13.3
Impact Factor
Theranostics 2023; 13(15):5483-5500. doi:10.7150/thno.85106 This issue Cite
Research Paper
Nanobody-mediated SPECT/CT imaging reveals the spatiotemporal expression of programmed death-ligand 1 in response to a CD8+ T cell and iNKT cell activating mRNA vaccine
1. Laboratory for Molecular and Cellular Therapy, Vrije Universiteit Brussel, Laarbeeklaan 103, B-1090 Brussels, Belgium.
2. Ghent research Group on Nanomedicines, Laboratory of Physical Pharmacy and General Biochemistry, Department of Pharmaceutics, Ghent University, Ottergemsesteenweg 460, B-9000 Gent, Belgium.
3. Cancer Research Institute Ghent (CRIG), Ghent University Hospital, Ghent University, Ghent B-9000, Belgium.
4. Biostatistics and Medical Informatics Research Group, Vrije Universiteit Brussel, Laarbeeklaan 103, B-1090 Brussels, Belgium.
5. Laboratory of Medicinal Chemistry, Department of Pharmaceutics, Ghent University, Ottergemsesteenweg 460, B-9000, Belgium.
6. Beta Cell Neogenesis (BENE), Vrije Universiteit Brussel, Laarbeeklaan 103, Brussels, Belgium.
7. Visual and Spatial Tissue Analysis (VSTA) Core Facility, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium
8. Universitair Ziekenhuis Brussel (UZ Brussel), Department of Pediatrics, Division of Pediatric Endocrinology, Brussels, Belgium.
9. Medical Imaging department, In Vivo Cellular and Molecular Imaging Laboratory, Vrije Universiteit Brussel, Laarbeeklaan 103, B-1090 Brussels, Belgium.
10. Nuclear Medicine Department, UZ Brussel, Laarbeeklaan 101, B-1090 Brussels, Belgium.
# The co-first authors contributed equally to this work.
* The co-last authors contributed equally to this work.
Abstract
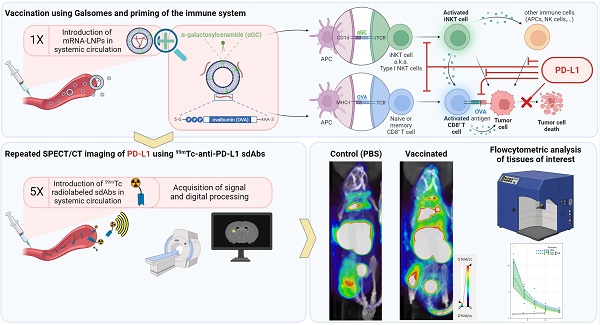
Rationale: Although promising responses are obtained in patients treated with immune checkpoint inhibitors targeting programmed death ligand 1 (PD-L1) and its receptor programmed death-1 (PD-1), only a fraction of patients benefits from this immunotherapy. Cancer vaccination may be an effective approach to improve the response to immune checkpoint inhibitors anti-PD-L1/PD-1 therapy. However, there is a lack of research on the dynamics of PD-L1 expression in response to cancer vaccination.
Methods: We performed non-invasive whole-body imaging to visualize PD-L1 expression at different timepoints after vaccination of melanoma-bearing mice. Mice bearing ovalbumin (OVA) expressing B16 tumors were i.v. injected with the Galsome mRNA vaccine: OVA encoding mRNA lipoplexes co-encapsulating a low or a high dose of the atypical adjuvant α-galactosylceramide (αGC) to activate invariant natural killer T (iNKT) cells. Serial non-invasive whole-body immune imaging was performed using a technetium-99m (99mTc)-labeled anti-PD-L1 nanobody, single-photon emission computerized tomography (SPECT) and X-ray computed tomography (CT) images were quantified. Additionally, cellular expression of PD-L1 was evaluated with flow cytometry.
Results: SPECT/CT-imaging showed a rapid and systemic upregulation of PD-L1 after vaccination. PD-L1 expression could not be correlated to the αGC-dose, although we observed a dose-dependent iNKT cell activation. Dynamics of PD-L1 expression were organ-dependent and most pronounced in lungs and liver, organs to which the vaccine was distributed. PD-L1 expression in lungs increased immediately after vaccination and gradually decreased over time, whereas in liver, vaccination-induced PD-L1 upregulation was short-lived. Flow cytometric analysis of these organs further showed myeloid cells as well as non-immune cells with elevated PD-L1 expression in response to vaccination. SPECT/CT imaging of the tumor demonstrated that the expression of PD-L1 remained stable over time and was overall not affected by vaccination although flow cytometric analysis at the cellular level demonstrated changes in PD-L1 expression in various immune cell populations following vaccination.
Conclusion: Repeated non-invasive whole-body imaging using 99mTc-labeled anti-PD-L1 nanobodies allows to document the dynamic nature of PD-L1 expression upon vaccination. Galsome vaccination rapidly induced systemic upregulation of PD-L1 expression with the most pronounced upregulation in lungs and liver while flow cytometry analysis showed upregulation of PD-L1 in the tumor microenvironment. This study shows that imaging using nanobodies may be useful for monitoring vaccine-mediated PD-L1 modulation in patients and could provide a rationale for combination therapy. To the best of our knowledge, this is the first report that visualizes PD-L1 expression upon cancer vaccination.
Keywords: melanoma, mRNA vaccine, programmed death-ligand 1, nanobody, single-photon emission computerized tomography/computed tomography
 Global reach, higher impact
Global reach, higher impact