13.3
Impact Factor
Theranostics 2023; 13(14):4993-5016. doi:10.7150/thno.87968 This issue Cite
Research Paper
Metabolite Neu5Ac triggers SLC3A2 degradation promoting vascular endothelial ferroptosis and aggravates atherosclerosis progression in ApoE-/-mice
1. College of Pharmacy, Chongqing Medical University, 400010, Chongqing, China.
2. Chongqing Key Laboratory for Pharmaceutical Metabolism Research, 400010, Chongqing, China.
3. Xi'an No.1 Hospital, The First Affiliated Hospital of Northwest University, Xi'an, 710002, Shaanxi, China.
Abstract
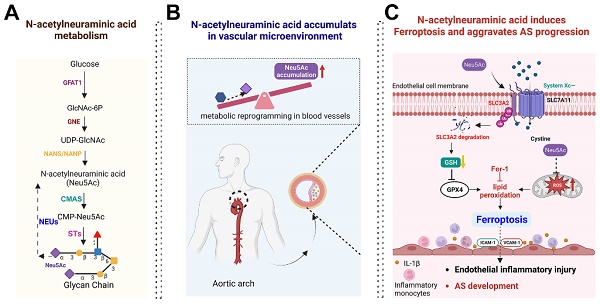
Background: Atherosclerosis (AS) is still the major cause of cardiovascular disease (CVD) as well as stroke. Endothelial metabolic disorder has been found to be activated and then promote endothelial cells (ECs) injury, which is regarded to initiate AS progression. N-acetylneuraminic acid (Neu5Ac), a metabolite produced by hexosamine-sialic acid pathway branching from glucose metabolism, was presented as a notable biomarker of CVD and is positively correlated with ECs function. However, few studies explain whether Neu5Ac regulate AS progression by affecting EC function as well as its involved mechanisms are still unknown.
Methods: Here, we mimicked an animal model in ApoE-/- mice which displaying similar plasma Neu5Ac levels with AS model to investigate its effect on AS progression.
Results: We found that Neu5Ac exacerbated plaques area and increased lipids in plasma in absence of HFD feeding, and ECs inflammatory injury was supposed as the triggering factor upon Neu5Ac treatment with increasing expression of IL-1β, ICAM-1, and promoting ability of monocyte adhesion to ECs. Mechanistic studies showed that Neu5Ac facilitated SLC3A2 binding to ubiquitin and then triggered P62 mediated degradation, further leading to accumulation of lipid peroxidation in ECs. Fer-1 could inhibit ECs injury and reverse AS progression induced by Neu5Ac in ApoE-/- mice. Interestingly, mitochondrial dysfunction was also partly participated in ECs injury after Neu5Ac treatment and been reversed by Fer-1.
Conclusions: Together, our study unveils a new mechanism by which evaluated metabolite Neu5Ac could promote SLC3A2 associated endothelial ferroptosis to activate ECs injury and AS plaque progression, thus providing a new insight into the role of Neu5Ac-ferroptosis pathway in AS. Also, our research revealed that pharmacological inhibition of ferroptosis may provide a novel therapeutic strategy for premature AS.
Keywords: atherosclerosis, endothelial inflammatory injury, Neu5Ac, ferroptosis, SLC3A2
 Global reach, higher impact
Global reach, higher impact