13.3
Impact Factor
Theranostics 2023; 13(11):3781-3793. doi:10.7150/thno.85323 This issue Cite
Research Paper
Platelet membrane-coated alterbrassicene A nanoparticle inhibits calcification of the aortic valve by suppressing phosphorylation P65 NF-κB
1. Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
2. Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China.
3. Department of Cardiovascular Surgery, Zhongnan Hospital of Wuhan University, Wuhan 430071, China.
†These authors have contributed equally to this work.
Abstract
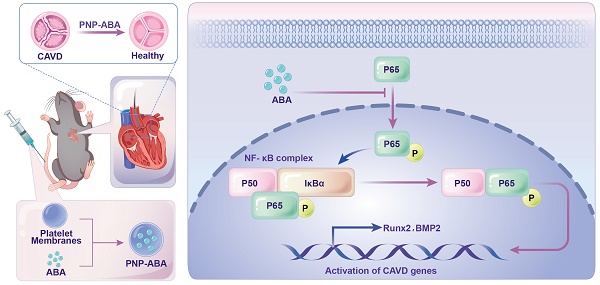
Rationale: Calcific aortic valve disease (CAVD) is a leading cause of cardiovascular mortality and morbidity with increasing prevalence and incidence. The pathobiology of CAVD involves valvular fibrocalcification, and osteogenic and fibrogenic activities are elevated in aortic valve interstitial cells (VICs) from diseased valves. It has been demonstrated that activated NF-κB pathway was present in the early stage of CAVD process. There is currently no effective clinical drugs targeting NF-κB pathway for CAVD treatment. Therefore, it is of great clinical significance to seek effective treatments for valve calcification.
Methods: In this study, we established immortal human valve interstitial cells (im-hVICs) with pGMLV-SV40T-puro lentivirus. Alizarin red staining and western blotting were performed to evaluate the calcification of immortal VICs supplemented with different compounds. The natural fusicoccane diterpenoid alterbrassicene A (ABA) was found to have potential therapeutic functions. Ribonucleic acid sequencing was used to identify the potential target of ABA. Platelet membrane-coated nanoparticle of ABA (PNP-ABA) was fabricated and the IBIDI pump was used to evaluate the adhesion ability of PNP-ABA. Murine wire-induced aortic valve stenosis model was conducted for in vivo study of PNP-ABA.
Results: The natural fusicoccane diterpenoid ABA was found to significantly reduce the calcification of human VICs during osteogenic induction via inhibiting the phosphorylation P65. Runt-related transcription factor 2 (Runx2) and bone morphogenetic protein-2 (BMP2) were down regulated with the treatment of ABA in human VICs. Additionally, molecular docking results revealed that ABA bound to RelA (P65) protein. Phosphorylation of P65 (Ser536) was alleviated by ABA treatment, as well as the nuclear translocation of P65 during osteogenic induction in human VICs. Alizarin red staining showed that ABA inhibited osteogenic differentiation of VICs in a dose-dependent manner. PNP-ABA attenuated aortic valve calcification in murine wire-induced aortic valve stenosis model in vivo.
Conclusions: The establishment of im-hVICs provides a convenient cell line for the study of CAVD. Moreover, our current research highlights a novel natural compound, ABA, as a promising candidate to prevent the progression of CAVD.
Keywords: natural product, calcific aortic valve disease, immortalization, NF-κB, nanoparticle
 Global reach, higher impact
Global reach, higher impact