13.3
Impact Factor
Theranostics 2023; 13(14):5057-5074. doi:10.7150/thno.84937 This issue Cite
Research Paper
Exosomal transfer leads to chemoresistance through oxidative phosphorylation-mediated stemness phenotype in colorectal cancer
1. Clinical Research Center (CRC), Medical Pathology Center (MPC), Cancer Early Detection and Treatment Center (CEDTC), Translational Medicine Research Center (TMRC), Chongqing University Three Gorges Hospital, Chongqing University, Wanzhou, Chongqing, China.
2. Longgang District Maternity & Child Healthcare Hospital of Shenzhen City (Longgang Maternity and Child Institute of Shantou University Medical College), Shenzhen 518172, China.
3. Hunan Zixing Intelligent Medical Technology Co., Ltd., Changsha 410221, China.
4. Richard Dimbleby Laboratory of Cancer Research, School of Cancer & Pharmaceutical Sciences, King's College London, London SE1 1UL, UK.
5. Oncology Department, Punan Hospital of Pudong New District, Shanghai 200125, China.
6. Department of Rheumatology and Immunology, Peking University People's Hospital, Beijing 100191, PR China.
7. Internet Medical and System Applications of National Engineering Laboratory, Zhengzhou, China.
8. Department of Pathology, Beijing Ditan Hospital, Capital Medical University, Beijing, China.
9. Department of General Surgery, Peking University Third Hospital, Beijing, China
10. Department of Chemistry, Physical & Theoretical Chemistry Laboratory, University of Oxford, South Parks Road, Oxford OX1 3QZ, United Kingdom
11. Cancer Institute, Paul O'Gorman Building, University College London, London, UK.
12. Chongqing University Medical School, Chongqing 400044, China.
13. Department of Interventional Radiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
* These authors contributed equally to this work
Abstract
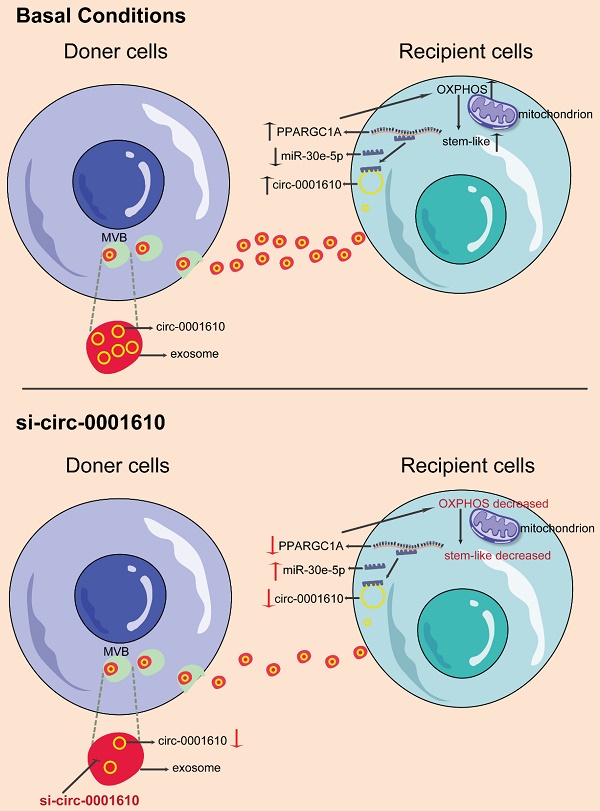
Background: Recently years have seen the increasing evidence identifying that OXPHOS is involved in different processes of tumor progression and metastasis and has been proposed to be a potential therapeutical target for cancer treatment. However, the exploration in oxidative phosphorylation-mediated chemoresistance is still scarce. In our study, we identify exosomal transfer leads to chemoresistance by reprogramming metabolic phenotype in recipient cells.
Methods: RNA sequencing analysis was used to screen altered targets mediating exosome transfer-induced chemoresistance. Seahorse assay allowed us to measure mitochondrial respiration. Stemness was measured by spheroids formation assay. Serum exosomes were isolated for circ_0001610 quantification.
Results: The induced oxidative phosphorylation leads to more stem-like properties, which is dependent on the transfer of exosomal circ_0001610. Exosome transfer results in the removal of miR-30e-5p-mediated suppression of PGC-1a, a master of mitochondrial biogenesis and function. Consequently, increased PGC-1a reshapes cellular metabolism towards oxidative phosphorylation, leading to chemoresistance. Inhibition of OXPHOS or exosomal si-circ_0001610 increases the sensitivity of chemotherapy by decreasing cell stemness in vitro and in vivo.
Conclusion: Our data suggests that exosomal circ_0001610-induced OXPHOS plays an important role in chemoresistance and supports a therapeutical potential of circ_0001610 inhibitors in the treatment of oxaliplatin-resistant colorectal cancer by manipulating cell stemness.
Keywords: Oxidative Phosphorylation, chemoresistance, PGC-1a, stemness, exosome
 Global reach, higher impact
Global reach, higher impact