13.3
Impact Factor
Theranostics 2023; 13(15):5305-5321. doi:10.7150/thno.84429 This issue Cite
Research Paper
Universal theranostic CRISPR/Cas13a RNA-editing system for glioma
1. Tianjin Neurological Institute, Tianjin Medical University General Hospital, Key Laboratory of Post-neurotrauma Neuro-repair and Regeneration in Central Nervous System, Ministry of Education, Tianjin City, Tianjin 300052, China.
2. Department of Dermatovenereology, Tianjin Medical University General Hospital, Tianjin 300052, China.
3. Tianjin Key Laboratory of Composite and Functional Materials, School of Materials Science and Engineering, Tianjin University, Tianjin, China.
4. College of Pharmacy, Kunming Medical University, Yunnan, China.
* These authors contributed equally to this work.
Abstract
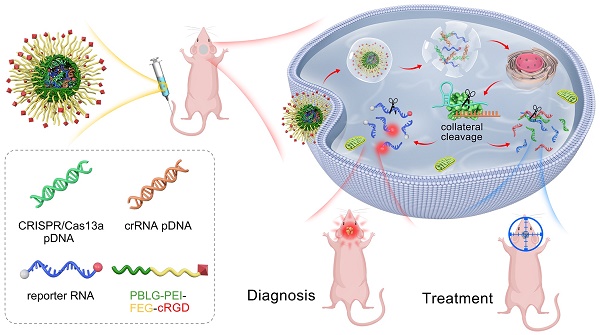
Background: The CRISPR/Cas13a system offers the advantages of rapidity, precision, high sensitivity, and programmability as a molecular diagnostic tool for critical illnesses. One of the salient features of CRISPR/Cas13a-based bioassays is its ability to recognize and cleave the target RNA specifically. Simple and efficient approaches for RNA manipulation would enrich our knowledge of disease-linked gene expression patterns and provide insights into their involvement in the underlying pathomechanism. However, only a few studies reported the Cas13a-based reporter system for in vivo molecular diagnoses.
Methods: A tiled crRNA pool targeting a particular RNA transcript was generated, and the optimally potential crRNA candidates were selected using bioinformatics modeling and in vitro biological validation methods. For in vivo imaging assessment of the anti-GBM effectiveness, we exploited a human GBM patient-derived xenograft model in nude mice.
Results: The most efficient crRNA sequence with a substantial cleavage impact on the target RNA as well as a potent collateral cleavage effect, was selected. In the xenografted GBM rodent model, the Cas13a-based reporter system enabled us in vivo imaging of the tumor growth. Furthermore, systemic treatments using this approach slowed tumor progression and increased the overall survival time in mice.
Conclusions: Our work demonstrated the clinical potential of a Cas13a-based in vivo imaging method for the targeted degradation of specific RNAs in glioma cells, and suggested the feasibility of a tailored approach like Cas13a for the modulation of diagnosis and treatment options in glioma.
Keywords: CRISPR/Cas13a, in vivo detection, IDH1, Glioblastoma, anti-tumor therapy
 Global reach, higher impact
Global reach, higher impact