alternative CT nanoprobe offering substantial x-ray attenuation [142]. For instance, Naha Pratap C. et al. fabricated dextran-wrapped CeO2 NPs (Dex-CeNPs) for diagnosing IBD [142]. Dextran is a polysaccharide derived from glucose that could enhance the biocompatibility, stability and water solubility of NPs. Compared with the Food and Drug Administration (FDA)-approved iodinated contrast agents (ICAs), Dex-Ce NPs exhibited greater accumulation at inflamed sites and had a CT contrast enhancement of 1.5 HU per mg/ml in a mouse model of dextran sodium sulfate (DSS)-induced colitis. Remarkably, 97.6% of oral doses were eliminated from the colitis mice within 24 hours, which reduced the risk of toxicity [142].
Bismuth (Bi) NPs have also emerged as promising agents for CT imaging because of their high density and atomic number (Z = 83), which give them strong X-ray attenuation power [143]. Furthermore, Bi NPs can be modified with various coatings or functional groups to enhance their biocompatibility, stability, solubility and targeting ability. Bi NPs can also be designed to have different sizes and surface properties, which influence their biodistribution, pharmacokinetics and targeting efficiency [143]. For instance, Rabin et al. employed a long-circulating bismuth sulfide nanocrystals (BPNPs) coated with polyvinylpyrrolidone (PVP) for CT imaging. Firstly, BPNPs had fivefold better X-ray absorption than iodine, which improved the contrast and resolution of CT images. Furthermore, BPNPs could enable the detection of small lymph nodes (< 1 mm) and liver lesions (<0.5 mm) in mice, which were difficult to image with ICAs. Additionally, they possessed a prolonged circulation times (> 2h) in vivo for targeted microvasculature imaging, holding significant potential for broadening the applications of X-ray CT [144]. Bi NPs can also provide higher CT values at lower doses and radiation parameters. For example, Tarighatnia et al. utilized diethylenetriaminepentaacetic acid (DTPA) as the chelating agent to synthesize a small-molecular bismuth chelate (Bi-DTPA) with high X-ray attenuation and low toxicity [145]. Compared with commercial iodine-based contrast agents, Bi-NPs demonstrated higher CT values with a 13-fold increase in CNR. Moreover, MUC-16 aptamer-targeted Bi NPs demonstrated a 6-fold increase in X-ray attenuation over non-targeted Bi-NPs in vitro [145]. Also, bismuth NPs can be used for dual-modality imaging, such as combining CT with single photon emission computed tomography (SPECT). For instance, Kevadiya et al. synthesized rilpivirine (RPV) loaded bismuth sulfide NPs (BiSNRs) and labeled them with lutetium-177 (LuBSNRs) to enable both SPECT and CT imaging [146].
RI nanoprobes
RI, also known as nuclear medicine imaging, is the earliest and most extensively used molecular imaging technique in clinical applications [147]. It uses molecular probes labeled with radioactive nuclides to detect their distribution and metabolism in the body, reflecting the location, degree and activity of inflammation. RI mainly includes SPECT and positron emission tomography (PET). SPECT relies on gamma-ray-emitting tracers to obtain detailed three-dimensional images, while PET utilizes positron-emitting radioactive tracers, commonly 18F-FDG, to highlight areas with high metabolic activity. [147]. By utilizing these methods, nuclear medicine provides valuable insights into physiological processes, enhancing our understanding of inflammation.
Despite high sensitivity of PET and SPECT, the range of medical contrast media accessible to clinicians remains constrained by ionizing radiation risk and low spatial resolution [106]. More importantly, the half-lives of radiopharmaceuticals (typically a few hours) also restrict quality control procedures [147]. NPs represent a promising approach enabling advanced and highly specialized contrast media. Their unique properties allow loading diverse radioactive tracers via various synthesis methods, enhancing targeting capabilities and specificity [106]. For instance, Senders et al. employed a high-density lipoprotein-derived nanotracer labelled with zirconium-89 (89Zr) to allow PET imaging and track the systemic dynamics of leukocytes in atherosclerotic mice [148]. The experimental findings demonstrated a significantly elevated PET signal in inflamed sites compared to the background [148]. Zhang et al. utilized polymer NPs doped with FDA approved diagnostic radioisotope technetium-99m (99mTc) for SPECT imaging of RA [149]. 99mTc had low toxicity and fast clearance from the body, which enhanced NPs' biocompatibility and safety. In vivo SPECT imaging results demonstrated that 99mTc-NPs accumulated in RA mice, showing the effective and targeted delivery of NPs [149].
PET and SPECT boast distinct advantages over alternative imaging modalities due to their exceptional sensitivity. However, the challenge lies in their constrained spatial resolution, necessitating the co-registration with CT or MRI to achieve accurate diagnosis [148]. Additionally, with the advances in nanotechnology, the use of combined PET or SPECT and CT will also broaden the scope of the imaging modality and reduce exposure to ionizing radiation, promoting clinical translation of SPECT and PET techniques [147].
MRI nanoprobes
MRI is a valuable tool for the non-invasive diagnostic imaging of inflammation-related diseases by providing high-resolution soft tissue images [150]. MRI is a technique that uses a strong magnetic field and radio waves to create detailed images of the organs and tissues in the body [151]. The fundamental MRI parameters, T1 and T2 relaxation times, describe the recovery and decay of magnetization in tissues after being disrupted by radiofrequency (RF) pulses [151]. Shorter T1 values in tissues accelerate longitudinal magnetization recovery, resulting in brighter T1-weighted images, while longer T2 values indicate slower decay, leading to darker images [152]. Typically, contrast agents employed in MRI are primarily paramagnetic agents such as gadolinium (Gd) or manganese (Mn), and superparamagnetic agents such as superparamagnetic iron oxide particles (SPIO) [152]. Paramagnetic agents mainly shorten T1 relaxation time, thereby enhancing signal intensity on T1-weighted images [153]. On the other hand, superparamagnetic agents mainly reduce T2 relaxation time, displaying T2 hypo signal. However, these contrast agents exhibit limitations such as suboptimal biocompatibility and targeting inefficiencies.
In recent years, nanotechnology-assisted modification of contrast agents has shown immense promise to mitigate these issues. For example, He et al. engineered a brain-targeted nanoconstruct (aAβ-BTRA-NC) activated by ROS for detecting and monitoring AD progression [154]. aAβ-BTRA-NC consisted of manganese oxide NPs (MnO2) for MRI imaging, a polymer/lipid core for stability and biocompatibility as well as an anti-amyloid-beta (Aβ) antibody for targeting. Due to the presence of targeting moieties, aAβ-BTRA-NC effectively penetrated the BBB and bound to Aβ plaques in the brain. Upon exposure to ROS, aAβ-BTRA-NC facilitated the local release of Mn2+, amplifying T1-weighted MR signals in the cerebrospinal fluid (CSF) by a factor of 1.51-2.24. In an AD mouse model, aAβ-BTRA-NC exhibited exceptional sensitivity (89%) and specificity (100%) in detecting early-stage AD [154].
Since the approval for clinical application by the FDA, SPIO NPs with small particle sizes have attracted tremendous scientific interest due to their exceptional chemical, magnetic and biocompatible properties [155]. Based on the selective aggregation-enhanced T2 effect of SPIO NPs, Tang et al. designed platelet-mimetic NPs (PTNPs) incorporating SPIO to monitor activated neutrophils in ischemic stroke [156]. Compared with PTNP control groups, SPIO-PTNP groups demonstrated superior biocompatibility, enhanced targeting efficiency and improved MRI contrast, enabling real-time monitoring of inflammatory progression [156].
Despite the advancements made with SPIO NPs, they encounter magnetization saturation at around 1.5 T, limiting MRI improvements at higher magnetic fields [157]. Dysprosium (Dy3+) has been identified for its substantial magnetic moment, short relaxation time and magnetic saturation surpassing 21 T via a Curie mechanism [157]. To augment the T2 relaxation rate, PAA-modified ultrasmall IO/Dy oxide NPs (IO-DyO NPs) were designed for precise liver fibrosis imaging [157]. Within IO-DyO NPs, IO showed magnetic characteristics conducive to MRI, while DyO served as a contrast agent to amplify the imaging signal. Specifically targeting the fibrotic regions in the liver, IO-DyO NPs boosted the sensitivity and specificity of MRI for detecting and characterizing liver fibrosis. In comparison to conventional SPIO NPs, IO-DyO NPs exhibited a threefold increase in r2 (1/T2 s-1) relaxivity, which was particularly evident under a 9.4 T MRI system. Furthermore, IO-DyO NPs significantly improved the spatial and temporal resolution of liver imaging, enabling accurate discrimination of fibrotic liver tissues and facilitating clear staging of the clinically consequential early and moderate liver fibrosis [157].
Multimodal imaging nanoprobes
Inflammation is a complex biological process that involves various tissues and cells in response to injury or infection. Imaging techniques can help to detect, monitor and characterize inflammation in different organs and systems. However, no single imaging modality can provide all the information needed for a comprehensive assessment of inflammation, such as high resolution, deep tissue penetration, high sensitivity, fast imaging and low toxicity [158]. Therefore, multiple imaging modalities are often combined to overcome the limitations of each individual technique and to obtain complementary information. Nanotechnology has sparked interest in combining various imaging methods on a nanoplatform. This integration harnesses their individual strengths, offering detailed information, enhanced sensitivity and specificity, improved spatial and temporal resolution and synergistic data interpretation [108]. However, this approach comes with challenges, such as technical complexities and the high costs [108].
As previously discussed, the PA nanoprobe demonstrates high spatial resolution and deep tissue penetrability, exhibiting considerable potential as a ROS imaging modality. In addition, CT is considered a powerful technique that provides images with high spatial and temporal resolution. Consequently, combining PA and CT on a nanoprobe presents a solution for ROS imaging at inflamed sites [159]. For example, Bouche M et al. devised a ROS-responsive hybrid nanoprobe (PPB NP) for dual CT/PA imaging by incorporating small AuNPs and polyphosphazene derivatives (PPB) nanogels [159]. Under ROS conditions, PPB NP selectively degraded, triggering a 73% reduction in PA signals, with CT signals remaining stable. This contrast between stable CT and diminishing PA signals effectively distinguished ROS-overproducing macrophages from non-inflamed ones, enabling the detection of endogenous ROS in inflamed macrophages [159]. Similarly, Dai et al. developed a type of NPs based on Gd-doped Prussian blue (GPB) for MRI/fluorescence dual-modality imaging, offering complementary information and improving the accuracy and sensitivity of AS plaque detection [160].
Beyond the combination of two imaging modalities, more imaging agents can also be utilized for accurate diagnosis. For instance, Gong et al. constructed a nanozyme-based ratio-metric nanoprobe (FeWOX NS) by co-loading 3,3,5,5-tetramethylbenzidine (TMB) and IR780 dye on FeWOX nanosheets (NSs) for PA/MRI/CT imaging of H2O2-related inflammation [128]. FeWOX NS exhibited high PA sensitivity (LOD: 0.5 µM) for H2O2 detection and the ratio-metric PA signal could distinguish the different levels of H2O2 in tumor and inflammation tissues. FeWOX NS also served as CT and MRI nanoprobes due to their high X-ray and MR contrast abilities, surpassing commercial iodine and Gd-based agents, respectively. Importantly, owing to their inherent biodegradability, FeWOX NSs could be cleared out from the body without any significant biotoxicity [128].
Summary
In summary, nanosensors and nanoprobes have played crucial roles in early inflammation detection and precise monitoring. Nanosensors have emerged with the benefits of low cost, high efficiency, sensitivity and specificity. Some of them have entered clinical trials, such as a nanosensor array for multiple sclerosis diagnosis (NCT04074629) [3]. Alongside accuracy and sensitivity, POCT platforms also emerge as a potential solution to meet the need for affordable nano biosensors with rapid tests and user-friendly panels.
Although biosensors can identify the presence of inflammation, they do not offer information about the exact location. Imaging methods show their advantages in visualizing tissue at both the structural and functional levels. In recent years, many nanoprobes have been developed for inflammation imaging and some of them are summarized in Table 2. Nanoprobes hold great promise in enhancing imaging modalities for clinical imaging, including US, CT, RI and MRI. In US, nanoprobes enhance acoustic signals for improved visualization of vascular structures. For CT, nanoprobes enhance X-ray attenuation, improving contrast in anatomical structures. In RI, nanoprobes act as effective tracers for precise detection of functional and molecular changes. In MRI, nanoprobes amplify magnetic properties, improving tissue and organ visibility. However, each imaging modality still has its own limitations, as shown in Figure 4. The choice depends on clinical requirements, the nature of information needed and considerations like radiation exposure and cost, collectively contributing to a comprehensive diagnostic toolkit.
Representative nanoprobes for imaging of inflammation in 2021-2024.
| Imaging modality | Nanoprobe | Inflammation | Route | Advantages | Disadvantages | Year | Refs. |
|---|---|---|---|---|---|---|---|
| FI | QMT-CBT | AD | I.V. | Enhanced fluorescence (AIE signal); Turn on and near-infrared imaging; Reduced autofluorescence interference | Limited stability and biocompatibility; Low clinical applicability | 2023 | [162 |
| Ir-CBM | Epilepsy | I.V. | Two-photon excitation; Ratiometric luminescence; Long-lived emission; High selectivity; Low cytotoxicity in vivo | Limited tissue penetration depth; Micellar environment restrictions | 2023 | [163] | |
| PCN-NP-HPZ | AS | I.V. | Simultaneous sensing and imaging of pH and phosphorylation; High-resolution images | Potential toxicity; Limited specificity | 2023 | [164] | |
| PAI | 1-PAIN | Liver inflammation | I.V. | Deep tissue penetration Real-time monitoring; High selectivity and low background; High biocompatibility | Relatively low PA signal; Endogenous •OH and H2S interference | 2022 | [104] |
| PA nanoagent | RA | I.V. | Deep tissue penetration High sensitivity and selectivity; Monitoring the therapeutic process; Enhance the PA conversion efficiency | Potential toxicity; Interference from other factors; Potential toxicity | 2024 | [165] | |
| L-CRP | AS | I.V. | High selectivity; Deep tissue penetration | Limited biodegradability | 2023 | [166] | |
| MRI | TMSN@PM | Inflammation | I.V. | High selectivity; Non-invasive imaging; Real-time monitoring | Potential toxicity; Limited resolution and contrast | 2022 | [167 |
| CT | PIDA nanofibers | IBD | Oral | Good compliance; Reduced scan time (within 2 h); Theranostics | Invasiveness; Limited penetration depth; Low spatial resolution | 2023 | [168] |
| Exitron nano 12000 | Abdominal aortic aneurysm | I.V. | Quantification of inflammation; Improved targeting and specificity | Invasiveness; Limited resolution and contrast | 2021 | [138] | |
| FI & PAI | QY-SN-H2O2 | IBD | Oral | Good compliance; High-resolution; Deep-penetration; ROS-responsiveness; Non-invasiveness | Limited stability and biocompatibilityy; Potential toxicity | 2022 | [169] |
| FI & PAI | MPN@CeOx | UC | Oral | Good compliance; ROS-responsiveness; Intestinal inflammation accumulation; Deep-penetration; | Potential toxicity (metal components) | 2023 | [170] |
| CT & MRI | BM@EP | UC | Oral | Good compliance; Colon-targeted delivery and controlled release; Quantitative and dynamic imaging; Improved accuracy and sensitivity | Limited stability and biocompatibility; Complexity for the synthesis | 2022 | [171] |
Nanoprobes also show promise in preclinical studies for FI and PAI that can provide high-resolution and real-time images, although not yet applied clinically. Firstly, NPs offer advantages over clinical organic dyes such as ICG and MB, with higher stability, lower toxicity, and increased sensitivity and selectivity [161]. In PAI, NPs also serve as contrast agents, delivering real-time images with deep tissue penetration. They can target inflammatory cells or detect changes in inflammatory biomarkers, such as excessive oxidative stress levels, for imaging inflammation. Finally, FI or PAI nanoprobes can integrate with other imaging modalities to provide complementary information and enhance the diagnostic accuracy. Despite these capabilities, challenges remain for clinical translation of nanoprobes in FI and PAI, including the imaging parameter optimization and standardization of imaging protocols. Therefore, further research and development are essential to overcome these challenges and to realize the full potential of FI and PAI for inflammation diagnosis in the clinic.
Anti-inflammatory nanotherapeutics
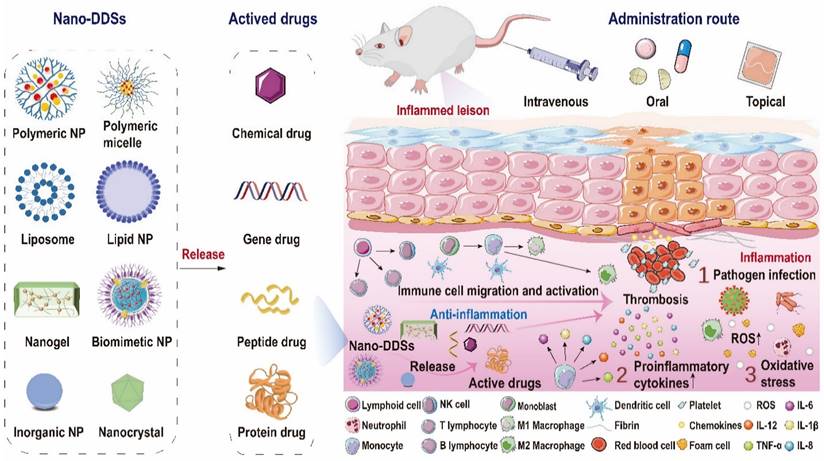
Over the decades, although multiple anti-inflammatory therapeutic agents have been widely developed and applied in the clinic, there are still several existing limitations for traditional DDSs including off-target biodistribution in the body, potential safety concerns of virus vectors and rapid clearance from the blood. Consequently, ongoing efforts have been put forward to develop novel DDSs to achieve satisfactory therapeutic outcomes [172]. Among them, nano-DDSs, characterized by inherent advantages such as site-specific drug delivery [55, 173], favorable bioavailability [174, 175] and time-controlled drug release [176, 177], have emerged as a promising therapeutic platform for the treatment of inflammation-related diseases (Figure 6). In this section, several emerging nano-DDSs for delivering chemical, gene and protein drugs as well as self-therapeutic NPs are mainly discussed.
Chemical drug nanotherapeutics
Recently, advanced nano-DDSs including lipid-based NPs, polymeric NPs, polymeric nanomicelles, nanogels, biomimetic nanomedicines and other nanoformulations, have opened up novel possibilities for improving the effectiveness of anti-inflammatory treatment while minimizing adverse effects [43, 178, 179]. A comprehensive summary of recent nanotherapeutics based on small-molecule chemical drugs is provided in Table 3.
Lipid-based NPs (LNPs) have long been employed as promising nano-vehicles for the delivery of therapeutic agents [180]. Comprising lipids such as phospholipids or solid lipids, LNPs demonstrate outstanding biocompatibility and stability [181]. LNPs enable the encapsulation of hydrophobic drugs within lipid bilayers and solubilization of hydrophilic drugs in aqueous cores [180]. Currently, significant attention is also focused on designing targeted nanomedicine by grafting specific ligands, such as peptides, onto the surface of LNPs to achieve active targeting [180]. For instance, Wu et al. devised peptides coupled celastrol (CLT)-phospholipid LNPs (PC-PLNs, 114.0 nm, 11.9 mV) to efficiently deliver CLT, a natural anti-inflammatory compound, to damaged endothelial cells and podocytes in the glomerulus for chronic kidney disease (CKD) treatment [182]. The PC-PLNs were prepared by self-assembly of CLT, phospholipids and a peptide (GLP) that can specifically bind to the glomerular basement membrane and facilitate the transcytosis of the NPs across the endothelial cells [182]. PC-PLNs demonstrated a robust therapeutic effect by selectively releasing CLT to the podocytes, which are the main target cells for CKD treatment, leading to inflammation reduction via nitric oxide upregulation and vascular cell adhesion molecule-1 (VCAM-1) expression inhibition. In vivo, PC-PLN treatment resulted in significant amelioration of CKD progression as well as reduced endothelial damage and CLT toxicity in a rat model of CKD induced by adenine [182].
Polymeric nanomedicines (PNs) also form a significant category of nano-DDS, including both dendrimers and polymer-drug conjugate [183]. PNs, characterized by their notable attributes of superior drug loading capacity, precise targeting to specific sites and regulated drug release, are emerging as an alternative approach for managing inflammatory diseases [184]. Recently, Shen et al. constructed a polymer-based nano-DDS (PPP-ACPP, 243 nm, 1.0 eV) to load the clinical anti-inflammatory drug etanercept (ET) for the treatment of spinal cord injury [54].
Representative small-molecule chemical drug-based nanotherapeutics in 2021-2023.
| Platform | NP sizes (nm) | Zeta Potential (mV) | Drug | Route | Animal models | Mechanism | Year | Refs. |
|---|---|---|---|---|---|---|---|---|
| Polymeric NPs | TEM: 91.4 | -17.4 | Cinnamaldehyde | I.V. | CIA mouse model and Colitis mouse model | Scavenge ROS; Suppress NF-κB signal pathway | 2023 | [199] |
| TEM: 171.7 | -22.3 | Magnolol | Oral | DSS-induced colitis mouse model | Scavenge ROS; Suppress NF-κB and signal pathway; Modulate gut health | 2023 | [72] | |
| DLS: 117.0 | -18.6 | Ginsenoside Rh2 | Oral | DSS-induced colitis mouse model | Scavenge ROS; Suppress STAT3/miR-214 signal pathway | 2022 | [73] | |
| Micelles | DLS: 190 | -50.0 | Rapamycin | I.V. | Atherosclerotic mouse mode | Scavenge ROS; Suppress pro-inflammatory factors | 2023 | [199] |
| DLS: 88.1 | -21.3 | Curcumin | Oral | DSS-induced colitis mouse model | Suppress pro-inflammatory factors | 2021 | [201] | |
| Nanogels | DLS: 124.2 | -19.2 | Phenytoin | I.V. | Status epilepsy rat model | Scavenge ROS | 2023 | [202] |
| / | / | Losmapimod | Topical | Diabetic wound mouse model | Scavenge ROS; Enhance M2-type macrophage polarization; Suppress pro-inflammatory factors | 2022 | [203] | |
| / | / | Psoralen | Intra-articular injection | CIA mouse model | Improve bone homeostasis; Regulate metabolism; Suppress pro-inflammatory factors | 2023 | [204] | |
| Liposomes | DLS: 85.6 | -6.0 | Celastrol | I.V. | Imiquimod-induced psoriasis mouse model | Inhibit maturation of DCs; Suppress pro-inflammatory factors | 2022 | [205] |
| Biomimetic NPs | DLS: 175.0 | -20.0 | Dexamethasone | I.V. | Endotoxin-induced lung inflammation murine model | Suppress pro-inflammatory factors | 2021 | [206] |
| TEM: ~100 | / | Indomethacin | Topical | CIA murine model | Suppress pro-inflammatory factors; Inhibit cyclooxygenase-2 | 2023 | [207] |
Schematic representation of the complex inflammatory microenvironment and the therapeutic nano-DDSs employed for the regulation of inflammation. The inflammatory microenvironment encompasses invasive pathogens, impaired cells, infiltrating immune cells and a plethora of pro-inflammatory molecules, with elevated levels of oxidative stress and intravascular thrombosis sometimes. Versatile nano-DDSs can be applied to deliver chemical, gene, peptide or protein drugs to the targeted sites to achieve satisfactory therapeutic outcomes.
PPP-ACPP was composed of a biocompatible polymer (PLGA-PEI-mPEG, or PPP) and an MMP-responsive molecule (activated cell-penetrating peptides, or ACPP), which could enhance the penetration of the NPs across the blood spinal cord barrier and the accumulation at the injured site. In vivo, ET@PPP-ACPP NPs accumulated at the lesion tissue and targeted inflammatory sites due to the activation of cell-penetrating ACPP by MMP-2 and MMP-9. Notably, ET within these particles moderated macrophage polarization from M1 to M2 phenotype, significantly reducing inflammation in the spinal cord injury mouse model, thereby protecting neurons and enhancing locomotor recovery. Such an activated target-based nanocarrier demonstrated considerable potential in improving the delivery efficiency of anti-inflammatory drugs [54].
Polymeric nanomicelles (PNMs) have attracted attention due to their cost-effectiveness, ease of preparation and reduced side effects [185]. PNMs consist of two functional components: a hydrophobic inner core and a hydrophilic outer shell [186]. The inner core is responsible for encapsulating hydrophobic drugs and maintaining the stability of nanomicelles. Additionally, the outer shell improves pharmacokinetic properties of nanodrugs, such as extended circulation time [185]. For example, Akshay Vyawahare et al. developed 9-aminoacridine (9AA)-encapsulated nanomicelles (9AA-NM, 190.0 nm, -20.6 mV) for the treatment of RA [187]. First, a promising amphiphilic block copolymer, methoxy polyethylene glycol polycaprolactone block copolymer (mPEG-b-PCL) was synthesized and then conjugated with the hydrophilic caffeic acid (CA). The 9AA drug, an FDA-approved anti-inflammatory drug, was eventually incorporated into the PNMs to activate the nuclear receptor subfamily 4 group A member (NR4A1), which has anti-inflammatory and protective effects in RA. Unlike nano-DDS with poor efficacy, the synthesized nanomicelles exhibited good biocompatibility and low toxicity, as they did not cause any adverse effects on the liver, kidney, or blood of the mice. In a rat model of RA, rats treated with 9AA-NMs demonstrated alleviated arthritic symptoms, along with a reduction in RA-associated inflammation [187].
Nanogels have a physically three-dimensional (3D) hydrogel structure with a particle size ranging from 20 to 250 nm [188]. Unlike conventional NPs, nanogel-based nano-vectors can form size-switchable 3D hydrogel networks by incorporating surfactants such as Tween®, Span®, polysorbic acid and sodium cholate into nanogels, providing a versatile platform for drug encapsulation and release due to their high water content and porosity of nanogels [189]. For example, triamcinolone acetonide (TAC) has fast clearance and adverse effects via intra-articular injection or oral administration [190]. To provide long-term effective therapy, Seo et al. developed injectable triamcinolone acetonide (TCA)-encapsulated polymeric hydrogel NPs (TePNs, -4.0 mV) for the sustainable and effective treatment of OA [190] (Figure 7). Polymeric NPs were successfully loaded with TCA via the interactions between the hydrophobic segments of the amphiphilic polymer and the hydrophobic TCA. Upon intra-articular administration at body temperature, the NPs transformed into a 3D hydrogel structure. In vitro, TePNs achieved long-term release for 6 weeks by inhibiting the expression of MMP. In vivo, the TePNs exhibited sustained anti-inflammatory effects without any skin irritation or systemic adverse effects in an early stage of rat model of OA [190].
Biomimetic NPs are a nascent category within the field of nanomedicine by emulating the properties and behaviors of native cells or exosomes, thereby enhancing biocompatibility and targeted drug delivery of NPs [191-193]. Among them, the red blood cell (RBC) membrane could help NPs evade the phagocytic system and achieve a prolonged circulation half-life in the circulation (about 120 days), which has gained attention for the development of nanocarriers [191, 194]. Wang et al. employed RBC membrane-coated biomimetic rapamycin-loaded PLGA nanocomplexes (97.4 nm, -28.7 mV) for AS treatment. The application of RBC membranes led to reduced macrophage-mediated phagocytosis in vivo, increased nanoparticle accumulation at atherosclerotic plaques, and decelerated AS progression [195]. Inflammatory effector cells, which exert substantial influence on the initiation and advancement of diverse inflammatory diseases, have been harnessed as biomimetic carriers for administering small-molecule pharmaceutical agents [196]. For instance, Song et al. synthesized macrophage cell membrane-camouflaged NPs (M-EC, 210.0 nm) containing epigallocatechin gallate (EGCG) and Ce4+ [197]. The macrophage cell membranes were beneficial to enhance the biocompatibility, immune evasion and targeting ability of the NPs to inflamed joints. In a mouse model of collagen-induced arthritis (CIA), M-EC resembling natural antioxidant enzyme sites, showed significant therapeutic effects by reducing the levels of pro-inflammatory cytokines, oxidative stress and cartilage erosion in the joints [197]. Combining anti-inflammatory and immune-evasive properties of M2 macrophages, the biocompatibility and long circulation of erythrocytes may produce desirable therapeutic efficacy. Most recently, Chen et al. developed a novel type of biomimetic nanosized liposome (USM[H]L, 139.2 nm, -13.1 mV) that was coated with hybrid membranes derived from M2 macrophages and erythrocytes [198]. USM[H]L were loaded with uricase and methotrexate, which could synergistically degrade uric acid, scavenge H2O2, produce photothermal effects and modulate immune responses. Upon being injected into a rat model of gouty arthritis (GA), USM[H]L showed enhanced therapeutic effects by reducing uric acid levels, ankle swelling, claw curling, inflammatory cytokines and oxidative stress, while increasing anti-inflammatory cytokines and reprogramming M1 macrophages to M2 phenotype. Notably, USM[H]L also exhibited low immunogenicity and toxicity, as well as high stability and targeting ability, compared to free uricase or other formulations, representing a promising strategy for the treatment of GA and other inflammatory-related diseases [198].
A one-time intra-articular injection of the TePN hydrogel system for long-term OA treatment. First, water-insoluble TCA was loaded into the hydrophobic core of PN by hydrophobic self-assembly, forming TePNs. Upon intra-articular administration at body temperature, the NPs formed into a 3D hydrogel structure and released TePNs for several months. TCA reduced the levels of MMP and pro-inflammatory cytokines, and increased those of anti-inflammatory cytokines in cartilages, treating OA effectively and safely in vitro and in vivo. Adapted with permission, from [190] Copyright 2022 Elsevier.
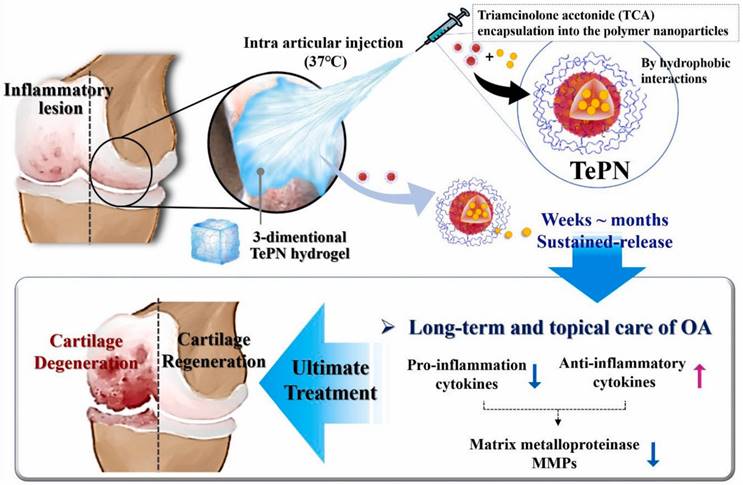
Gene drug nanotherapeutics
The era of gene therapy has emerged with the rapid development of genomics and gene technology. Gene therapy, compared to chemical drugs, may offer a more target-specific and safe therapy by inhibiting gene expression or cleaving abnormal messenger RNA (mRNA) [208]. Gene drugs mainly consist of small interfering RNA (siRNA), mRNA and plasmid DNA (pDNA). However, naked gene drugs are highly susceptible to degradation by nucleases [209].
Virus vectors are effective for delivering genes into cells, but they also have several disadvantages, including safety concerns, immune responses, complex preparation and limited drug loading capabilities. Consequently, there is an urgent need for suitable carriers to protect gene drugs and overcome these challenges. In the case of non-viral vehicles, such as cationic LNPs, cationic polymers and other NPs have been extensively explored for gene-based nanomedicines. These carriers present a myriad of advantages, including sample preparation, high safety, easy modification and superior stability, making them ideal for gene therapy [210].
As a type of cationic LNPs, cationic liposomes present a versatile nano-DDS for gene delivery due to their capability to bind to negatively charged nucleic acids [211]. Notably, multiple reports have demonstrated that cationic liposomes can fuse with cellular plasma membranes, facilitating the direct release of cargos into the cytoplasm and thereby improving the speed and efficiency of drug delivery [212]. Furthermore, the downregulation of pro-inflammatory cytokines, particularly TNF-α, may assume a pivotal role in inflammation treatment [213]. Recently, LNPs loaded with either siRNA or mRNA have emerged as a promising strategy for modulating the immune system and treating inflammation. For example, Guo et al. developed a ROS-responsive cationic liposome (ZnDPA-R, ∼100 nm) to deliver TNF-α siRNA for colitis treatment by taking thioketal as a linker to bind the hydrophobic tails and the metal complex headgroup [213]. The targeted liposomes were prepared by spraying the organic phase containing the lipid and the siRNA onto the aqueous phase containing the edge activators, which could overcome the limitations of conventional therapies for colitis, such as low bioavailability, poor specificity, and severe side effects. In vivo, ZnDPA-R could accumulate in the intestine, respond to the high ROS level by breaking the thioketal bond, silence the expression of TNF-α and related inflammatory factors, as well as alleviate the inflammatory symptoms, ultimately ameliorating colitis symptoms in a mouse model of colitis [213].
In addition to enhancing intracellular delivery via cell membrane fusion, LNPs can also be modified with various molecules to target specific tissues or cells with gene drugs [214]. For instance, Tao et al. rationally designed S2P-conjugated lipid-based NPs (S2P50-siCamk2g NPs) to specifically deliver siRNA to atherosclerotic lesional macrophages [214]. S2P50-siCamk2g NPs were prepared by encapsulating siRNA/cationic lipid complexes into PLGA polymer, followed by coating DSPE-PEG and plaque macrophage-targeting peptides (S2P) on the surface. By integrating targeting peptides onto their surface, S2P50-siCamk2g NPs demonstrated targeting capability in both cultured macrophages and in vivo. Owing to their unique attributes, such as prolonged circulation, selective targeting, superior plaque permeation and effective gene silencing, S2P50-siCamk2g NPs played an important role in silencing plaque-destabilizing gene (CaMKII) expression in macrophages as well as promoting plaque stabilization both in vitro and in vivo [214].
Similarly, Ralvenius et al. developed a novel microglia LNP (MG-LNP, 60-80 nm) to efficiently deliver RNA to microglia for treating neuroinflammation and neurodegeneration [215]. PU.1 is a potential therapeutic target for reducing neuroinflammation in AD and other neurological disorders. Therefore, reducing PU.1 expression in microglia may have beneficial effects on reducing neuroinflammation and improving cognitive function in AD. MG-LNP was optimized for microglia delivery by screening different lipid compositions and ratios (DLin-MC3-DMA: 1,2-distearoyl-sn-glycero-3-phosphocholine: cholesterol: PEG = 50:10:38.5:1.5). Instead of systemic injection, MG-LNP containing anti-PU.1 siRNA exhibited low toxicity and enhanced delivery to activated microglia via a local intracerebroventricular injection into the cerebrospinal fluid, which bypassed the BBB. In vivo, MG-LNP-mediated delivery of anti-PU.1 siRNA successfully exhibited efficiency at transfecting microglia and reduced PU.1 expression in a mouse model of neuroinflammatory conditions, offering a promising avenue for targeted gene therapies against neuroinflammation [215].
Cationic lipid-like materials are also useful in nanomedicine because they can enhance the delivery and expression of mRNA in target cells [216]. Gao et al. developed a novel mRNA nanomedicine (IL-10 mRNA@M-HNPs, 122 nm, 2.33 mV) that selectively delivered IL-10 mRNA to macrophages in AS plaques [216]. Targeted nanocarrier consisted of a cationic lipid-like material and a mannose-coated PLGA-PEG that bound to the mannose receptor (CD206) on the surface of macrophages, enhancing the uptake and accumulation of the mRNA nanomedicine in the plaques. The nanomedicine induced the expression of IL-10 in macrophages, promoting their polarization to the M2 anti-inflammatory phenotype. This created a positive feedback loop that modulated plaque inflammation by blocking several pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 and slowed down AS. The nanomedicine also protected the mRNA from degradation and immunogenicity issues. In a western diet-fed Ldlr-/- mouse model, the outcomes demonstrated that the selective delivery of IL-10 mRNA to macrophage-rich plaques and induced IL-10 played important roles in reducing AS [216].
Functional polymers, characterized by diverse chemical components and topological structures, are being increasingly utilized for the intracellular delivery of nucleic acids [217]. Among them, cationic polymers are emerging as ideal nucleic acid drug delivery carriers due to their low immunogenicity and easy structure modification [209]. As shown in Figure 8, Jeon et al. developed a potent siRNA-based therapeutic approach for the treatment of inflamed lungs by targeting silencing of TNF-α [218]. The synthesized cationic polymer, poly (oxanorbornene imide)-guanidinium (PONI-Guan), exhibited the capacity to spontaneously assemble into well-defined nanoscale polyplexes (∼170 nm) in conjunction with siRNA. Notably, this approach achieved efficient silencing (>70%) by using significantly lower siRNA dosages (0.14-0.28 mg/kg) in contrast to those used in current clinical studies, thereby mitigating the risk of off-target effects. Furthermore, the inherent modularity of the PONI system enabled prospective modifications to target additional organs. In vivo, PONI-Guan/siRNA polyplexes with a minimal siRNA dosage (0.28 mg/kg), achieved a threefold increase in pulmonary accumulation compared to control mice in an LPS mouse model, resulting in an effective knockdown of serum TNF-α (>80%) [218].
Although classical cationic lipids or cationic polymers enable nucleic acids delivery with high efficiency, they are still suffering from cytotoxicity or immunogenicity [219]. Therefore, there is a need for novel noncationic carriers that can deliver nucleic acids safely, efficiently and specifically to the diseased sites. For instance, Bai et al. invented a novel three-dimensional spherical noncationic nucleic acid nanostructure (miR-146a-SPIONs, 72.7 nm, -8.2 mV), consisting of a PEG-coated SPION NP core and a nucleic acid shell containing phosphorothioate (PS)-modified microRNA-146a for treating AS [219]. Such a nanocarrier demonstrated several advantages. Firstly, miR-146a-SPIONs were non-ionic and did not cause cytotoxicity or immune response. Next, miR-146a-SPIONs naturally targeted the class A scavenger receptor (SR-A) in the atherosclerotic plaque in vivo, improving the drug delivery efficiency. Furthermore, miR-146a-SPIONs entered the cell without a transfection agent, released microRNA-146a and suppressed the NF-κB signaling pathway, reducing inflammation and stabilizing the plaques. In a mouse model of apolipoprotein E knockout (ApoE-/-), repeated injections of miR-146a-SPIONs showed improved delivery efficiency, reduced plaque size and stabilized plaque morphology without causing severe toxicity, providing a safe and effective method for AS treatment [219].
The PONI-Guan/siRNA polyplex self-assembly and its membrane fusion-like siRNA delivery mechanism. Upon systemic administration of PONI-Guan/siRNA polyplexes, the nanocomplexes were preferentially distributed to inflamed lungs with priority and exhibited potent anti-inflammatory activity via efficient TNF-α knockdown by siRNA. Adapted with permission, from [218] Copyright 2023 American Chemical Society.
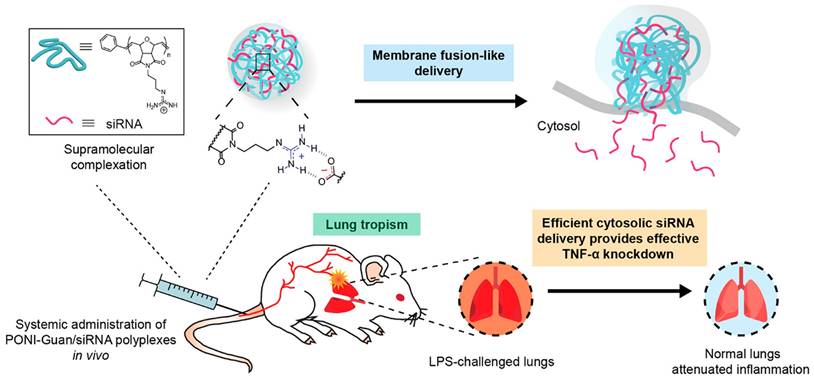
Protein or peptide drug nanotherapeutics
Therapeutic proteins and peptide drugs represent new frontiers of drug research and development, as they show better target affinity and safety than chemical drugs [74, 220]. However, their clinical efficacy of them is often hindered by several drawbacks, including high plasma clearance rates, short circulation duration, instability, and side effects resulting from frequent administration [221]. Thus, the utilization of nanotechnology and pharmaceutical technology to create long-acting, stable and effective formulations holds substantial clinical value [222].
Cytokines, immune cell-secreted proteins acting as intercellular messengers, are crucial for normal physiological functions and serve as therapeutic targets [223]. For instance, IL10 has protective properties against atherosclerotic inflammation, but suffers from rapid in vivo clearance by proteases [224]. To overcome the constraint, Kim et al. employed a cyclic arginine-glycine-aspartic acid (cRGD)-conjugated pluronic-based nano-carrier (NC) to encapsulate IL10 and iron oxide NPs for targeted delivery to AS lesions [224]. The therapeutic IL-10 was deftly loaded into NC in relatively mild conditions and enabled sustained release, showing equivalent efficacy to free IL10 in inhibiting the generation of pro-inflammatory cytokines and ROS in vitro. Of note, the IL10-loaded NC showed vastly improved circulation time, drastically high accumulation at targeted sites and significantly reduced atherosclerotic plaque regression in an apolipoprotein E-knockout mouse model [224].
Recently, cytokine-conjugated antibodies have shown promise in targeting and selectively accumulating at chronic inflammatory sites, achieving considerable progress in anti-inflammation therapy [225]. However, the premature degradation of cytokine-conjugated antibodies remains a formidable challenge. To overcome this obstacle, Liu et al. devised an immunological nanonut, termed anti-TNF-α antibody-sheltered immunological nanonut (AINUT, ~200 nm, ~-20 mV) to mitigate RA progression [225] (Figure 9). AINUT, constructed with zeolitic imidazolate framework-8 (ZIF8) encapsulating the anti-TNF-α antibody (TNFi), was enveloped in a nanocarrier derived from anti-inflammatory macrophages (M2NVs). The nut-like structure preserved the structural integrity and biological functionality of TNFi, even in the presence of proteases. In addition, M2NVs modulated the immune microenvironment by redirecting inflammatory macrophages to anti-inflammatory pathways. Targeted delivery and dose reduction are the main methods to reduce the toxic side effects of AINUT. In vivo, AINUTs preferentially accumulated in acidic RA joints, enabling the sustained release of TNFi to neutralize TNF-α, thereby potently alleviating RA symptoms in a mouse model [225].
The fabrication and anti-inflammatory mechanism of AINUT. AINUT, a nanocomposite constructed with ZIF8 encapsulating the anti-TNF-α antibody TNFi, was enveloped in a nanocarrier derived from anti-inflammatory macrophages (M2NVs). The zeolitic imidazolate framework preserved the structural integrity and biological functionality of TNFi, even in the presence of proteases. In addition, the M2NV reduced the inflammatory response of the immune cells and attenuated toxic side effects. Adapted with permission, from [225] Copyright 2023 Elsevier.
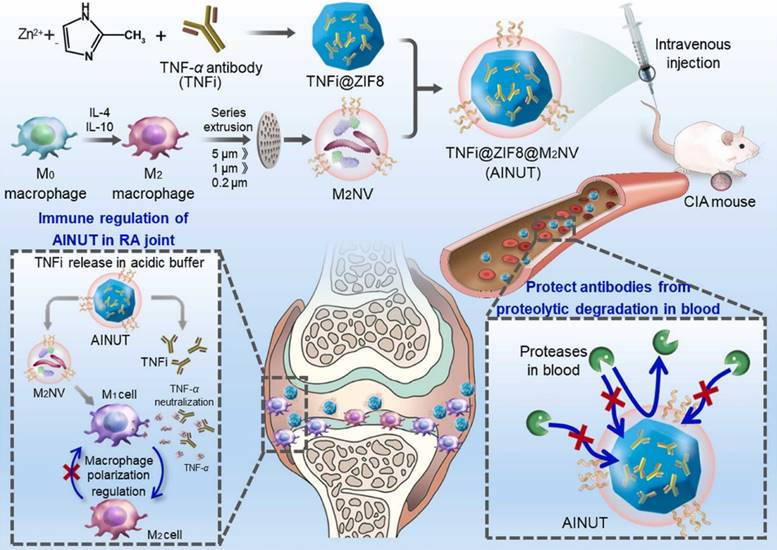
In addition to the protective cytokine, some antioxidant enzymes also play essential roles in inflammatory diseases [226, 227]. The free radicals and other oxidants, which are critical yet damaging factors in the pathogenesis of inflammation, can be neutralized by these antioxidant enzymes [228]. Notably, superoxide dismutase (SOD), as an antioxidant and anti-inflammatory enzyme, not only transforms superoxide into H2O2 and oxygen but also influences immune responses [228]. To enrich SOD at inflammatory lesions without degrading protein drugs, Sun et al. synthesized several pH-sensitive copolymer-based nanomicelles to deliver SOD in a rat peritonitis model [227]. Among them, nanomicelles composed of mPEG-poly (cyclohexane-1,4-dial acetone dimethyleneketal) (mPEG-PCADK) exhibited the most favorable properties, including high encapsulation efficiency, long blood circulation and accelerated acidolysis under acidic conditions. In vivo, mPEG-PCADK nanomicelles significantly suppressed leukocyte proliferation, providing a potent nano-carrier for the delivery of unstable protein drugs [227].
Peptide-based drugs have also garnered substantial attention due to several unparalleled advantages including quick synthesis and limited side effects [229, 230]. Moreover, most therapeutic peptides can selectively target pathological lesions and regulate signaling pathways both in vitro and in vivo. However, the high polarity and small size of peptides generally lead to poor pharmacokinetics and availability [231]. Formulating peptides into NPs offers a potential solution to these drawbacks. For instance, Keum et al. designed biomimetic disc-shaped lipid NPs encapsulated with cell-penetrating 9-arginine-modified peptides (APTstat3-9R@DLNP, ∼20-30 nm) [232]. APTstat3-9R@DLNP demonstrated excellent colloidal stability over a period of two weeks, without any noticeable alteration in its hydrodynamic size. Additionally, the presence of biomimetic lipid components facilitated transmembrane permeation, resulting in enhanced penetration effects. In a mouse model of pulmonary fibrosis, APTstat3-9R@DLNPs effectively mitigated pulmonary inflammation via the inhibition of multiple inflammatory mediators and STAT3 activation. Such a therapeutic nanoparticle serves as a safe and efficient nanoplatform for delivering peptide drugs [232].
Self-therapeutic nanotherapeutics
Self-therapeutic nanomaterials are a type of nanomaterials that modulate inflammation without external drugs, acting as anti-inflammatory agents or interacting with the biological environment. Inorganic NPs such as Au NPs [233], Pt NPs [234] and CeO2 NPs [235] and organic nanomaterials such as hydrogel possess intrinsic therapeutic properties against inflammation [236]. Some nanomaterials show antioxidant-like property to mimic the functions of natural ntioxidant-like catalase (CAT), glutathione peroxidase (GPx) and SOD, which can hinder oxidation in inflamed tissues or cells by trapping free radicals or reducing oxidation initiation [237]. Notably, the self-therapeutic nanomaterials simplifies design complexity, eliminating concerns about drug encapsulation and release [71]. Consequently, they hold potential cost and translational advantages, requiring no extra chemical or biological components that might complicate large-scale production and purification [71].
AuNPs have emerged as fascinating candidates for self-therapeutic nanoplatforms with unique physicochemical properties, including cell targeting and cytokine expression regulation [233]. AuNPs can inhibit pro-inflammatory cytokines and their tunable properties for precise adjustment of therapeutic outcomes [233]. To study the impact of NPs' size and alkylation on efficacy, Han et al. proposed a series of alkyl-terminated Au NPs for the prevention and treatment of psoriasis [233]. Upon comparing different Au NPs, they found that Au NPs (Au3@PEG-octadecyl30%) with 3 nm gold cores modified with polyethylene glycol with 30% octadecyl chains could effectively penetrate into the epidermis and interact with keratinocytes. In contrast, unalkylated PEG-coated Au cores are >20 nm, hindering skin penetration. Additionally, 3 nm PEG-coated cores with >30 mol% octadecyl chains mildly aggregate to form ~35 nm clusters, limiting effective skin penetration. Furthermore, Au3@PEG-octadecyl30% worked by inhibiting key genes associated with the IL-17 signaling pathway, known for its connection to excessive skin cell growth and inflammation. Compared with commercial steroid and vitamin D analog treatments, Au3@PEG-octadecyl30% exhibited comparable efficacy with fewer side effects such as hair loss and skin wrinkling, providing a safe and efficient therapy for psoriasis [233]. Au NPs can also inhibit other inflammatory signaling pathways. For instance, Chan et al. introduced a sub-10-nm Au NP (~7 nm) coated with PEG and folic acid (FA) for kidney fibrosis [238]. The outcomes demonstrated that the Au NP was more effective in reducing tissue degeneration and inhibiting the p38α enzyme compared to standard Captopril therapy [238]. Shifting macrophage polarization from M1 to M2 plays key roles in resolving inflammation [239]. Lu et al. invented glutathione-protected gold nanoclusters (GA) for treating IBD [239]. Remarkably, GA exhibited targeted accumulation in the colon. GA not only facilitated M2 differentiation of IL-4-treated peritoneal macrophages but also shifted macrophage polarization from M1 to M2 in a pro-inflammatory setting [239].
Apart from common metal compounds, transition metal selenides such as ruthenium (Ru) has emerged as promising catalytic materials [237]. Ru is a transition metal element with multiple oxidation states, and the interconversion between Ru2+/Ru3+ redox pairs can be used to eliminate ROS [240]. For instance, Deng et al. introduced a cobalt selenide-based biocatalyst with an amorphous Ru@CoSe nanolayer (thickness: 2.5 nm, -25.8 mV) for rapid ROS elimination [237]. The nanolayer, created by doping Ru atoms onto CoSe nanorods through a solvothermal method and subsequent high-energy ball milling, offered enriched electrons and unoccupied orbitals from Ru, enabling outstanding pseudo-peroxidase kinetics. These Ru species served as “regulators,” adjusting Co site electron states and oxygen interactions, enhancing reversible redox properties at catalytic sites, outperforming many existing metal compound-based ROS biocatalysts [237]. In a diabetic mouse model with skin wounds, Ru@CoSe demonstrated superior ROS-scavenging capabilities in inflammatory diabetic wounds [237]. Additionally, Ru complexes have shown efficacy in neutralizing reactive nitrogen species (RNS) [240]. Wang et al. incorporated transition metal atoms (Ru and Ni) to create ultrathin trimetallic two-dimensional nanosheets (TMNSs, thickness: 2.4-4.8 nm, -21.5 mV) [241]. The modification facilitated RNS and ROS elimination by forming Ru-O and Ni-O bonds on the TMNSs' surface, effectively addressing chronic colitis inflammation [241].
Some metal oxide NPs have also been used as self-therapeutic agents, including iron oxide (Fe3O4) [242], CeO2 [234] and zinc oxide [243]. Among them, CeO2 is recognized for its antioxidative and ROS scavenging properties by switching between two oxidation states, Ce3+ and Ce4+ [71]. CeO2 NPs can mimic the functions of natural antioxidases, such as CAT and SOD, allowing the decomposition of ROS and achieving anti-inflammatory efficacy [71]. For instance, Min et al. developed a straightforward noble metal deposition strategy, depositing Au NPs, Ag NPs (5-10 nm) and Pt NPs on the surface of hollow mesoporous CeO2 nanospheres [234]. These hybrid structures, combining the multienzyme-like activities of CeO2 and ROS scavenging capacities of metal NPs, demonstrate high efficacy in IBD and ear inflammatory mice models via the downregulation IL-1β and TNF-α [234]. Among them, CeO2@Ag displayed exceptional therapeutic effectiveness at a dosage of 0.5 mg/kg [234].
Metal-organic frameworks (MOFs) integrated with nanozymes show outstanding antioxidant abilities, offering potential as anti-neuroinflammatory agents for treating inflammation. Jiang et al. deliberately chiral MOFs incorporated with nanozymes by incorporating ultra-small Pt nanozymes (Ptzymes, 6-8 nm) into L- and D-chiral imidazolate zeolite frameworks (Ptzyme@L-ZIF (82.2 nm, 25.1 mV) and Ptzyme@D-ZIF (70.4 nm, 18.4 mV)) through shell-ligand exchange reactions [244]. Both Ptzyme@L-ZIF and Ptzyme@D-ZIF showed strong SOD- and CAT-like activities. Compared to Ptzyme@L-ZIF, Ptzyme@D-ZIF exhibited increased accumulation in the brain lesions of Parkinson's disease (PD) mouse model due to its prolonged plasma retention time and diverse pathways across the BBB [244]. Furthermore, Ptzyme@D-ZIFs effectively inhibited neuroinflammation-induced apoptosis and ferroptosis, leading to superior therapeutic effects [244]. Additionally, Liu et al. developed a Ru-based metal-organic framework (RuMOF, 141.6 nm) as a nano-antioxidant for treating inflammation-related diseases [240]. Such a Ru-MOF system demonstrated excellent biocompatibility and potent catalytic activity, which eliminated excessive reactive oxygen and nitrogen species (RONS) and blocked TNF-related inflammatory pathways in both inflammation-related conditions (LPS-induced endotoxemia and dextran sulfate sodium (DDS)-induced colitis) [240].
Additionally, some organic nanomaterials also play important roles in fighting inflammation. For instance, hydrogel can hinder oxidation in inflamed tissues or cells by trapping free radicals or reducing oxidation initiation [236]. Moreover, hydrogel can also provide mechanical support and promote tissue regeneration by mimicking the extracellular matrix (ECM) [236]. Conley et al. developed a dynamic nanohybrid peptide hydrogel (NHPH) to modulate the inflammatory and inhibitory microenvironment of the intervertebral disc (IVD) and promote its repair and regeneration [236]. The NHPH, formed through self-assembly of peptide amphiphiles with biodegradable 2D nanomaterials, exhibited desirable enzyme-like functions. Notably, NHPH could scavenge ROS, remodel the ECM and provide sustained delivery of growth and differentiation factors. In a rat nucleotomy model, the NHPH demonstrated improved nucleus pulposus cell differentiation and inflammation reduction, suggesting potential for treating IVD degeneration and fibrocartilaginous injuries [236].
Despite their potential, self-therapeutic nanomaterials encounter challenges including toxicity, immunogenicity and biodistribution [245]. Addressing these issues requires further studies to comprehend the mechanisms of specific inflammation, optimize the synthesis and assess the safety tests for self-therapeutic nanomaterials.
Summary
In summary, nano-DDSs have significant advantages in enhancing drug efficacy and reducing side effects. For chemical drugs, nano-DDSs improve their solubility, bioavailability and stability, while minimizing side effects via targeted delivery. Gene drugs demonstrate high targeting and specificity, enabling the regulation of gene expression for challenging-to-treat diseases, such as autoimmune diseases and viral infections. However, they face challenges, such as membrane crossing, easy degradation and high immunogenicity. Encapsulating gene drugs into NPs facilitates intracellular release, improving the biological activity and safety. Protein or peptide drugs, known for their high biocompatibility and biological activity, can simulate or regulate physiological functions, but with stability concerns. Nano-DDSs serve as biocompatible vehicles, precisely delivering various protein or peptide agents to inflammatory areas for effective treatments. There are also self-therapeutic nanomaterials that can act as anti-inflammatory or antioxidant agents independently to treat inflammation, eliminating concerns about drug encapsulation and release.
Nano-DDSs, while offering advantages, also face challenges including complex preparation, high cost, uneven biological distribution, unclear clearance mechanisms and insufficient toxicity evaluation. For example, liposomes have good application potential, but the lipid bilayer structure is not stable, leading to hydrolysis and leakage during the transport of chemical or gene ingredients [246]. Biodegradable polymers such as PLGA are commonly used for RNA drug delivery, but the in vivo metabolism and toxicity after inhalation are often overlooked, which may lead to challenges for anti-inflammatory nanomedicine, especially in chronic conditions requiring repetitive administrations [247].
Despite the challenges, there has been continuous progress in developing nano-DDSs for anti-inflammatory treatments. To manage the rational design of novel nano-drugs, researchers need to explore various approaches, including: i) Identifying pathologic hallmarks or pathways in different inflammatory diseases, focusing on nanomaterials sensitive to biochemical signals like pH, ROS and enzymes at diseased sites. ii) Modifying the surface of NPs to enhance uptake by target cells and impart diverse biological functions. iii) Co-delivering multiple therapeutic agents with different targets to enhance biomedical performance. Additionally, from a translational perspective, achieving biodegradable, large-scale, high-throughput manufacturing processes for nano-DDSs is also crucial.
Combination strategies
With advances in medical treatment and technology, an increasing number of scientists are devoted to developing diagnostic and therapeutic agents with reduced treatment frequencies [248]. Theranostics, a term combining therapy and diagnostics, offering imaging-guided treatment and therapeutic effect monitoring, has gained significant attention. In addition, compared with single therapy modalities, combined therapies exhibit better therapeutic efficacy in inflammation therapy [249]. Recently, nanomaterials with appropriate size, outstanding targeting capabilities and controllable release, have become optimal carriers for co-delivering two or more components [5, 250, 251]. As depicted in Figure 10, in this section, we will introduce several representative nanocarriers for theranostics and combined therapy.
Theranostic nanosystem
Most inflammatory disorders are prevalent chronic diseases with clinical silence and delayed therapy [252]. Thus, timely detection and treatment of inflammation are imperative. Theranostic NPs, which provide both diagnostic and therapeutic functions in a single dose, have emerged as significant contributors to the biomedical field [253]. Recently, a series of nano-DDSs have been developed for inflammation theranostics [152, 254-256]. For example, Liu et al. created a peanut-shaped Janus nanoplatform named Janus cationic polymer-silica (Janus-CPS, ~100 nm), for the early theranostics of RA [257] (Figure 11). This Janus nanoplatform consisted of a CeO2-Pt nanozyme subunit on one side and a high-capacity periodic mesoporous organosilica (PMO) on the other. Notably, the Janus nanostructure enhanced ROS scavenging with increased active sites, showing augmented capabilities compared to core-shell NPs. Moreover, Janus-CPS loaded with ICG demonstrated remarkable NIR-II imaging capabilities, enabling early detection of RA in a CIA mouse model. Additionally, micheliolide (MCL), a plant-derived compound with anti-osteoclastogenesis effects, was also loaded to synergize anti-inflammatory properties for effective treatment of RA via macrophage polarization, ROS scavenging and antiosteoclast properties [257].
Schematic representation of multiple strategies for anti-inflammatory nanotheranostics and combination nanotherapeutics.
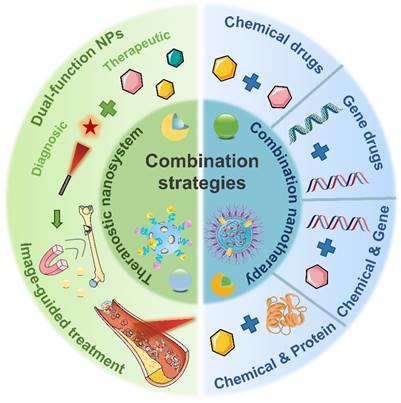
In addition to FL, MRI examination is also deemed as a potent tool for monitoring therapeutic effects via the measurement of relaxation times [79]. To visualize inflamed lesions, NPs have been extensively employed to enhance MRI signals [79]. For instance, Liu et al. developed dual-functional arginine (Arg) and Mn2+-doped polydopamine NPs (DAMM NPs, 229.1 nm) to load Dex for diagnosing and treating OA [79]. In vitro, DAMM NPs continuously released Dex effectively, inhibiting synovial inflammation and toll-like receptor 3 (TLR-3) production in chondrocytes and chondrocyte apoptosis through the TLR-3/NF-κB pathway. Additionally, DAMM NPs played a significant role in removing ROS generated in chondrocytes, thereby inhibiting ROS-induced M1 macrophage polarization and chondrocyte apoptosis. Notably, DAMM NPs, when chelated with Mn2+, exerted robust T1-T2 MRI contrast that enabled the potential tracking of nanoparticle retention at the injured cartilage. In vivo, DAMM NPs demonstrated enhanced MRI contrast capabilities and desirable performance in ameliorating OA development in the joint cavity of mice, enabling traceable delivery of nanomedicines [79].
The structure and theranostic mechanism of Janus-CPS. Janus-CPS was a peanut-shaped Janus nanoplatform with CeO2-Pt nanozymes on one side and PMO on the other side. The CeO2-Pt nanozymes could scavenge ROS and the PMO could load an anti-inflammatory drug to regulate inflammation. In vivo, Janus-CPS loaded with ICG, a NIR-II fluorophore, could visualize the inflamed joints and blood vessels in a CIA mouse model. Moreover, Janus-CPS loaded with MCL, an anti-osteoclast drug, could reduce the bone erosion and inflammation in the CIA mice by inhibiting the formation and activity of osteoclasts. Adapted with permission, from [257] Copyright 2023 American Chemical Society.
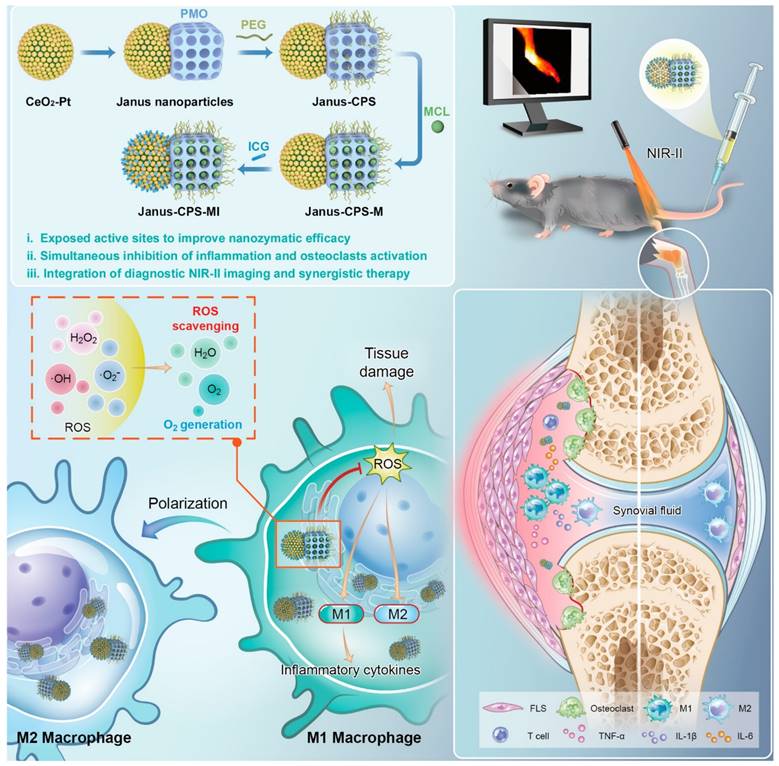
Combination nanotherapeutics
Notwithstanding significant advancements in inflammatory treatment, the effectiveness of monotherapy is limited. Combination nanotherapy for inflammation employs multifunctional nanosystems and a variety of therapeutic agents (such as chemical drugs, gene drugs and protein drugs) to amplify the effectiveness of anti-inflammatory interventions [258, 259].
The combination of two chemical drugs simultaneously via a nanocarrier has been demonstrated to enhance drug delivery, increase therapeutic effectiveness and mitigate dose escalation-induced toxicity [260]. For instance, ticagrelor is an antiplatelet agent that inhibits platelet aggregation, while simvastatin is a statin that curtails the polarization of M1-type macrophages and down-regulate inflammatory-associated cytokines [259]. To achieve the synergistic AS therapeutic effect, Zhao et al. developed a fibronectin-targeted nanoparticle to co-deliver simvastatin and ticagrelor [259]. Firstly, a ROS-responsive nanoprodrug (TPTS, 185 nm, -4 mV) was synthesized by conjugating an α-tocopherol polyethylene glycol derivative and simvastatin with a thioketal linker. Subsequently, CREKA-containing lipopeptides, ticagrelor and TPTS were co-assembled into NPs (TPTS/C/T) via hydrogen bonding interactions. With the assistance of CREKA peptides, TPTS/C/T could specifically target atherosclerotic lesions and release both ticagrelor and simvastatin in conditions of excessive ROS, fostering a synergistic anti-inflammatory effect. Compared with TPTS and free statin groups, TPTS/C/T exhibited superior anti-inflammatory efficacy and a better safety profile in an apolipoprotein E-deficient (ApoE-/-) mouse model, providing a novel avenue for synergistic treatment of inflammation [259]. Various chemical drugs-based combination strategies for anti-inflammatory nanotherapy are also explored in Table 4.
Aside from the suboptimal efficacy of mono-chemical drugs in treating inflammation, single-gene therapy, which primarily targets individual inflammatory pathways, is also insufficient for reversing the progression of inflammation [250, 265, 266]. Inflammation is typically regulated by intricate signaling pathways, such as NF-κB, MAPK and JAK/STAT [265]. Therefore, delivering different gene-drug combinations targeting separate pathways or multiple different inflammatory mediators effectively dampens the inflammatory cascade, leading to a more comprehensive and sustained therapeutic outcome. For example, the pathogenesis of immunoglobulin A nephropathy (IgAN) is associated with the pivotal involvement of the p38 MAPK and NF-κB signaling pathways. To achieve concurrent inhibition, Wang et al. reported a liposomal siRNA delivery system (2i@DuaLR, ~110 nm, 6.6 mV) to alleviate glomerulonephritis-related inflammation by simultaneously delivering p38α MAPK inhibitor and p65 siRNA in the glomerulus [267]. With the assistance of PEI, p65 siRNA and p38α MAPK siRNA were simultaneously encapsulated into a liposomal nanoparticle and further modified with PEG and octa-arginine. Owing to the suitable size and special charge reversal, 2i@DuaLR could achieve superior glomerulus targeting and selective accumulation at the targeted sites. More importantly, a mere 4 nmol/kg 2i@DuaLR was found to be as efficient as a high dosage of traditional anti-inflammatories in an IgAN mouse model, while maintaining low systemic toxicity. Such a strategy offers an effective therapeutic nanoplatform for treating glomerulus-related inflammation [267].
In addition to the aforementioned combination therapies, therapeutic gene agents can also synergize with chemical drugs to exert a potent anti-inflammatory response [5]. Among them, gene therapies are capable of modifying or silencing the expression of pro-inflammatory genes, while chemical drugs can inhibit the activity of inflammatory mediators. Concurrent use of gene-based and chemical drugs may have a synergistic effect, surpassing the sum of their contributions [5]. As shown in Figure 12, Lan et al. strategically engineered ROS-ultrasensitive mesoporous silica NPs (MSNs, ~200 nm, -18 mV) to simultaneously deliver RAGE siRNA (siRAGE) and Dex for the synergistic treatment of myocardial inflammation [5]. Upon the encapsulation of Dex within the MSN cavities, a PGE2-PEG-modified tellurium-crosslinked polyethyleneimine (PPTP) was employed to encapsulate the MSNs for preventing Dex pre-leakage and facilitating siRNA condensation.
Representative chemical drugs-based combination strategies for anti-inflammatory nanotherapeutics.
| Platform | Drug 1 | Drug 2 | Route | Animal models | Mechanism | Year | Refs. |
|---|---|---|---|---|---|---|---|
| Cationic polypeptide nanomicelles | Losmapimod | Tempo | Topical | Dry eye mouse model- | Scavenge ROS; Suppress MAPK signal pathway | 2022 | [261] |
| ROS-responsive prodrug nanomicelles | Dexamethasone | Artesunate | I.V. | Adjuvant-induced arthritis rat model | Scavenge ROS; Suppress HIF-1α/NF-κB signal pathway; Enhance M2-type macrophage polarization | 2022 | [262] |
| mPEG-b-PCL nanomicelles | 9-aminoacridine | Caffeic acid | I.V. | CIA rat model | Suppress HIF-1α/NF-κB signal pathway | 2022 | [187] |
| Self-assembled peptide hydrogel | 3,5-dihydroxybenzoic acid | Ibuprofen | I.V. | Endotoxin-induced uveitis rabbit model | Scavenge ROS; Suppress pro-inflammatory factors (e.g., IL-1β, IL-6 and TNF-α); Suppress NF-κB and JAK-STAT signal pathways | 2022 | [263] |
| Injectable polymeric aggregate-embodied copolymer hydrogel | Dexamethasone | Tempo | Intra-articular injection | RA mouse model | Scavenge RNS an ROS; Upregulate immune cells | 2021 | [264] |
The ROS-ultrasensitive MSNs to concurrently deliver siRAGE and Dex for the synergistic treatment of myocardial inflammation. The MSNs were coated with PPTP, which further complexed with siRAGE and suppressed the pre-leakage of Dex. Upon injection into a myocardial IR-injured rat model, the nanomedicine selectively targeted the inflamed cardiomyocytes, reduced myocardial infarct size, improved cardiac function and attenuated inflammation via RAGE silencing (72%) and a synergistic anti-inflammatory therapeutic effect. Adapted with permission, from [5] Copyright 2022 Springer Nature.
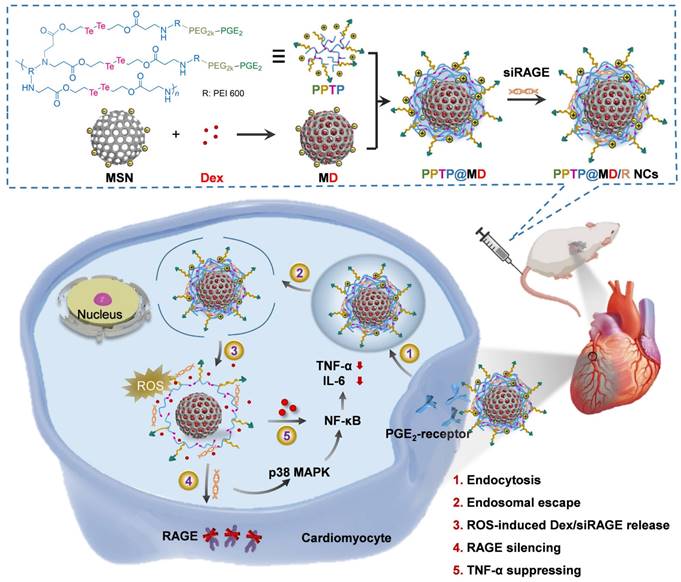
Intracellularly, PPTP underwent degradation into smaller segments in response to excessive ROS, thereby facilitating the release of siRAGE and Dex. This mechanism led to the achievement of efficient receptor for advanced glycation end-products (RAGE) silencing (72%) and a synergistic anti-inflammatory therapeutic effect. Upon intravenous administration, the nanomedicine selectively targeted the inflamed cardiomyocytes, reduced myocardial infarct size, improved cardiac function and attenuated inflammation in a myocardial ischemia-reperfusion (IR)-injured rat model [5].
Compared with chemical and gene drugs, protein drugs exhibit superior target affinity and safety. Nevertheless, relying solely on protein therapy might be insufficient for complex inflammatory responses [268]. The combination of protein drugs and chemical drugs could be a favorable strategy. For example, to sustain the mechanical function of the IVD and mitigate inflammatory reactions, Han et al. constructed a biomimetic core-shell nanofibrous scaffold to co-load ibuprofen (IBU) and transforming growth factor beta 3 (TGFβ3) by using electrospinning technology [269]. Specifically, hyaluronic acid (HA) hydrosol was employed to encapsulate TGFβ3 and then was transformed into an emulsion to load IBU by dispersing in a spinning formulation. During the process of electrospinning, HA micro-droplets within the emulsion were further encapsulated into Poly (L-lactic acid) (PLLA) fibers to attain a dual-drug co-loaded core-shell nanofibrous scaffold. The dual-drug delivery system was able to assemble into an angle-ply structure, forming a highly biomimetic annulus fibrosus (AF). In vivo, due to the rapid release of IBU and sustained release of TGFβ3, the inflammatory lesions were improved and a nascent ECM was formed. Notably, the excellent anti-inflammatory capabilities and biocompatibility of this biomimetic nanofibrous scaffold were demonstrated in two rat tail defect models, suggesting potential applications for IVD repair [269].
Summary
Based on the above-mentioned, various multifunctional nanoplatforms have been developed for inflammation thernostics and combination therapy. For theranostics, NPs play a crucial role in image-guided inflammation therapy, enabling real-time visualization of drug accumulation in inflamed sites and optimizing the dose of drug treatment. For combination therapy, co-encapsulating multiple bioactive anti-inflammatory agents in nano-DDSs enhances therapeutic efficacy, reduces drug resistance and dose-related toxicity, provides insights into pharmacokinetics and supports personalized medicine therapy. Given that each imaging and therapeutic modality has unique advantages and limitations, selecting an optimal combination and dose levels are crucial, as they can ensure enhanced therapeutic efficacy and reduced toxicity.
Conclusions and perspectives
This review focuses on the recent and notable advances of various nanomaterials in the diagnosis and treatment of inflammation. So far, there have been lots of nano-DDSs developed for inflammation diagnosis and therapy. Notably, some of the NPs have been approved by the FDA. For example, ferumoxytol (Feraheme®) is a SPIO NP (30 nm), which was approved by the FDA in 2009 for the treatment of iron deficiency anemia [270]. In addition, ferumoxytol is also explored as an off-label MRI contrast agent, particularly for the clear imaging of blood vessels [268]. For the treatment of inflammation, fenofibrate (Tricor®) is a nanocrystal product approved in 2004 for preventing AS, which show a 9% increase in oral bioavailability compared to micronized Tricor [271]. More recently, the rapid development of LNPs has facilitated the systematic siRNA and mRNA delivery, with the FDA approving for mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) to prevent COVID-19 [272].
Indeed, the clinical application of nanomaterials still faces challenges. First, safety stands as a fundamental criterion for clinical nanoformulations. For instance, the widely used biodegradable polymer PLGA may trigger inflammation in vivo due to its degradation byproducts and interactions with macrophages. Second, animal models of chronic inflammation often fall short of clinical standards. Most in vivo studies testing inflammation diagnosis and therapy rely on early-stage animal models, with limited involvement of larger animals or clinical trials. Finally, scaling up nanomedicine production encounters hurdles in manufacturing and controls (CMC) as well as regulatory requirements, which influence clinical translation.
Despite the challenges in clinical translation, nanomaterials still hold great promise for advancing the inflammation diagnosis and treatment. Thus, the optimization of nanomaterials is urgently needed. For diagnosis, targeting biomedical materials is essential to enhance concentration in the target organ for better resolution. Additionally, ensuring rapidly metabolizing doses or degradable formulations to avoid excessive nanomaterials accumulation in the body is also crucial. In terms of treatment, designing biochemical stimuli-DDSs based on the inflammatory types and sites could be helpful to minimize side effects and improve efficacy. Furthermore, considering the varying levels of oxidative stress and the different signaling pathways in different inflammation-related diseases and the various stages of inflammation, targeting multiple signaling pathways could be effective. Notably, it is very necessary to build clinically applicable in vivo studies to identify therapeutic efficiency and address the potential risks. Finally, optimizing the regulatory frameworks as well as ensuring the consistent manufacturing and toxicology assessments are vital for expediting the clinical research and approvals. For example, the regular calibration of nanosensors and nanoprobes is the guarantee for inflammation diagnosis accuracy. Additionally, the accurate and consistent compositions, sizes, synthesis methods, dosages and administration routes of nano-DDSs are also key to ensure the inflammation therapeutic efficacy.
In conclusion, recognizing and addressing the challenges in nano-DDSs for the diagnosis and treatment of inflammation is crucial for efficient clinical translations. In the future, collaborative efforts from diverse fields will be beneficial to advance nanomaterials from the lab to clinical applications, ultimately improving the life quality and saving more lives.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 82161138029), the Basic Research Projects of Liaoning Provincial Department of Education (LJKZZ20220109), and the Shenyang Youth Science and Technology Innovation Talents Program (No. RC210452).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309-324
2. Feehan K T, Gilroy D W. Is resolution the end of inflammation? Trends Mol Med. 2019;25:198-214
3. He R, Li L, Zhang T, Ding X, Xing Y, Zhu S. et al. Recent advances of nanotechnology application in autoimmune diseases-a bibliometric analysis. Nano Today. 2023;48:101694
4. Liu Z, Liu H, Cheng J, Wang H, Yang Y, Ye J. et al. Strategies and opportunities of micro/nano delivery systems for targeted therapy of ulcerative colitis: Focus on underlying mechanisms and future perspectives. Chinese Chem Lett. 2023;35:109074
5. Lan M, Hou M, Yan J, Deng Q, Zhao Z, Lv S. et al. Cardiomyocyte-targeted anti-inflammatory nanotherapeutics against myocardial ischemia reperfusion (IR) injury. Nano Res. 2022;15:1-10
6. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis—from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20:589-610
7. Lee Y S, Wollam J, Olefsky J M. An integrated view of immunometabolism. Cell. 2018;172:22-40
8. Kronsten V T, Tranah T H, Pariante C, Shawcross D L. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J Hepatol. 2022;76:665-680
9. Davidson S, Coles M, Thomas T, Kollias G, Ludewig B, Turley S. et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21:704-717
10. Black R J, Cross M, Haile L M, Culbreth G T, Steinmetz J D, Hagins H. et al. Global, regional, and national burden of rheumatoid arthritis, 1990-2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e594-e610
11. Figus F A, Piga M, Azzolin I, McConnell R, Iagnocco A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun Rev. 2021;20:102776
12. England B R, Thiele G M, Anderson D R, Mikuls T R. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018;361:k1036
13. Wang R, Li Z, Liu S, Zhang D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. J BMJ open. 2023;13:e065186
14. Doran A C. Inflammation resolution: Implications for atherosclerosis. Circ Res. 2022;130:130-148
15. Zhang S, Liu Y, Cao Y, Zhang S, Sun J, Wang Y. et al. Targeting the microenvironment of vulnerable atherosclerotic plaques: an emerging diagnosis and therapy strategy for atherosclerosis. Adv Mater. 2022;34:e2110660
16. Wright J D, Folsom A R, Coresh J, Sharrett A R, Couper D, Wagenknecht L E. et al. The ARIC (atherosclerosis risk in communities) study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77:2939-2959
17. Thomas D, McDonald V M, Pavord I D, Gibson P G. Asthma remission: what is it and how can it be achieved? Eur Respir J. 2022;60:2102583
18. Mozafari N, Azadi S, Mehdi-Alamdarlou S, Ashrafi H, Azadi A. Inflammation: A bridge between diabetes and COVID-19, and possible management with sitagliptin. J Medical hypotheses. 2020;143:110111
19. Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nature Reviews Neurology. 2021;17:157-172
20. Matyas C, Haskó G, Liaudet L, Trojnar E, Pacher P. Interplay of cardiovascular mediators, oxidative stress and inflammation in liver disease and its complications. J Nature Reviews Cardiology. 2021;18:117-135
21. Jiang W-j, Xu C-t, Du C-l, Dong J-h, Xu S-b, Hu B-f. et al. Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics. 2022;12:324
22. Shen Y, Teng L, Qu Y, Liu J, Zhu X, Chen S. et al. Anti-proliferation and anti-inflammation effects of corilagin in rheumatoid arthritis by downregulating NF-κB and MAPK signaling pathways. J Ethnopharmacol. 2022;284:114791
23. Hou M, Wei Y, Zhao Z, Han W, Zhou R, Zhou Y. et al. Immuno-engineered nanodecoys for the multi-target anti-inflammatory treatment of autoimmune diseases. Adv Mater. 2022;34:2108817
24. Morris G, Gevezova M, Sarafian V, Maes M. Redox regulation of the immune response. Cell Mol Immunol. 2022;19:1079-1101
25. Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5:209
26. Bharadwaj U, Kasembeli M M, Robinson P, Tweardy D J. Targeting janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: rationale, progress, and caution. Pharmacol Rev. 2020;72:486-526
27. Fitzgerald K A, Kagan J C. Toll-like receptors and the control of immunity. Cell. 2020;180:1044-1066
28. Bora G, Yaba A. The role of mitogen-activated protein kinase signaling pathway in endometriosis. J Obstet Gynaecol Res. 2021;47:1610-1623
29. Lippi G, Targher G, Montagnana M, Salvagno G L, Zoppini G, Guidi G C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628-632
30. Ridker P M, Bhatt D L, Pradhan A D, Glynn R J, MacFadyen J G, Nissen S E. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401:1293-1301
31. Lippi G, Salvagno G L, Gelati M, Pucci M, Cascio C L, Demonte D. et al. Two-center comparison of 10 fully-automated commercial procalcitonin (PCT) immunoassays. Clin Chem Lab Med. 2020;58:77-84
32. Cush J J. Rheumatoid arthritis: early diagnosis and treatment. Rheum Dis Clin. 2022;48:537-547
33. Williams C M, Noll J E, Bradey A L, Duggan J, Wilczek V J, Masavuli M G. et al. Myeloperoxidase creates a permissive microenvironmental niche for the progression of multiple myeloma. Br J Haematol. 2023;203:614-624
34. Horný M, Saindane A M, Duszak Jr R. Clinical characteristics of most frequent use of iodinated contrast media for CT. AJR Am J Roentgenol. 2022;219:825-826
35. Zhang J, Dai L, He L, Bhattarai A, Chan C-M, Tai W C-S. et al. Design and synthesis of chiral DOTA-based MRI contrast agents with remarkable relaxivities. Commun Chem. 2023;6:251
36. Buchert R, Wegner F, Huppertz H J, Berding G, Brendel M, Apostolova I. et al. Automatic covariance pattern analysis outperforms visual reading of 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) in variant progressive supranuclear palsy. Mov Disord. 2023;38:1901-1913
37. Liu R, Tang J, Xu Y, Dai Z. Bioluminescence imaging of inflammation in vivo based on bioluminescence and fluorescence resonance energy transfer using nanobubble ultrasound contrast agent. ACS Nano. 2019;13:5124-5132
38. Meng Z, Zhou X, She J, Zhang Y, Feng L, Liu Z. Ultrasound-responsive conversion of microbubbles to nanoparticles to enable background-free in vivo photoacoustic imaging. Nano Lett. 2019;19:8109-8117
39. MacRitchie N, Frleta-Gilchrist M, Sugiyama A, Lawton T, McInnes I B, Maffia P. Molecular imaging of inflammation-Current and emerging technologies for diagnosis and treatment. Pharmacol Ther. 2020;211:107550
40. Wang J, Jia Y, Wang Q, Liang Z, Han G, Wang Z. et al. An ultrahigh-field-tailored T1-T2 dual-mode MRI contrast agent for high-performance vascular imaging. Adv Mater. 2021;33:2004917
41. Katz J N, Arant K R, Loeser R F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325:568-578
42. Stiller C O, Hjemdahl P. Lessons from 20 years with COX-2 inhibitors: Importance of dose-response considerations and fair play in comparative trials. J Intern Med. 2022;292:557-574
43. Tu Z, Zhong Y, Hu H, Shao D, Haag R, Schirner M. et al. Design of Therapeutic Biomaterials to Control Inflammation. Nat Rev Mater. 2022;7:557-574
44. Booth B J, Nourreddine S, Katrekar D, Savva Y, Bose D, Long T J. et al. RNA editing: Expanding the potential of RNA therapeutics. Mol Ther. 2023;31:1533-1549
45. You H, Jones M K, Gordon C A, Arganda A E, Cai P, Al-Wassiti H. et al. The mRNA vaccine technology era and the future control of parasitic infections. Clin Microbiol Rev. 2023;36:e00241-21
46. Zhang Q, Wang L, Wang S, Cheng H, Xu L, Pei G. et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7:78
47. Sands B E, Irving P M, Hoops T, Izanec J L, Gao L-L, Gasink C. et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn's disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet. 2022;399:2200-2211
48. Smolen J S, Feist E, Fatenejad S, Grishin S A, Korneva E V, Nasonov E L. et al. Olokizumab versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2022;387:715-726
49. Singh S, Murad M H, Fumery M, Sedano R, Jairath V, Panaccione R. et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn's disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:1002-1014
50. Maher S, Brayden D J. Formulation strategies to improve the efficacy of intestinal permeation enhancers. Adv Drug Deliv Rev. 2021;177:113925
51. Muttenthaler M, King G F, Adams D J, Alewood P F. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021;20:309-325
52. Cao S-j, Lv Z-q, Guo S, Jiang G-p, Liu H-l. An Update-Prolonging the action of protein and peptide drugs. J Drug Deliv Sci Technol. 2021;61:102124
53. Chen M, Li H, Shi Z, Peng W, Qin Y, Luo R. et al. High fluorescence quenching probe-based reverse fluorescence enhancement LFTS coupling with IS-primer amplification reaction for the rapid and sensitive Parkinson Disease-associated MicroRNA detection. Biosens Bioelectron. 2020;165:112278
54. Shen K, Sun G, Chan L, He L, Li X, Yang S. et al. Anti-inflammatory nanotherapeutics by targeting matrix metalloproteinases for immunotherapy of spinal cord injury. Small. 2021;17:e2102102
55. Zhao Z, Zhang X, Zhang H, Shan X, Bai M, Wang Z. et al. Elaborately engineering a self-indicating dual-drug nanoassembly for site-specific photothermal-potentiated thrombus penetration and thrombolysis. Adv Sci. 2022;9:e2104264
56. Zhang Y, Yang H, Wei D, Zhang X, Wang J, Wu X. et al. Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration. 2021;1:20210115
57. Chen Y, Zhao T, Bai M, Gu T, Sun J, He Z. et al. Emerging small molecule-engineered hybrid nanomedicines for cancer therapy. Chem Eng J. 2022;435:135160
58. Wang Z, Zhang S, Kong Z, Li S, Sun J, Zheng Y. et al. Self-adaptive nanoassembly enabling turn-on hypoxia illumination and periphery/center closed-loop tumor eradication. Cell Rep Med. 2023;4:101014
59. Jo D H, Kim J H, Lee T G, Kim J H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine. 2015;11:1603-11
60. Xu M, Qi Y, Liu G, Song Y, Jiang X, Du B. Size-dependent in vivo transport of nanoparticles: implications for delivery, targeting, and clearance. ACS Nano. 2023;17:20825-20849
61. Aldayel A M, Hufnagel S, O'Mary H L, Valdes S A, Alzhrani R F, Xu H. et al. Effect of nanoparticle size on their distribution and retention in chronic inflammation sites. Discov Nano. 2023;18:105
62. Hu B, Liu R, Liu Q, Lin Z, Shi Y, Li J. et al. Engineering surface patterns on nanoparticles: new insights into nano-bio interactions. J Mater Chem B. 2022;10:2357-2383
63. Xie X, Liao J, Shao X, Li Q, Lin Y. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci Rep. 2017;7:3827
64. Di J, Gao X, Du Y, Zhang H, Gao J, Zheng A. Size, shape, charge and “stealthy” surface: Carrier properties affect the drug circulation time in vivo. Asian J Pharm Sci. 2021;16:444-458
65. Wang Q, Qin X, Fang J, Sun X. Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies. Acta Pharm Sin B. 2021;11:1158-1174
66. Li D F, Yang M F, Xu H M, Zhu M Z, Zhang Y, Tian C M. et al. Nanoparticles for oral delivery: targeted therapy for inflammatory bowel disease. J Mater Chem B. 2022;10:5853-5872
67. Wang Y, Li S, Wang X, Chen Q, He Z, Luo C. et al. Smart transformable nanomedicines for cancer therapy. Biomaterials. 2021;271:120737
68. Matthews P M. Chronic inflammation in multiple sclerosis—seeing what was always there. Nat Rev Neurol. 2019;15:582-593
69. Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L. et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13:1182-1190
70. Jung E, Lee J, Jeong L, Park S, Lee M, Song C. et al. Stimulus-activatable echogenic maltodextrin nanoparticles as nanotheranostic agents for peripheral arterial disease. Biomaterials. 2019;192:282-291
71. Han R, Xiao Y, Bai Q, Choi C H J. Self-therapeutic metal-based nanoparticles for treating inflammatory diseases. Acta Pharm Sin B. 2023;13:1847-1865
72. Fan X, Zhang Z, Gao W, Pan Q, Luo K, He B. et al. An engineered butyrate-derived polymer nanoplatform as a mucosa-healing enhancer potentiates the therapeutic effect of magnolol in inflammatory bowel disease. ACS nano. 2024;18:229-244
73. Xu Y, Zhu B-W, Sun R, Li X, Wu D, Hu J-N. Colon-targeting angelica sinensis polysaccharide nanoparticles with dual responsiveness for alleviation of ulcerative colitis. ACS Appl Mater Interfaces. 2023;15:26298-26315
74. Ozer I, Slezak A, Sirohi P, Li X, Zakharov N, Yao Y. et al. An injectable PEG-like conjugate forms a subcutaneous depot and enables sustained delivery of a peptide drug. Biomaterials. 2023;294:121985
75. Watson N, Ding B, Zhu X, Frisina R D. Chronic inflammation-inflammaging-in the ageing cochlea: a novel target for future presbycusis therapy. Ageing Res Rev. 2017;40:142-148
76. Chetrit M, Xu B, Kwon D H, Ramchand J, Rodriguez R E, Tan C D. et al. Imaging-guided therapies for pericardial diseases. JACC Cardiovasc Imaging. 2020;13:1422-1437
77. Xu Z, Jiang Y, Fan M, Tang S, Liu M, Law W C. et al. Aggregation-induced emission nanoprobes working in the NIR-II region: from material design to fluorescence imaging and phototherapy. Adv Opt Mater. 2021;9:2100859
78. Palaniyandi T, B K, Prabhakaran P, Viswanathan S, Rahaman Abdul Wahab M, Natarajan S. et al. Nanosensors for the diagnosis and therapy of neurodegenerative disorders and inflammatory bowel disease. Acta Histochem. 2023;125:151997
79. Liu S, Zhang C, Zhou Y, Zhang F, Duan X, Liu Y. et al. MRI-visible mesoporous polydopamine nanoparticles with enhanced antioxidant capacity for osteoarthritis therapy. Biomaterials. 2023;295:122030
80. Polat E O, Cetin M M, Tabak A F, Bilget Güven E, Uysal B Ö, Arsan T. et al. Transducer technologies for biosensors and their wearable applications. Biosensors. 2022;12:385
81. Naresh V, Lee N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors. 2021;21:1109
82. Altug H, Oh S-H, Maier S A, Homola J. Advances and applications of nanophotonic biosensors. Nat Nanotechnol. 2022;17:5-16
83. Jin C, Wu Z, Molinski J H, Zhou J, Ren Y, Zhang J X. Plasmonic nanosensors for point-of-care biomarker detection. Materials Today Bio. 2022;14:100263
84. Zhou D, Yin Y, Zhu Z, Gao Y, Yang J, Pan Y. et al. Orally administered platinum nanomarkers for urinary monitoring of inflammatory bowel disease. ACS Nano. 2022;16:18503-18514
85. Tao Y, Zhao Y, Wang L, Huang J, Chen Y, Huang Q. et al. Flexible amperometric immunosensor based on colloidal quantum dots for detecting the myeloperoxidase (MPO) systemic inflammation biomarker. Biosensors. 2023;13:255
86. Zhao G, Wang Y, Wang H, Bai G, Zhang N, Wang Y. et al. Ultrasensitive photoelectrochemical immunoassay strategy based on Bi2S3/Ag2S for the detection of the inflammation marker procalcitonin. Biosensors. 2023;13:366
87. Wang L, Liu Y, Yan J, Li H, Tu Y. Novel electrochemiluminescent immunosensor using dual amplified signals from a CoFe Prussian blue analogue and Au nanoparticle for the detection of Lp-PLA2. ACS Sens. 2023;8:2859-2868
88. Atta N F, Gawad S A A, Galal A, Razik A A, El-Gohary A R. Efficient electrochemical sensor for determination of H2O2 in human serum based on nano iron-nickel alloy/carbon nanotubes/ionic liquid crystal composite. J Electroanal. 2021;881:114953
89. Mansuriya B D, Altintas Z. Enzyme-free electrochemical nano-immunosensor based on graphene quantum dots and gold nanoparticles for cardiac biomarker determination. Nanomaterials. 2021;11:578
90. Zhang X, Li S, Ma H, Wang H, Zhang R, Zhang X-D. Activatable NIR-II organic fluorescent probes for bioimaging. Theranostics. 2022;12:3345-3371
91. Gao F, Liu C, Yao Y, Lei C, Li S, Yuan L. et al. Quantum dots' size matters for balancing their quantity and quality in label materials to improve lateral flow immunoassay performance for C-reactive protein determination. Biosens Bioelectron. 2022;199:113892
92. Qin D, Meng S, Wu Y, Luo Z, Deng B. Construction of efficient electrochemiluminescence resonance energy transfer sensor based on SnO2/SnS2QDs-Ru@ IRMOF-3 composite for sensitive detection of procalcitonin. Microchimica Acta. 2022;189:430
93. Cai Q, Li H, Dong W, Jie G. Versatile photoelectrochemical biosensor based on AIS/ZnS QDs sensitized-WSe2 nanoflowers coupled with DNA nanostructure probe for “On- Off” assays of TNF-α and MTase. Biosens Bioelectron. 2023;241:115704
94. Lv Y, Wang P, Li J, Li N, Xu D, Wu R. et al. Establishment of a Ca (II) ion-quantum dots fluorescence signal amplification sensor for high-sensitivity biomarker detection. Anal Chim Acta. 2023;1237:340534
95. Liu B, Lu S, Yang K, Dou X, Feng X, Cui H. et al. Two strategies for rapid and sensitive detection of procalcitonin using carbon dots-encapsulated nanocapsule and magnetic carbon dots as coupled labels. Chem Eng J. 2023;472:145038
96. Yola M L, Atar N. Novel voltammetric tumor necrosis factor-alpha (TNF-α) immunosensor based on gold nanoparticles involved in thiol-functionalized multi-walled carbon nanotubes and bimetallic Ni/Cu-MOFs. Anal Bioanal Chem. 2021;413:2481-2492
97. Hong J M, Lee H, Menon N V, Lim C T, Lee L P, Ong C W. Point-of-care diagnostic tests for tuberculosis disease. Sci Transl Med. 2022;14:eabj4124
98. Li P, Lee G H, Kim S Y, Kwon S Y, Kim H R, Park S. From diagnosis to treatment: Recent advances in patient-friendly biosensors and implantable devices. ACS Nano. 2021;15:1960-2004
99. Macovei D G, Irimes M B, Hosu O, Cristea C, Tertis M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal Bioanal Chem. 2023;415:1033-1063
100. Tan M, Xu Y, Gao Z, Yuan T, Liu Q, Yang R. et al. Recent advances in intelligent wearable medical devices integrating biosensing and drug delivery. Adv Mater. 2022;34:2108491
101. Wang Z, Hao Z, Wang X, Huang C, Lin Q, Zhao X. et al. A flexible and regenerative aptameric graphene-nafion biosensor for cytokine storm biomarker monitoring in undiluted biofluids toward wearable applications. Adv Funct Mater. 2020;31:2005958
102. Chudal L, Santelli J, Lux J, Woodward A, Hafeez N, Endsley C. et al. In vivo ultrasound imaging of macrophages using acoustic vaporization of internalized superheated nanodroplets. ACS Applied Materials Interfaces. 2023;15:42413-42423
103. He L, He L H, Xu S, Ren T B, Zhang X X, Qin Z J. et al. Engineering of Reversible NIR-II Redox-Responsive Fluorescent Probes for Imaging of Inflammation In Vivo. Angew Chem Int Ed Engl. 2022;61:e202211409
104. Wu L, Zeng W, Ishigaki Y, Zhang J, Bai H, Harimoto T. et al. A Ratiometric Photoacoustic Probe with a Reversible Response to Hydrogen Sulfide and Hydroxyl Radicals for Dynamic Imaging of Liver Inflammation. Angew Chem Int Ed Engl. 2022;61:e202209248
105. Sarkar S, Matsukuma K E, Spencer B, Chen S, Olson K A, Badawi R D. et al. Dynamic positron emission tomography/computed tomography imaging correlate of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2021;19:2441-2443
106. Scheinberg D A, Grimm J, Heller D A, Stater E P, Bradbury M, McDevitt M R. Advances in the clinical translation of nanotechnology. Curr Opin Biotechnol. 2017;46:66-73
107. Revel M P, Chassagnon G. Use of MRI to measure bronchial inflammation in cystic fibrosis. Radiology. 2020;294:197-198
108. Rosenkrans Z T, Ferreira C A, Ni D, Cai W. Internally responsive nanomaterials for activatable multimodal imaging of cancer. Adv Healthc Mater. 2021;10:e2000690
109. Zhao Z, Yang F, Zhang X, Sun J, He Z, Luo C. Emerging nanotherapeutics for antithrombotic treatment. Biomaterials. 2020;255:120200
110. Ouyang J, Xie A, Zhou J, Liu R, Wang L, Liu H. et al. Minimally invasive nanomedicine: nanotechnology in photo-/ultrasound-/radiation-/magnetism-mediated therapy and imaging. Chem Soc Rev. 2022;51:4996-5041
111. Li M, Xuan Y, Zhang W, Zhang S, An J. Polydopamine-containing nano-systems for cancer multi-mode diagnoses and therapies: A review. Int J Biol Macromol. 2023;247:125826
112. Lv W, Liu Y, Li S, Lv L, Lu H, Xin H. Advances of nano drug delivery system for the theranostics of ischemic stroke. J Nanobiotechnology. 2022;20:248
113. Minopoulou I, Kleyer A, Yalcin-Mutlu M, Fagni F, Kemenes S, Schmidkonz C. et al. Imaging in inflammatory arthritis: progress towards precision medicine. Nat Rev Rheumatol. 2023;19:650-665
114. Kaur A, New E J, Sunde M. Strategies for the molecular imaging of amyloid and the value of a multimodal approach. ACS Sens. 2020;5:2268-2282
115. Bai F, Du W, Liu X, Su L, Li Z, Chen T. et al. A NO-responsive ratiometric fluorescent nanoprobe for monitoring drug-induced liver injury in the second near-infrared window. Anal Chem. 2021;93:15279-15287
116. Liu S, Yan A, Guo W, Fang Y, Dong Q, Li R. et al. Human neutrophil elastase activated fluorescent probe for pulmonary diseases based on fluorescence resonance energy transfer using CdSe/ZnS quantum dots. ACS Nano. 2020;14:4244-4254
117. Aizik G, Waiskopf N, Agbaria M, Levi-Kalisman Y, Banin U, Golomb G. Delivery of liposomal quantum dots via monocytes for imaging of inflamed tissue. ACS Nano. 2017;11:3038-3051
118. Guo Z, Yan C, Zhu W H. High-performance quinoline-malononitrile core as a building block for the diversity-oriented synthesis of AIEgens. Angew Chem Int Ed Engl. 2020;59:9812-9825
119. Xu L, Jiang X, Liang K, Gao M, Kong B J A. Frontier luminous strategy of functional silica nanohybrids in sensing and bioimaging: From ACQ to AIE. Aggregate. 2022;3:e121
120. Xie H, Zhang J, Chen C, Sun F, Liu H, He X. et al. Sensitive and specific detection of peroxynitrite and in vivo imaging of inflammation by a “simple” AIE bioprobe. Mater Chem Front. 2021;5:1830-1835
121. Chen T, He B, Tao J, He Y, Deng H, Wang X. et al. Application of Förster Resonance Energy Transfer (FRET) technique to elucidate intracellular and in vivo biofate of nanomedicines. Adv Drug Deliv Rev. 2019;143:177-205
122. Liang T, Zhang D, Hu W, Tian C, Zeng L, Wu T. et al. A dual lock-and-key two photon fluorescence probe in response to hydrogen peroxide and viscosity: Application in cellular imaging and inflammation therapy. Talanta. 2021;235:122719
123. Zhang X, Wang W, Su L, Ge X, Ye J, Zhao C. et al. Plasmonic-fluorescent janus Ag/Ag2S nanoparticles for in situ H2O2-activated NIR-II fluorescence imaging. Nano Lett. 2021;21:2625-2633
124. Liu Q, Zhong Y, Su Y, Zhao L, Peng J. Real-time imaging of hepatic inflammation using hydrogen sulfide-activatable second near-infrared luminescent nanoprobes. Nano Lett. 2021;21:4606-4614
125. Su Y, Yu B, Wang S, Cong H, Shen Y J B. NIR-II bioimaging of small organic molecule. Biomaterials. 2021;271:120717
126. Cao C, Zhou X, Xue M, Han C, Feng W, Li F. Dual near-infrared-emissive luminescent nanoprobes for ratiometric luminescent monitoring of ClO- in living organisms. ACS Appl Mater Interfaces. 2019;11:15298-15305
127. Pei P, Hu H, Chen Y, Wang S, Chen J, Ming J. et al. NIR-II ratiometric lanthanide-dye hybrid nanoprobes doped bioscaffolds for in situ bone repair monitoring. Nano Lett. 2022;22:783-791
128. Gong F, Yang N, Wang Y, Zhuo M, Zhao Q, Wang S. et al. Oxygen-deficient bimetallic oxide FeWOX nanosheets as peroxidase-like nanozyme for sensing cancer via photoacoustic imaging. Small. 2020;16:2003496
129. Ahmad A, Ahsan H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J Immunoassay Immunochem. 2020;41:257-271
130. Bhatt S, Nagappa A N, Patil C R. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270-1276
131. Chen Q, Liang C, Sun X, Chen J, Yang Z, Zhao H. et al. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc Natl Acad Sci U S A. 2017;114:5343-5348
132. Ma B, Xiao Y, Lv Q, Li G, Wang Y, Fu G. Targeting theranostics of atherosclerosis by dual-responsive nanoplatform via photoacoustic imaging and three-in-one integrated lipid management. Adv Mater. 2023;35:e2206129
133. Li Q, Ge X, Ye J, Li Z, Su L, Wu Y. et al. Dual ratiometric SERS and photoacoustic core-satellite nanoprobe for quantitatively visualizing hydrogen peroxide in inflammation and cancer. Angew Chem Int Ed Engl. 2021;60:7323-7332
134. Afshari M J, Cheng X, Duan G, Duan R, Wu S, Zeng J. et al. Vision for ratiometric nanoprobes: in vivo noninvasive visualization and readout of physiological hallmarks. ACS Nano. 2023;17:7109-7134
135. He C, Zhu J, Zhang H, Qiao R, Zhang R. Photoacoustic imaging probes for theranostic applications. Biosensors. 2022;12:947
136. Ye J, Li Z, Fu Q, Li Q, Zhang X, Su L. et al. Quantitative photoacoustic diagnosis and precise treatment of inflammation in vivo using activatable theranostic nanoprobe. Adv Funct Mater. 2020;30:2001771
137. Li Y, Younis M H, Wang H, Zhang J, Cai W, Ni D. Spectral computed tomography with inorganic nanomaterials: State-of-the-art. Adv Drug Deliv Rev. 2022;189:114524
138. Toczek J, Boodagh P, Sanzida N, Ghim M, Salarian M, Gona K. et al. Computed tomography imaging of macrophage phagocytic activity in abdominal aortic aneurysm. Theranostics. 2021;11:5876-5888
139. Faucon A L, Bobrie G, Clement O. Nephrotoxicity of iodinated contrast media: From pathophysiology to prevention strategies. Eur J Radiol. 2019;116:231-241
140. Luo D, Wang X, Burda C, Basilion J P. Recent development of gold nanoparticles as contrast agents for cancer diagnosis. Cancers. 2021;13:1825
141. Yu C, Chen Z, Li X, Bao H, Wang Y, Zhang B. et al. pH-triggered aggregation of gold nanoparticles for enhanced labeling and long-term CT imaging tracking of stem cells in pulmonary fibrosis treatment. Small. 2021;17:2101861
142. Naha P C, Hsu J C, Kim J, Shah S, Bouche M, Si-Mohamed S. et al. Dextran-coated cerium oxide nanoparticles: a computed tomography contrast agent for imaging the gastrointestinal tract and inflammatory bowel disease. ACS Nano. 2020;14:10187-10197
143. Fu J J, Guo J J, Qin A P, Yu X Y, Zhang Q, Lei X P. et al. Bismuth chelate as a contrast agent for X-ray computed tomography. J Nanobiotechnology. 2020;18:110
144. Rabin O, Manuel Perez J, Grimm J, Wojtkiewicz G, Weissleder R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater. 2006;5:118-22
145. Tarighatnia A, Fouladi M R, Tohidkia M R, Johal G, Nader N D, Aghanejad A. et al. Engineering and quantification of bismuth nanoparticles as targeted contrast agent for computed tomography imaging in cellular and animal models. J Drug Deliv Sci Technol. 2021;66:102895
146. Kevadiya B D, Ottemann B, Mukadam I Z, Castellanos L, Sikora K, Hilaire J R. et al. Rod-shape theranostic nanoparticles facilitate antiretroviral drug biodistribution and activity in human immunodeficiency virus susceptible cells and tissues. Theranostics. 2020;10:630-656
147. Liu C, Fan Z, He D, Chen H, Zhang S, Guo S. et al. Designer functional nanomedicine for myocardial repair by regulating the inflammatory microenvironment. Pharmaceutics. 2022;14:758
148. Senders M L, Meerwaldt A E, van Leent M M T, Sanchez-Gaytan B L, van de Voort J C, Toner Y C. et al. Probing myeloid cell dynamics in ischaemic heart disease by nanotracer hot-spot imaging. Nat Nanotechnol. 2020;15:398-405
149. Zhang Q, Li D, Zhong J, Wu Y, Shi Y, Yang H. et al. SPECT imaging and highly efficient therapy of rheumatoid arthritis based on hyperbranched semiconducting polymer nanoparticles. Biomater Sci. 2021;9:1845-1854
150. Zhou I, Catalano O A, Caravan P. Advances in functional and molecular MRI technologies in chronic liver diseases. J Hepatol. 2020;73:1241-1254
151. Ottens T, Barbieri S, Orton M R, Klaassen R, van Laarhoven H W M, Crezee H. et al. Deep learning DCE-MRI parameter estimation: Application in pancreatic cancer. Med Image Anal. 2022;80:102512
152. Zhao J, Chen X, Ho K-H, Cai C, Li C-W, Yang M. et al. Nanotechnology for diagnosis and therapy of rheumatoid arthritis: Evolution towards theranostic approaches. Chinese Chem Lett. 2021;32:66-86
153. Wang C, Sun W, Zhang J, Zhang J, Guo Q, Zhou X. et al. An electric-field-responsive paramagnetic contrast agent enhances the visualization of epileptic foci in mouse models of drug-resistant epilepsy. Nat Biomed Eng. 2021;5:278-289
154. He C, Ahmed T, Abbasi A Z, Li L, Foltz W, Cai P. et al. Multifunctional bioreactive-nanoconstructs for sensitive and accurate MRI of cerebrospinal fluid pathology and intervention of Alzheimer's disease. Nano Today. 2020;35:100965
155. Wu B, Deng K, Lu S T, Zhang C J, Ao Y W, Wang H. et al. Reduction-active Fe3O4-loaded micelles with aggregation-enhanced MRI contrast for differential diagnosis of Neroglioma. Biomaterials. 2021;268:120531
156. Tang C, Wang C, Zhang Y, Xue L, Li Y, Ju C. et al. Recognition, intervention, and monitoring of neutrophils in acute ischemic stroke. Nano Lett. 2019;19:4470-4477
157. Balachandran Y L, Wang W, Yang H, Tong H, Wang L, Liu F. et al. Heterogeneous iron oxide/dysprosium oxide nanoparticles target liver for precise magnetic resonance imaging of liver fibrosis. ACS Nano. 2022;16:5647-5659
158. Senders M L, Calcagno C, Tawakol A, Nahrendorf M, Mulder W J, Fayad Z A. PET/MR imaging of inflammation in atherosclerosis. Nat Biomed Eng. 2023;7:202-220
159. Bouche M, Puhringer M, Iturmendi A, Amirshaghaghi A, Tsourkas A, Teasdale I. et al. Activatable hybrid polyphosphazene-AuNP nanoprobe for ROS detection by bimodal PA/CT imaging. ACS Appl Mater Interfaces. 2019;11:28648-28656
160. Dai Y, Sha X, Song X, Zhang X, Xing M, Liu S. et al. Targeted therapy of atherosclerosis vulnerable plaque by ROS-scavenging nanoparticles and MR/fluorescence dual-modality imaging tracing. Int J Nanomedicine. 2022;17:5413-5429
161. Zhang L, Liu Y, Huang H, Xie H, Zhang B, Xia W. et al. Multifunctional nanotheranostics for near infrared optical imaging-guided treatment of brain tumors. Adv Drug Deliv Rev. 2022;190:114536
162. Xu L, Gao H, Zhan W, Deng Y, Liu X, Jiang Q. et al. Dual aggregations of a near-infrared aggregation-induced emission luminogen for enhanced imaging of Alzheimer's disease. J Am Chem Soc. 2023;145:27748-27756
163. Gao X, Zhang W, Dong Z, Ren J, Song B, Zhang R. et al. FRET luminescent probe for the ratiometric imaging of peroxynitrite in rat brain models of epilepsy-based on organic dye-conjugated iridium (III) complex. Anal Chem. 2023;95:18530-18539
164. Li J, Zhao N, Zhang W, Li P, Yin X, Zhang W. et al. Assessing the progression of early atherosclerosis mice using a fluorescence nanosensor for the simultaneous detection and imaging of pH and phosphorylation. Angew Chem Int Ed Engl. 2023;62:e202215178
165. Zhang Y, Kang X, Li J, Song J, Li X, Li W. et al. Inflammation-responsive nanoagents for activatable photoacoustic molecular imaging and tandem therapies in rheumatoid arthritis. ACS Nano. 2024;18:2231-2249
166. Ma Y, Shang J, Liu L, Li M, Xu X, Cao H. et al. Rational design of a double-locked photoacoustic probe for precise in vivo imaging of cathepsin B in atherosclerotic plaques. J Am Chem Soc. 2023;145:17881-17891
167. Li X, Liu Y, Qi X, Xiao S, Xu Z, Yuan Z. et al. Sensitive activatable nanoprobes for real-time ratiometric magnetic resonance imaging of reactive oxygen species and ameliorating inflammation in vivo. Adv Mater. 2022;34:e2109004
168. Yin M, Chen Y, Liu X, Tian S, Zhao L, Bai Y. et al. Targeted computed tomography visualization and healing of inflammatory bowel disease by orally delivered bacterial-flagella-inspired polydiiododiacetylene nanofibers. ACS Nano. 2023;17:3873-3888
169. Sun L, Ouyang J, Zeng F, Wu S. An AIEgen-based oral-administration nanosystem for detection and therapy of ulcerative colitis via 3D-MSOT/NIR-II fluorescent imaging and inhibiting NLRP3 inflammasome. Biomaterials. 2022;283:121468
170. Deng Z, Ma W, Ding C, Wei C, Gao B, Zhu Y. et al. Metal polyphenol network/cerium oxide artificial enzymes therapeutic nanoplatform for MRI/CT-aided intestinal inflammation management. Nano Today. 2023;53:102044
171. Zeng Z, Ouyang J, Sun L, Zeng F, Wu S. A biomarker-responsive nanosystem with colon-targeted delivery for ulcerative colitis's detection and treatment with optoacoustic/NIR-II fluorescence imaging. Adv Healthc Mater. 2022;11:e2201544
172. Placha D, Jampilek J J P. Chronic inflammatory diseases, anti-inflammatory agents and their delivery nanosystems. Pharmaceutics. 2021;13:64
173. Li Y, Li L, Jin Q, Liu T, Sun J, Wang Y. et al. Impact of the amount of PEG on prodrug nanoassemblies for efficient cancer therapy. Asian J Pharm Sci. 2022;17:241-252
174. Wang C, Xu M, Fan Q, Li C, Zhou X. Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases. Asian J Pharm Sci. 2023;18:100772
175. Zhang X, Xiong J, Wang K, Yu H, Sun B, Ye H. et al. Erythrocyte membrane-camouflaged carrier-free nanoassembly of FRET photosensitizer pairs with high therapeutic efficiency and high security for programmed cancer synergistic phototherapy. Bioact Mater. 2021;6:2291-2302
176. Liu Y, Wang X, Wang Z, Liao R, Qiu Q, Wang Y. et al. Reduction-responsive stearyl alcohol-cabazitaxel prodrug nanoassemblies for cancer chemotherapy. Pharmaceutics. 2023;15:262
177. Luo C, Sun B, Wang C, Zhang X, Chen Y, Chen Q. et al. Self-facilitated ROS-responsive nanoassembly of heterotypic dimer for synergistic chemo-photodynamic therapy. J Control Release. 2019;302:79-89
178. Zhao Y, Zhang Z, Pan Z, Liu Y. Advanced bioactive nanomaterials for biomedical applications. Exploration. 2021;1:20210089
179. Xue X, Qu H, Li Y. Stimuli-responsive crosslinked nanomedicine for cancer treatment. Exploration. 2022;2:20210134
180. Xu Y, Fourniols T, Labrak Y, Preat V, Beloqui A, des Rieux A. Surface modification of lipid-based nanoparticles. ACS Nano. 2022;16:7168-7196
181. Böttger R, Pauli G, Chao P, Fayez N, Hohenwarter L, Li S. Lipid-based nanoparticle technologies for liver targeting. Adv Drug Deliv Rev. 2020;154:79-101
182. Wu Q, Wang J, Wang Y, Xiang L, Tan Y, Feng J. et al. Targeted delivery of celastrol to glomerular endothelium and podocytes for chronic kidney disease treatment. Nano Res. 2022;15:3556-3568
183. Åslund A K, Vandebriel R J, Caputo F, de Jong W H, Delmaar C, Hyldbakk A. et al. A comparative biodistribution study of polymeric and lipid-based nanoparticles. Drug Deliv Transl Res. 2022;12:2114-2131
184. Zu M, Ma Y, Cannup B, Xie D, Jung Y, Zhang J. et al. Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Adv Drug Deliv Rev. 2021;176:113887
185. Ghezzi M, Pescina S, Padula C, Santi P, Del Favero E, Cantù L. et al. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J Control Release. 2021;332:312-336
186. An L, Li Z, Shi L, Wang L, Wang Y, Jin L. et al. Inflammation-targeted celastrol nanodrug attenuates collagen-induced arthritis through NF-κB and Notch1 pathways. Nano Lett. 2020;20:7728-7736
187. Vyawahare A, Prakash R, Jori C, Ali A, Raza S S, Khan R. Caffeic acid modified nanomicelles inhibit articular cartilage deterioration and reduce disease severity in experimental inflammatory arthritis. ACS Nano. 2022;16:18579-18591
188. Scotti A, Schulte M F, Lopez C G, Crassous J J, Bochenek S, Richtering W. How softness matters in soft nanogels and nanogel assemblies. Chem Rev. 2022;122:11675-11700
189. Yin Y, Hu B, Yuan X, Cai L, Gao H, Yang Q. Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics. 2020;12:290
190. Seo B B, Kwon Y, Kim J, Hong K H, Kim S E, Song H R. et al. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact Mater. 2022;7:14-25
191. Jan N, Madni A, Khan S, Shah H, Akram F, Khan A. et al. Biomimetic cell membrane-coated poly (lactic-co-glycolic acid) nanoparticles for biomedical applications. Bioeng Transl Med. 2022;8:e10441
192. Zinger A, Soriano S, Baudo G, De Rosa E, Taraballi F, Villapol S. Biomimetic nanoparticles as a theranostic tool for traumatic brain injury. Adv Funct Mater. 2021;31:2100722
193. Han Y, Pang X, Pi G. Biomimetic and bioinspired intervention strategies for the treatment of rheumatoid arthritis. Adv Funct Mater. 2021;31:2104640
194. Nguyen P H D, Jayasinghe M K, Le A H, Peng B, Le M T N. Advances in drug delivery systems based on red blood cells and their membrane-derived nanoparticles. ACS Nano. 2023;17:5187-5210
195. Wang Y, Zhang K, Qin X, Li T, Qiu J, Yin T. et al. Biomimetic nanotherapies: red blood cell based core-shell structured nanocomplexes for atherosclerosis management. Adv Sci. 2019;6:1900172
196. Gao C, Huang Q, Liu C, Kwong C H T, Yue L, Wan J B. et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11:2622
197. Song X, Zheng Z, Ouyang S, Chen H, Sun M, Lin P. et al. Biomimetic epigallocatechin gallate-cerium assemblies for the treatment of rheumatoid arthritis. ACS Appl Mater Interfaces. 2023;15:33239-33249
198. Chen R, Yang J, Wu M, Zhao D, Yuan Z, Zeng L. et al. M2 macrophage hybrid membrane-camouflaged targeted biomimetic nanosomes to reprogram inflammatory microenvironment for enhanced enzyme-thermo-immunotherapy. Adv Mater. 2023;35:e2304123
199. Zhang Y, Liu L, Wang T, Mao C, Shan P, Lau C S. et al. Reactive oxygen species-responsive polymeric prodrug nanoparticles for selective and effective treatment of inflammatory diseases. Adv Healthc Mater. 2023;12:2301394
200. Wang Q, Duan Y, Jing H, Wu Z, Tian Y, Gong K. et al. Inhibition of atherosclerosis progression by modular micelles. J Control Release. 2023;354:294-304
201. Wang Y, Li Y, He L, Mao B, Chen S, Martinez V. et al. Commensal flora triggered target anti-inflammation of alginate-curcumin micelle for ulcerative colitis treatment. Colloids Surf B Biointerfaces. 2021;203:111756
202. Zhou Z, Li K, Guo Y, Liu P, Chen Q, Fan H. et al. ROS/electro dual-reactive nanogel for targeting epileptic foci to remodel aberrant circuits and inflammatory microenvironment. ACS nano. 2023;17:7847-7864
203. Tu C, Lu H, Zhou T, Zhang W, Deng L, Cao W. et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597
204. Wang K, Yin C, Ye X, Chen Q, Wu J, Chen Y. et al. A metabolic driven bio-responsive hydrogel loading psoralen for therapy of rheumatoid arthritis. Small. 2023;19:2207319
205. Xi L, Lin Z, Qiu F, Chen S, Li P, Chen X. et al. Enhanced uptake and anti-maturation effect of celastrol-loaded mannosylated liposomes on dendritic cells for psoriasis treatment. Acta Pharm Sin B. 2022;12:339-352
206. Park J H, Jiang Y, Zhou J, Gong H, Mohapatra A, Heo J. et al. Genetically engineered cell membrane-coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci Adv. 2021;7:eabf7820
207. Lin Y, Chen Y, Deng R, Qin H, Li N, Qin Y. et al. Delivery of neutrophil membrane encapsulated non-steroidal anti-inflammatory drugs by degradable biopolymer microneedle patch for rheumatoid arthritis therapy. Nano Today. 2023;49:101791
208. Zhu Y, Zhu L, Wang X, Jin H. RNA-based therapeutics: an overview and prospectus. Cell Death Dis. 2022;13:644
209. He C, Yue H, Xu L, Liu Y, Song Y, Tang C. et al. siRNA release kinetics from polymeric nanoparticles correlate with RNAi efficiency and inflammation therapy via oral delivery. Acta Biomater. 2020;103:213-222
210. Yonezawa S, Koide H, Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv Drug Deliv Rev. 2020;154:64-78
211. Puchkov P A, Maslov M A. Lipophilic polyamines as promising components of Liposomal gene delivery systems. Pharmaceutics. 2021;13:920
212. Wang Y, Zhang C, Yang K, Wang Y, Shan S, Yan Y. et al. Transportation of AIE-visualized nanoliposomes is dominated by the protein corona. Natl Sci Rev. 2021;8:nwab068
213. Guo Y, He X, Zhao R M, Yang H Z, Huang Z, Zhang J. et al. Zn-dipicolylamine-based reactive oxygen species-responsive lipids for siRNA delivery and in vivo colitis treatment. Acta Biomater. 2022;147:287-298
214. Tao W, Yurdagul Jr A, Kong N, Li W, Wang X, Doran A C. et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Science translational medicine. 2020;12:eaay1063
215. Ralvenius W T, Andresen J L, Huston M M, Penney J, Bonner J M, Fenton O S. et al. Nanoparticle-mediated delivery of Anti-PU. 1 siRNA via localized intracisternal administration reduces neuroinflammation. Adv Mater. 2023 e2309225
216. Gao M, Tang M, Ho W, Teng Y, Chen Q, Bu L. et al. Modulating plaque inflammation via targeted mRNA nanoparticles for the treatment of atherosclerosis. ACS Nano. 2023;17:17721-17739
217. Wang Y, Zhang Z, Luo J, Han X, Wei Y, Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20:33
218. Jeon T, Luther D C, Goswami R, Bell C, Nagaraj H, Cicek Y A. et al. Engineered polymer-siRNA polyplexes provide effective treatment of lung inflammation. ACS Nano. 2023;17:4315-4326
219. Bai Q, Xiao Y, Hong H, Cao X, Zhang L, Han R. et al. Scavenger receptor-targeted plaque delivery of microRNA-coated nanoparticles for alleviating atherosclerosis. Proc Natl Acad Sci U S A. 2022;119:e2201443119
220. Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G. et al. Therapeutic peptides: Current applications and future directions. Signal Transduct Target Ther. 2022;7:48
221. Varanko A, Saha S, Chilkoti A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv Drug Deliv Rev. 2020;156:133-187
222. Kianfar E. Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles. J Nanobiotechnology. 2021;19:159
223. Gonçalves A, Machado R, Gomes A C, Costa A. Nanotechnology solutions for controlled cytokine delivery: an applied perspective. Appl Sci. 2020;10:7098
224. Kim M, Sahu A, Hwang Y, Kim G B, Nam G H, Kim I-S. et al. Targeted delivery of anti-inflammatory cytokine by nanocarrier reduces atherosclerosis in Apo E-/-mice. Biomaterials. 2020;226:119550
225. Liu L, Zhang Y, Mao C, Chen H, Zhang Y, Wang J. et al. Antibody-sheltered immunological nanonut (AINUT) for rheumatoid arthritis-targeted efficient alleviation. Nano Today. 2022;47:101640
226. Costa T J, Barros P R, Arce C, Santos J D, da Silva-Neto J, Egea G. et al. The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic Biol Med. 2021;162:615-635
227. Sun X, Yu K, Zhou Y, Dong S, Hu W, Sun Y. et al. Self-assembled pH-sensitive polymeric nanoparticles for the inflammation-targeted delivery of Cu/Zn-superoxide dismutase. ACS Appl Mater Interfaces. 2021;13:18152-18164
228. Nguyen N H, Tran G-B, Nguyen C T. Anti-oxidative effects of superoxide dismutase 3 on inflammatory diseases. J Mol Med. 2020;98:59-69
229. Guan T, Li J, Chen C, Liu Y. Self-assembling peptide-based hydrogels for wound tissue repair. Adv Sci. 2022;9:e2104165
230. Qin X, He L, Fan D, Liang W, Wang Q, Fang J. Targeting the resolution pathway of inflammation using Ac2-26 peptide-loaded PEGylated lipid nanoparticles for the remission of rheumatoid arthritis. Asian J Pharm Sci. 2021;16:483-493
231. Liu P, Zhang T, Chen Q, Li C, Chu Y, Guo Q. et al. Biomimetic dendrimer-peptide conjugates for early multi-target therapy of Alzheimer's disease by inflammatory microenvironment modulation. Adv Mater. 2021;33:2100746
232. Kim J Y, Ahn J, Kim J, Choi M, Jeon H, Choe K. et al. Nanoparticle-assisted transcutaneous delivery of a signal transducer and activator of transcription 3-inhibiting peptide ameliorates psoriasis-like skin inflammation. ACS nano. 2018;12:6904-6916
233. Han R, Ho L W C, Bai Q, Chan C K W, Lee L K C, Choi P C. et al. Alkyl-terminated gold nanoparticles as a self-therapeutic treatment for psoriasis. Nano Lett. 2021;21:8723-8733
234. Muhammad F, Huang F, Cheng Y, Chen X, Wang Q, Zhu C. et al. Nanoceria as an electron reservoir: Spontaneous deposition of metal nanoparticles on oxides and their anti-inflammatory activities. ACS Nano. 2022;16:20567-20576
235. Zhou F, Li M, Chen M, Chen M, Chen X, Luo Z. et al. Redox homeostasis strategy for inflammatory macrophage reprogramming in rheumatoid arthritis based on ceria oxide nanozyme-complexed biopolymeric micelles. ACS Nano. 2023;17:4358-4372
236. Conley B M, Yang L, Bhujel B, Luo J, Han I, Lee K B. Development of a nanohybrid peptide hydrogel for enhanced intervertebral disc repair and regeneration. ACS Nano. 2023;17:3750-3764
237. Deng Y, Gao Y, Li T, Xiao S, Adeli M, Rodriguez R D. et al. Amorphizing metal selenides-based ROS biocatalysts at surface nanolayer toward ultrafast inflammatory diabetic wound healing. ACS Nano. 2023;17:2943-2957
238. Chan C K W, Szeto C C, Lee L K C, Xiao Y, Yin B, Ding X. et al. A sub-10-nm, folic acid-conjugated gold nanoparticle as self-therapeutic treatment of tubulointerstitial fibrosis. Proc Natl Acad Sci U S A. 2023;120:e2305662120
239. Lu C, Xue L, Luo K, Liu Y, Lai J, Yao X. et al. Colon-accumulated gold nanoclusters alleviate intestinal inflammation and prevent secondary colorectal carcinogenesis via nrf2-dependent macrophage reprogramming. ACS nano. 2023;17:18421-18432
240. Liu J, Shi L, Wang Y, Li M, Zhou C, Zhang L. et al. Ruthenium-based metal-organic framework with reactive oxygen and nitrogen species scavenging activities for alleviating inflammation diseases. Nano Today. 2022;47:101627
241. Wang Y, Dai X, Wu L, Xiang H, Chen Y, Zhang R. Atomic vacancies-engineered ultrathin trimetallic nanozyme with anti-inflammation and antitumor performances for intestinal disease treatment. Biomaterials. 2023;299:122178
242. Seaberg J, Clegg J R, Bhattacharya R, Mukherjee P. Self-therapeutic nanomaterials: Applications in biology and medicine. Mater Today (Kidlington). 2023;62:190-224
243. Xu J, Chen H, Chu Z, Li Z, Chen B, Sun J. et al. A multifunctional composite hydrogel as an intrinsic and extrinsic coregulator for enhanced therapeutic efficacy for psoriasis. J Nanobiotechnology. 2022;20:155
244. Jiang W, Li Q, Zhang R, Li J, Lin Q, Li J. et al. Chiral metal-organic frameworks incorporating nanozymes as neuroinflammation inhibitors for managing Parkinson's disease. Nat Commun. 2023;14:8137
245. Seaberg J, Clegg J R, Bhattacharya R, Mukherjee P. Self-therapeutic nanomaterials: Applications in biology and medicine. Mater Today. 2023;62:190-224
246. Large D E, Abdelmessih R G, Fink E A, Auguste D T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851
247. Paunovska K, Loughrey D, Dahlman J E. Drug delivery systems for RNA therapeutics. Nat Rev Drug Discov. 2022;23:265-280
248. Dormont F, Brusini R, Cailleau C, Reynaud F, Peramo A, Gendron A. et al. Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Sci Adv. 2020;6:eaaz5466
249. Chen W, Schilperoort M, Cao Y, Shi J, Tabas I, Tao W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19:228-249
250. Zhou L, Li Y, Liang Q, Liu J, Liu Y. Combination therapy based on targeted nano drug co-delivery systems for liver fibrosis treatment: a review. J Drug Target. 2022;30:577-588
251. Gao J, Wang S, Dong X, Leanse L G, Dai T, Wang Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun Biol. 2020;3:680
252. Deng Z, Liu S. Inflammation-responsive delivery systems for the treatment of chronic inflammatory diseases. Drug Deliv Transl Res. 2021;11:1475-1497
253. Jung W, Lee D Y, Moon E, Jon S. Nanoparticles derived from naturally occurring metal chelators for theranostic applications. Adv Drug Deliv Rev. 2022;191:114620
254. Kim W Y, Won M, Koo S, Zhang X, Kim J S. Mitochondrial H2Sn-mediated anti-inflammatory theranostics. Nanomicro Lett. 2021;13:168
255. Ahamad N, Prabhakar A, Mehta S, Singh E, Bhatia E, Sharma S. et al. Trigger-responsive engineered-nanocarriers and image-guided theranostics for rheumatoid arthritis. Nanoscale. 2020;12:12673-12697
256. Ma B, Xu H, Wang Y, Yang L, Zhuang W, Li G. et al. Biomimetic-coated nanoplatform with lipid-specific imaging and ROS responsiveness for atherosclerosis-targeted theranostics. ACS Appl Mater Interfaces. 2021;13:35410-35421
257. Liu Y, Chen L, Chen Z, Liu M, Li X, Kou Y. et al. Multifunctional janus nanoplatform for efficiently synergistic theranostics of rheumatoid arthritis. ACS nano. 2023;17:8167-8182
258. Hou M, Wu X, Zhao Z, Deng Q, Chen Y, Yin L. Endothelial cell-targeting, ROS-ultrasensitive drug/siRNA co-delivery nanocomplexes mitigate early-stage neutrophil recruitment for the anti-inflammatory treatment of myocardial ischemia reperfusion injury. Acta Biomater. 2022;143:344-355
259. Zhao R, Ning X, Wang M, Wang H, Xing G, Wang L. et al. A ROS-responsive simvastatin nano-prodrug and its fibronectin-targeted co-delivery system for atherosclerosis treatment. ACS Appl Mater Interfaces. 2022;14:25080-25092
260. Li X, Gu J, Xiao Q, Liu Y, Zhou P, Fan L. et al. Liposomal codelivery of inflammation inhibitor and collagen protector to the plaque for effective anti-atherosclerosis. Chinese Chem Lett. 2022;34:107483
261. Li S, Lu Z, Huang Y, Wang Y, Jin Q, Shentu X. et al. Anti-oxidative and anti-inflammatory micelles: break the dry eye vicious cycle. Adv Sci. 2022;9:2200435
262. Li Y, Liang Q, Zhou L, Cao Y, Yang J, Li J. et al. An ROS-responsive artesunate prodrug nanosystem co-delivers dexamethasone for rheumatoid arthritis treatment through the HIF-1α/NF-κB cascade regulation of ROS scavenging and macrophage repolarization. Acta Biomater. 2022;152:406-424
263. Deng J, Lin D, Ding X, Wang Y, Hu Y, Shi H. et al. Multifunctional supramolecular filament hydrogel boosts anti-inflammatory efficacy in vitro and in vivo. Adv Funct Mater. 2022;32:2109173
264. Kim T, Suh J, Kim W J. Polymeric aggregate-embodied hybrid nitric-oxide-scavenging and sequential drug-releasing hydrogel for combinatorial treatment of rheumatoid arthritis. Adv Mater. 2021;33:2008793
265. Mendell J R, Al-Zaidy S A, Rodino-Klapac L R, Goodspeed K, Gray S J, Kay C N. et al. Current clinical applications of in vivo gene therapy with AAVs. Mol Ther. 2021;29:464-488
266. Liu X, Chen S, Yan Y, Liu L, Chen Y. Nanoparticulate DNA scavenger loading methotrexate targets articular inflammation to enhance rheumatoid arthritis treatment. Biomaterials. 2022;286:121594
267. Wang Y, Wu Q, Wang J, Li L, Sun X, Zhang Z. et al. Co-delivery of p38α MAPK and p65 siRNA by novel liposomal glomerulus-targeting nano carriers for effective immunoglobulin a nephropathy treatment. J Control Release. 2020;320:457-468
268. Song Y, Li M, Song N, Liu X, Wu G, Zhou H. et al. Self-amplifying assembly of peptides in macrophages for enhanced inflammatory treatment. J Am Chem Soc. 2022;144:6907-6917
269. Han F, Yu Q, Chu G, Li J, Zhu Z, Tu Z. et al. Multifunctional nanofibrous scaffolds with angle-ply microstructure and co-delivery capacity promote partial repair and total replacement of intervertebral disc. Adv Healthc Mater. 2022;11:2200895
270. Lu C-H, Hsiao J-K. Diagnostic and therapeutic roles of iron oxide nanoparticles in biomedicine. Tzu Chi Med J. 2022;35:p11-17
271. Chirinos J A, Lopez-Jaramillo P, Giamarellos-Bourboulis E J, Davila-del-Carpio G H, Bizri A R, Andrade-Villanueva J F. et al. A randomized clinical trial of lipid metabolism modulation with fenofibrate for acute coronavirus disease 2019. Nat Metab. 2022;4:1847-1857
272. Wilson B, Geetha K M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J Drug Deliv Sci Technol. 2022;74:103553
Author contact
![]() Corresponding authors: Yuequan Wang, Ph.D. and Cong Luo, Ph.D., Department of Pharmaceutics, Wuya College of Innovation, Shenyang Pharmaceutical University, No. 103 Wenhua Road, Shenyang 110016, China; E-mail address: wangyuequancom; luocongedu.cn.
Corresponding authors: Yuequan Wang, Ph.D. and Cong Luo, Ph.D., Department of Pharmaceutics, Wuya College of Innovation, Shenyang Pharmaceutical University, No. 103 Wenhua Road, Shenyang 110016, China; E-mail address: wangyuequancom; luocongedu.cn.
 Global reach, higher impact
Global reach, higher impact