13.3
Impact Factor
Theranostics 2023; 13(14):4711-4729. doi:10.7150/thno.85663 This issue Cite
Research Paper
Tuning ultrasmall theranostic nanoparticles for MRI contrast and radiation dose amplification
1. Department of Physics, Northeastern University, Boston 02115, USA.
2. Department of Radiation Oncology, Brigham and Women's Hospital, Dana-Farber Cancer Institute, and Harvard Medical School, Boston 02115, USA.
3. NH TherAguix, Meylan 38240, France.
4. Institut Lumière-Matière, UMR 5306, Université Lyon1-CNRS, Université de Lyon, Villeurbanne Cedex 69100, France.
5. Department of Physics and Applied Physics, University of Massachusetts Lowell, Lowell 01854, USA.
6. Department of Pathology, Harvard Medical School and Dana-Farber Cancer Institute, Boston 02115, USA.
7. John B. Little Center for Radiation Sciences, Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, 02115, USA.
8. Laboratory of Systems Pharmacology, Harvard Program in Therapeutic Science, Department of Systems Biology, Harvard Medical School, Boston, MA, 02115, USA.
9. Molecular and Integrative Physiological Sciences Program, Harvard T.H. Chan School of Public Health, Boston, MA, 02115, USA.
10. Department of Medical Oncology, Dana-Farber Cancer Institute/ Harvard Cancer Center, Boston, MA, 02115, USA.
11. Institut des Sciences Analytiques, Université de Lyon, CNRS, Université Claude Bernard Lyon 1, UMR 5280, 69100, Villeurbanne, France.
12. Institut Universitaire de France (IUF), Paris 75005, France.
#These authors contributed equally to this work.
Abstract
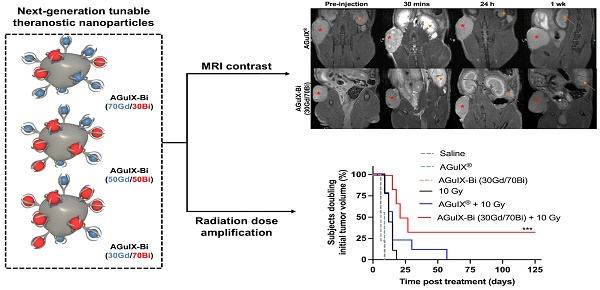
Background: The introduction of magnetic resonance (MR)-guided radiation treatment planning has opened a new space for theranostic nanoparticles to reduce acute toxicity while improving local control. In this work, second-generation AGuIX® nanoparticles (AGuIX-Bi) are synthesized and validated. AGuIX-Bi are shown to maintain MR positive contrast while further amplifying the radiation dose by the replacement of some Gd3+ cations with higher Z Bi3+. These next-generation nanoparticles are based on the AGuIX® platform, which is currently being evaluated in multiple Phase II clinical trials in combination with radiotherapy.
Methods: In this clinically scalable methodology, AGuIX® is used as an initial chelation platform to exchange Gd3+ for Bi3+. AGuIX-Bi nanoparticles are synthesized with three ratios of Gd/Bi, each maintaining MR contrast while further amplifying radiation dose relative to Bi3+. Safety, efficacy, and theranostic potential of the nanoparticles were evaluated in vitro and in vivo in a human non-small cell lung cancer model.
Results: We demonstrated that increasing Bi3+ in the nanoparticles is associated with more DNA damage and improves in vivo efficacy with a statistically significant delay in tumor growth and 33% complete regression for the largest Bi/Gd ratio tested. The addition of Bi3+ by our synthetic method leads to nanoparticles that present slightly altered pharmacokinetics and lengthening of the period of high tumor accumulation with no observed evidence of toxicity.
Conclusions: We confirmed the safety and enhanced efficacy of AGuIX-Bi with radiation therapy at the selected ratio of 30Gd/70Bi. These results provide crucial evidence towards patient translation.
Keywords: nanoparticle, bismuth, theranostics, magnetic resonance, radiation therapy
 Global reach, higher impact
Global reach, higher impact