13.3
Impact Factor
Theranostics 2023; 13(11):3826-3843. doi:10.7150/thno.82543 This issue Cite
Research Paper
Therapeutic silencing of lncRNA RMST alleviates cardiac fibrosis and improves heart function after myocardial infarction in mice and swine
1. Translational Medical Center for Stem Cell Therapy & Institute for Regenerative Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200123, China.
2. Department of Thoracic Cardiovascular Surgery, The Eighth Affiliated Hospital of Sun Yat-sen University, Shenzhen, Guangdong 518033, China.
3. Department of Cardiovascular and Thoracic Surgery, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, China.
4. Department of Radiology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, China.
5. The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning 121001, China.
6. Department of Respiratory Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, China.
7. Shanghai Institute of Stem Cell Research and Clinical Translation, Shanghai East Hospital, Tongji University, Shanghai 200120, China.
# Equal contribution authors
Abstract
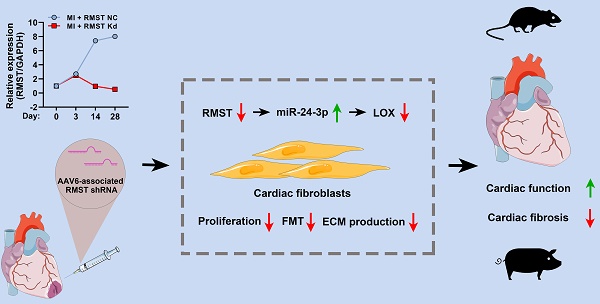
Rationale: Cardiac fibrosis is an adverse consequence of aberrant fibroblast activation and extracellular matrix (ECM) deposition following myocardial infarction (MI). Recently, long noncoding RNAs (lncRNAs) have been reported to participate in multiple cardiac diseases. However, the biological functions of lncRNA rhabdomyosarcoma 2-associated transcript (RMST) in cardiac fibrosis remain largely unknown.
Methods: The role of RMST in regulating cardiac fibroblast (CF) proliferation, fibroblast-to-myofibroblast transition (FMT), and ECM production, which were induced by transforming growth factor-β1, was evaluated through immunofluorescence staining, cell contraction assay, cell migration assay, qRT-PCR, and western blot. The therapeutic effect of RMST silencing was assessed in murine and porcine MI models.
Results: The present study showed that RMST expression was upregulated and associated with cardiac fibrosis in murine and porcine MI models. Further loss-of-function studies demonstrated that RMST silencing in vitro significantly inhibited CF proliferation, FMT, and ECM production. Accordingly, RMST knockdown in vivo alleviated cardiac fibrosis and improved cardiac contractile function in MI mice. Moreover, RMST acted as a competitive endogenous RNA of miR-24-3p. miR-24-3p inhibition abolished, while miR-24-3p agomir reproduced, the RMST knockdown-mediated effects on CF fibrosis by regulating the lysyl oxidase signaling pathway. Finally, the therapeutic potential of RMST knockdown was evaluated in a porcine MI model, and local RMST knockdown significantly inhibited cardiac fibrosis and improved myocardial contractile function in pigs after MI.
Conclusion: Our findings identified RMST as a crucial regulator of cardiac fibrosis, and targeting RMST may develop a novel and efficient therapeutic strategy for treating fibrosis-related cardiac diseases.
Keywords: cardiac fibrosis, lncRNA RMST, miR-24-3p, lysyl oxidase, pig
 Global reach, higher impact
Global reach, higher impact