13.3
Impact Factor
Theranostics 2023; 13(13):4482-4496. doi:10.7150/thno.84921 This issue Cite
Research Paper
Restoration of CPT1A-mediated fatty acid oxidation in mesothelial cells protects against peritoneal fibrosis
1. Division of Nephrology, Nanfang Hospital, Southern Medical University, State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of Nephrology, 1838 North Guangzhou Ave, Guangzhou 510515, P. R. China
2. Division of Nephrology, The Second Affiliated Hospital of Guangzhou Medical University, Changgang East Road, Guangzhou 510260, P.R. China
3. Department of Urology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong medicine and Health Key Laboratory of Organ Transplantation and Nephrosis, Shandong Institute of Nephrology, Jinan 250013, P.R. China
#These authors contributed equally to this work.
Abstract
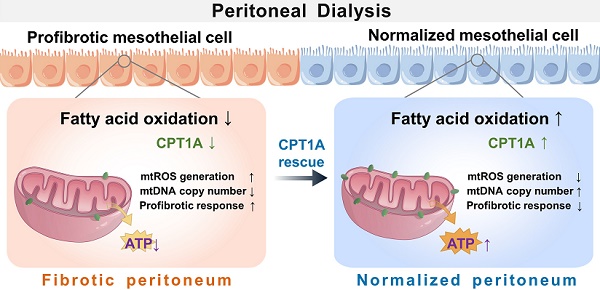
Background: Peritoneal dialysis (PD) is limited by gradual fibrotic remodeling in the peritoneum, a process involving profibrotic response of mesothelial cells. However, the role of fatty acid oxidation (FAO) and carnitine palmitoyltransferase 1A (CPT1A) in this process remains unexplored.
Methods: FAO and CPT1A expression were characterized in mesothelial cells from patients on long-term PD and from a mouse model of PD using multiple experimental methods, including single-cell sequencing, seahorse assay, real-time quantitative PCR, Western blot, and immunofluorescence staining. Overexpression of CPT1A was achieved in a human mesothelial cell line and in primary mouse mesothelial cells. Finally, genetic and pharmacological manipulations of CPT1A were performed in a mouse model of PD.
Results: Herein, FAO and CPT1A expression were reduced in mesothelial cells from patients on long-term PD, which negatively correlated with expression of fibrogenic markers in these cells. This was corroborated in PD mice, as well as in mouse and human mesothelial cells incubated with transforming growth factor (TGF) β1. CPT1A overexpression in mesothelial cells, which prevented TGFβ1-induced suppression of mitochondrial respiration, restored cellular ATP levels and downregulated the expression of fibrogenic markers. Furthermore, restoration of FAO by overexpressing CPT1A in PD mice reversed profibrotic phenotype in mesothelial cells and reduced fibrotic lesions in the peritoneum. Treatment with the CPT1A activator C75 induced similar therapeutic benefit in PD mice. In contrast, inhibition of FAO with a CPT1 inhibitor caused more severe fibrosis in PD mice.
Conclusions: A defective FAO is responsible for the profibrotic response of mesothelial cells and thus the peritoneal fibrogenesis. This aberrant metabolic state could be improved by modulating CPT1A in mesothelial cells, suggesting FAO enhancement in mesothelial cells is a potential treatment of peritoneal fibrosis.
Keywords: peritoneal fibrosis, mesothelial cell, fatty acid oxidation, carnitine palmitoyltransferase 1A, peritoneal dialysis
 Global reach, higher impact
Global reach, higher impact