13.3
Impact Factor
Theranostics 2023; 13(13):4356-4375. doi:10.7150/thno.84655 This issue Cite
Research Paper
TRPM2 protects against cisplatin-induced acute kidney injury and mitochondrial dysfunction via modulating autophagy
1. Kidney Disease Center, The First Affiliated Hospital, Zhejiang University School of Medicine; Institute of Nephrology, Zhejiang University; Key Laboratory of Kidney Disease Prevention and Control Technology, Zhejiang Province; Zhejiang Clinical Research Center of Kidney and Urinary System Disease, Hangzhou 310003, China.
2. Department of Infectious Disease, Sir Run Run Shaw Hospital, Zhejiang University School of medicine, Hangzhou 310003, China.
3. International Institutes of Medicine, The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu 322000, China.
4. Department of Biophysics, and Department of Neurology of the Fourth Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China.
5. The Children's Hospital, Zhejiang University School of medicine, Hangzhou 310003, China.
6. Sino-UK Joint Laboratory of Brain Function and Injury of Henan Province, and Department of Physiology and Pathophysiology, Xinxiang Medical University, P.R. China.
7. A4245-Transplantation, Immunology and Inflammation, Faculty of Medicine, University of Tours, France.
8. School of Biomedical Sciences, Faculty of Biological Sciences, University of Leeds, UK.
* These authors contributed equally to this work.
Abstract
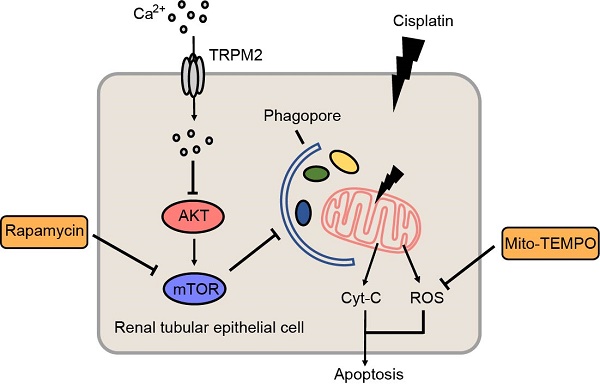
Background: Cisplatin is a widely used anti-tumor agent but its use is frequently limited by nephrotoxicity. Transient receptor potential melastatin 2 (TRPM2) is a non-selective cation channel which is generally viewed as a sensor of oxidative stress, and increasing evidence supports its link with autophagy, a critical process for organelle homeostasis.
Methods: Cisplatin-induced cell injury and mitochondrial damage were both assessed in WT and Trpm2-knockout mice and primary cells. RNA sequencing, immunofluorescence staining, immunoblotting and flowcytometry were applied to interpret the mechanism of TRPM2 in cisplatin nephrotoxicity.
Results: Knockout of TRPM2 exacerbates renal dysfunction, tubular injury and cell apoptosis in a model of acute kidney injury (AKI) induced by treatment with cisplatin. Cisplatin-caused tubular mitochondrial damage is aggravated in TRPM2-deficient mice and cells and, conversely, alleviated by treatment with Mito-TEMPO, a mitochondrial ROS scavenger. TRPM2 deficiency hinders cisplatin-induced autophagy via blockage of Ca2+ influx and subsequent up-regulation of AKT-mTOR signaling. Consistently, cisplatin-induced tubular mitochondrial damage, cell apoptosis and renal dysfunction in TRPM2-deficient mice are mitigated by treatment with a mTOR inhibitor.
Conclusion: Our results suggest that the TRPM2 channel plays a protective role in cisplatin-induced AKI via modulating the Ca2+-AKT-mTOR signaling pathway and autophagy, providing novel insights into the pathogenesis of kidney injury.
Keywords: TRPM2, autophagy, mitochondria, cisplatin, acute kidney injury
 Global reach, higher impact
Global reach, higher impact