13.3
Impact Factor
Theranostics 2023; 13(11):3655-3674. doi:10.7150/thno.85823 This issue Cite
Review
LncRNAs associated with oxidative stress in diabetic wound healing: Regulatory mechanisms and application prospects
1. Key Laboratory of Medical Electrophysiology, Ministry of Education, Drug Discovery Research Center, Southwest Medical University, Luzhou, China.
2. Laboratory for Cardiovascular Pharmacology, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, China.
3. Luzhou Municipal Key Laboratory of Thrombosis and Vascular Biology, Luzhou, Sichuan, China.
4. Department of General Surgery (Thyroid Surgery), the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
5. Metabolic Vascular Diseases Key Laboratory of Sichuan Province, Luzhou, Sichuan, China.
6. Department of Pharmacy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
# Qinzhi Yang and Dan Fang have contributed equally to this work.
Received 2023-5-3; Accepted 2023-6-12; Published 2023-6-26
Abstract
Diabetes is a group of chronic diseases with blood glucose imbalance, and long-term hyperglycaemia causes sustained damage to various organs of the body, resulting in vascular lesions, neuropathy and impaired wound healing. Diabetic wound formation involves a variety of complex mechanisms, and they are characterized by a persistent chronic inflammatory response, degradation of angiogenesis and imbalance of extracellular matrix regulation, all of which are related to oxidative stress. Additionally, repair and healing of diabetic wounds require the participation of a variety of cells, cytokines, genes, and other factors, which together constitute a complex biological regulatory network. Recent studies have shown that long noncoding RNAs (lncRNAs) can be involved in the regulation of several key biological pathways and cellular functions demonstrating their critical role in diabetic wound healing. LncRNAs are a major family of RNAs with limited or no protein-coding function. Numerous studies have recently reported a strong link between oxidative stress and lncRNAs. Given that both lncRNAs and oxidative stress have been identified as potential drivers of diabetic wound healing, their link in diabetic wound healing can be inferred. However, the specific mechanism of oxidative stress related to lncRNAs in diabetic wound healing is still unclear, and elucidating the functions of lncRNAs in these processes remains a major challenge. This article reviews the mechanisms of lncRNAs related to oxidative stress in several stages of diabetic wound healing and discusses diagnostic and treatment potential of lncRNAs to treat diabetic wounds by improving oxidative stress, as well as the challenges of using lncRNAs for this purpose. It is hoped that these results will provide new targets and strategies for the diagnosis and treatment of impaired wound healing in diabetic patients.
Keywords: diabetes, wound healing, oxidative stress, lncRNAs, therapeutic application
Introduction
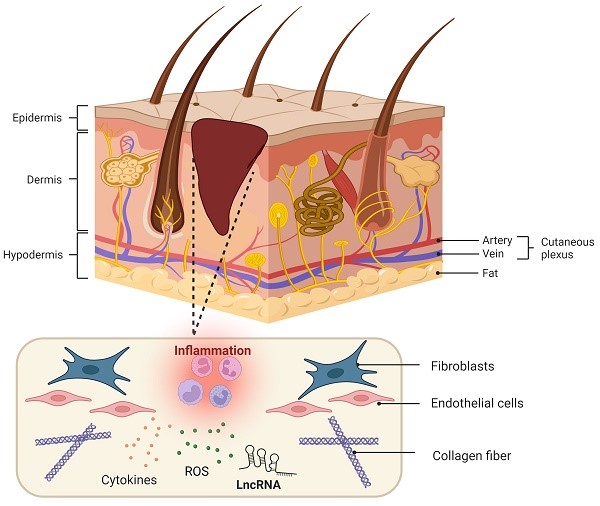
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycaemia resulting from the interaction of genetic and environmental factors. DM leads to insulin resistance and islet dysfunction and causes high morbidity and disability that pose a serious threat to human health [1]. Diabetic cutaneous ulcers (DCU) are caused by long-term poor blood glucose control in diabetic patients, which leads to neuropathy and various degrees of peripheral vascular lesions, resulting in lower limb infection, ulceration, and/or deep tissue destruction [2, 3]. DCU is the most common injury leading to lower limb amputation and is one of the serious complications that can result in the death and disability of diabetic patients [4]. However, the pathogenesis of DCU has not been fully understood. Hyperglycaemia-induced DCU has been associated with lipid peroxidation, oxidative stress (OS), cell apoptosis and a variety of signal transduction pathways [5, 6]. For diabetic patients, a long-term high glucose environment promotes the production of reactive oxygen species, thereby promoting the inflammation cascade reaction and stimulating oxidative stress, thus forming a vicious circle and eventually leading to difficulty healing wounds [6]. Additionally, diabetic patients with hyperglycaemia have increased lipid peroxidation-associated oxidative stress and reduced antioxidant capacity, stimulating the formation of advanced glycation end products and autooxidative glycosylation, thus enhancing polyol pathway activity and aggravating destructive processes in the feet of diabetic patients [7]. Here, the pathological characteristics of diabetic wounds are shown in Figure 1, which shows that a lack of spontaneous neovascularization, bacterial infection of the refractory wound surface, and poorly differentiated dermal cells are the main causes of DCU healing difficulty, and all of these factors are closely related to oxidative stress.
Pathological characteristics of diabetic wound healing. Diabetic wounds are characterized by difficulty in hemostasis, chronic inflammation, impaired angiogenesis, slowed cell proliferation and delayed extracellular matrix remodeling. After injury, wounded tissue must establish haemostasis via coagulation and clot formation. Injury is quickly followed by immune cell infiltration and inflammation as a means to clear the wound of damaged tissue and microbes, thus preventing infection and facilitating granulation. Subsequently, fibroblasts, keratinocytes and endothelial cells not only proliferate and migrate to the wounds to promote angiogenesis but also deposit ECM and repopulate the injury site, further facilitating wound closure. Finally, matrix deposition and clearance regulate scar formation. However, for diabetic patients, a long-term high glucose environment promotes high ROS levels in inflammatory cells, causing adhesion of white blood cells to the vascular endothelium and further promoting the inflammation cascade reaction. In turn, the inflammatory reaction stimulates the generation of oxidative stress, thus forming a vicious cycle. Therefore, under the combined action of various factors, these events influence each other and ultimately inhibit diabetic wound repair.
Diabetic wounds characterized by persistent inflammation and the excessive production of reactive oxygen species, thus resulting in impaired wound healing, are unlike typical wounds in that they are slower to heal, causing treatment with conventional topical medications to be a difficult process [8-10]. LncRNAs are a class of RNA molecules that do not encode proteins featuring large number and wide variety, with transcripts over 200 nucleotides in length [11]. Recently, the abnormal expression of long noncoding RNAs (lncRNAs) in diabetic wounds has received widespread attention since it is an important factor in the development of diabetic wounds [12]. Currently, lncRNAs are increasingly recognized as risk factors for oxidative stress and diabetic wound healing. However, the specific regulatory details and mechanisms are still unclear and require further investigation. In this review, we summarize the current findings on lncRNA-mediated gene regulation of oxidative stress and diabetic wound healing. Furthermore, we describe the specific molecular mechanism underlying the regulation of lncRNA-dependent signaling pathways in different cellular processes. These findings may provide a novel theoretical basis and important targets for the clinical application of various diseases including diabetes mellitus and its complication.
LncRNAs participate in oxidative stress
Biology research deepens the understanding of lncRNAs and their regulatory role in oxidative stress
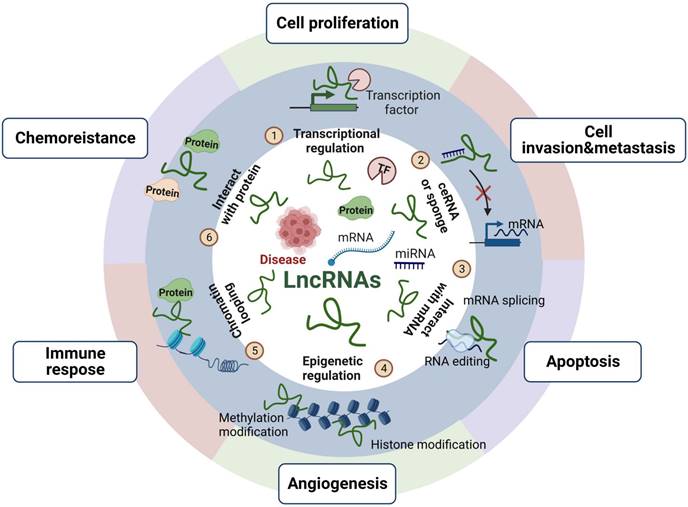
LncRNAs, located in the nucleus and cytoplasm of eukaryotic cells, are a class of noncoding RNAs (>200 bp) that interact with different molecules, such as DNA, RNA or proteins, depending on their subcellular distribution, to modulate gene transcription and kinase cascades [13, 14]. LncRNAs have cis- or trans-regulation, with cis- regulation acting on adjacent genes and trans-regulation acting on genes far away from their transcription sites [15]. Moreover, despite lacking an obvious open reading framework, lncRNAs mainly exert their function via epigenetic modification, transcriptional control and translational regulation, as shown in Figure 2, which provides an overview of the mechanisms and functions of lncRNAs. LncRNAs can act as scaffolds to bind a variety of proteins that perform biological functions and can participate in the synthesis and reconstruction of nucleic acid sequences by soliciting or impounding certain protein factors, thereby being involved in a variety of physiological and pathological activities [16]. Additionally, lncRNAs can also act as molecular sponges of miRNAs to regulate the biological activities of cells [17].
LncRNAs play key roles in various cell biological processes, such as cell proliferation, differentiation and apoptosis [18]. Recent studies have demonstrated the importance of lncRNAs in regulating gene expression in various cells under oxidative stress, including macrophages (MФs), endothelial cells (ECs), and vascular smooth muscle cells (VSMCs). An overview of lncRNAs, their targets in oxidative stress and the roles of oxidative stress-associated lncRNAs in different cells and structures is shown in Table 1.
The mechanisms and functions of lncRNAs. LncRNAs play roles in cell proliferation, invasion and metastasis, apoptosis, angiogenesis, immune response and chemoresistance mainly through epigenetic modification, transcriptional control and translational regulation. We reported the functions of lncRNAs as follows: ① recruiting transcription factors to activate or repress gene expression; ② acting as competing endogenous RNAs (ceRNAs) or miRNA sponges to regulate miRNA targets and affect their expression; ③ directly binding to mRNA and regulating mRNA stability; ④ regulating epigenetic modifications include histone and DNA methylation, histone acetylation, and ubiquitination; ⑤ promoting chromatin looping; ⑥ interacting with proteins and controlling protein phosphorylation, acetylation, and ubiquitination at the posttranslational level.
Roles of oxidative stress associated lncRNAs in different cells and structures.
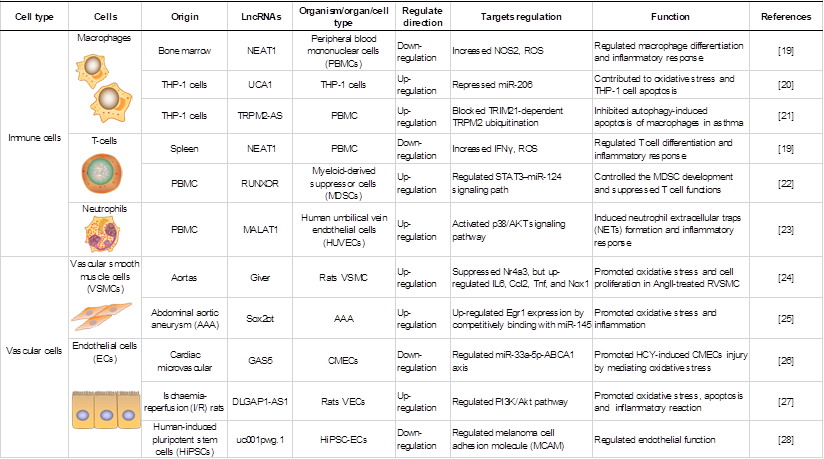
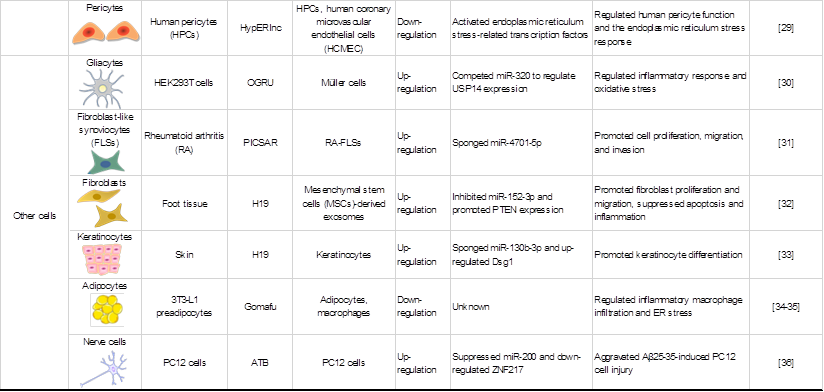
LncRNAs in immune cells are associated with oxidative stress
The activation of immune cells requires the coordinated action of both catabolic and anabolic pathways [37]. To accomplish the pathways, activated immune cells shift towards efficient aerobic glycolysis [38]. Early reports have shown that the lncRNA HULC is involved in the regulation of the cellular glycolytic metabolic pathway by interacting with LDHA and PKM2, two key enzymes in the glycolytic pathway [39]. Liu J et al. [40] also identified an important molecule of lncRNA Actin Gamma 1 PseudoGene (AGPG) that affects the glycolytic activity and cell proliferation of esophageal cancer by binding the metabolic enzyme PFKFB3. Inefficient glucose utilization reduces ATP production, which is closely related to oxidative damage, indicating that oxidative damage to enzymes caused by the abnormal expression of lncRNAs may be an important reason for the inefficiency of glucose utilization, thus affecting the activity of immune cells. In addition, oxidative damage to mitochondrial DNA induced by lncRNAs may also lead to impaired energy production. For example, studies have shown that deletion of the lncRNA NORAD can lead to genomic instability and mitochondrial dysfunction in mice [41].
Endogenous antioxidant molecules play a central role in the activation of immune cells and inflammatory responses. MФs are indispensable in pathophysiology through their diverse roles in metabolism and the immune response [42]. Oxidative phosphorylation (OXPHOS) is inhibited in proinflammatory and activated MФs. The receptors of proinflammatory cytokines such as TNF-α and others on macrophages are coupled to NADPH oxidase (NOX). NOX activation leads to reactive oxygen species (ROS) production within macrophages to counteract the pathogen response or cellular stress response. Previous studies have shown that lncRNAs may be important regulators of TNF-α-mediated oxidative stress in macrophages, suggesting that abnormal expression of lncRNAs can lead to the abnormal expression of NOX regulated by TNF-α, resulting in excessive ROS production. Moreover, Hu et al. [20] found that lncRNA UCA1 sponges miR-206 to aggravate oxidative stress and apoptosis in ox-LDL-induced human macrophages, thus contributing to atherosclerosis. Notably, immune cells are very sensitive to oxidative stress. The high concentration of unsaturated fatty acids in the cell membrane of immune cells and their high sensitivity to peroxidation reactions decreases the immune function of the cells after being stimulated by excessive free radicals, thereby affecting the immune system [43]. Overall, abnormally expressed lncRNAs are involved in various functions of immune cells in the body, which may potentially influence the oxidative stress response of immune cells.
LncRNAs in vascular cells participate in oxidative stress
The two most distinct cell types in the vascular system are endothelial cells (ECs) and mural cells, which include pericytes (PCs) and vascular smooth muscle cells (VSMCs). Interactions of VSMCs, ECs and PCs are important aspects of vascular homeostasis [44]. Recent findings have shown that oxidative damage to vascular cells is correlated with lncRNAs. For example, angiotensin II (AngII) is a major inducer of ROS production in VSMCs. Das S et al. [24] found that knockdown of the lncRNA Giver attenuated AngII-induced intracellular ROS production in rat VSMCs (RVSMCs), thus confirming the functional roles of Giver in oxidative stress. In addition, other studies have shown that overexpressed lncRNA Sox2ot aggravated the oxidative stress and inflammation of VSMCs treated with Ang II, and its mechanism is related to the upregulation of early growth response factor-1 (Egr1) expression by competitively binding with miR-145 [25].
A substantial amount of evidence supports that lncRNAs play an important role in the oxidative damage of endothelial cells. After overexpression of the lncRNA growth-arrest specific transcript 5 (GAS5), Diao et al. [26] observed decreased GAS5 expression levels in endothelial cells subjected to homocysteine (HCY)-induced cardiac microvascular endothelial cell (CMEC) injury. GAS5 upregulation attenuated HCY-induced CMEC injury by mediating oxidative stress, and its mechanism was related to the upregulation of ABCA1 expression by competitively binding with miR-33a-5p. Similarly, Shen et al. [27] found that lncRNA DLGAP1 antisense RNA 1 (DLGAP1-AS1) silencing inhibits the expression of inflammatory factors, reduces malondialdehyde (MDA) levels and decreases vascular endothelial cell (VEC) apoptosis. The findings suggest that DLGAP1-AS1 silencing can inhibit oxidative stress and exert an antiapoptotic effect. Another study confirmed the involvement of lncRNAs in oxidative damage of ECs induced by NO, and it was shown that the lncRNA uc001pwg.1 could increase endothelial nitric oxide synthase (eNOS) phosphorylation and NO production in vitro [28].
A recent study found that lncRNAs could regulate human pericyte function and were involved in the endoplasmic reticulum (ER) stress response. In 2017, Bischoff FC et al. [29] demonstrated the regulatory feedback role between the ER stress response and HypERlnc expression that affects pericyte function. Endoplasmic reticulum stress-related transcription factors were prominently activated by HypERlnc knockdown, while induction of ER stress significantly lowered HypERlnc levels and induced pericyte dedifferentiation. These observations suggest that lncRNAs may control the occurrence of disease by regulating the level of oxidative stress and reducing endoplasmic reticulum stress.
LncRNAs in a variety of cells play a role in the regulation of oxidative stress
Several studies have shown that lncRNAs are also involved in the functional regulation of other cells during oxidative stress. For example, the lncRNA OGRU mediates diabetic retinopathy (DR) by competing for miR-320 to promote inflammation and induce the production of ROS by regulating ubiquitin-specific peptidase 14 (USP14) expression in high glucose (HG)-incubated Müller cells [30]. Similarly, the suppression of the lncRNA PICSAR reduced IL-6 and IL-8 production in rheumatoid arthritis (RA)-fibroblast-like synoviocytes (FLSs), indicating that PICSAR affects an important function of RA-FLSs [31]. In addition, keratinocytes [33], platelets and adipocytes [34, 35] have been revealed to be affected by lncRNA-mediated oxidative stress.
LncRNAs as regulators of oxidative stress in diabetic wound healing
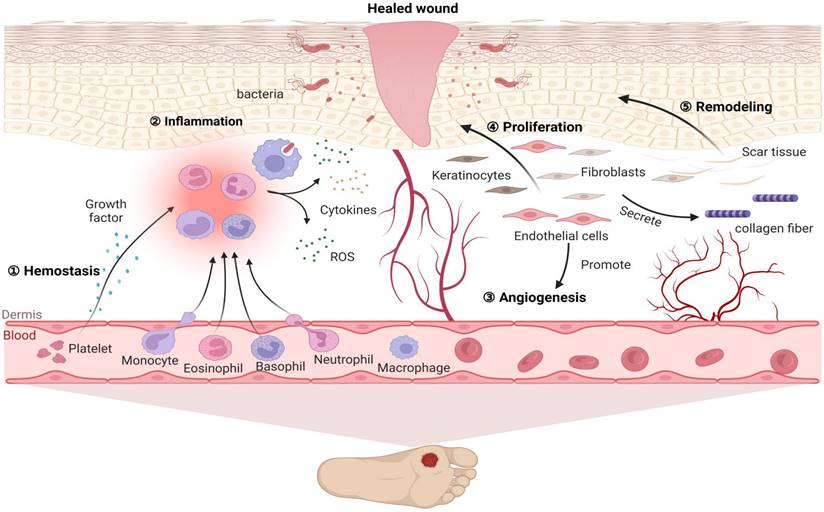
Wound healing is a complex and orderly biological process that mainly includes five stages: haemostasis, inflammation, angiogenesis, proliferation and remodelling [45]. Oxidative stress is one of the complex mechanisms that causes diabetic wound healing difficulties [5]. Multiple studies have shown that lncRNAs are involved in various stages of diabetes wound healing and play a critical role in oxidative stress. Thus, it is necessary to understand how the abnormal regulation of expressed lncRNAs delays or accelerates the repair process of wound healing. This part mainly reviews the lncRNAs in each stage of diabetic wound healing and their roles in each phase impacted by oxidative stress, as shown in Table 2 and Figure 3, which exhibit the biogenesis and regulation mechanism of oxidative stress associated lncRNA function and that play a vital role in diabetic wound healing.
Haemostasis
Platelets are essential components of hemostasis that provides prompt control of blood loss due to vascular injury [67]. Platelets from diabetic patients with poor blood glucose control tend to aggregate [68]. Immediately after trauma, haemostasis occurs first, and then platelets aggregate to form fibrin clots via intrinsic mechanisms. This clot formation is followed by platelets releasing large amounts of growth factors, which constrict blood vessels, limit bleeding and attract inflammatory cells to migrate to the wound site and disinfect the wound surface [69]. This process is associated with the chemotaxis of many cell types, such as macrophages, white blood cells, vascular smooth muscle cells and the fibroblasts mentioned above, and the dysfunction caused by the oxidative damage of these cells is closely related to lncRNAs. In addition, under diabetic conditions, platelets have been shown to be less responsive to NO released from the vascular endothelium, which normally reduces platelet aggregation after vascular injury, and previous studies have shown that lncRNAs also regulate NO expression in vascular cells [70]. Therefore, platelet dysfunction in diabetic patients can not only hinder wound healing by reducing coagulation during haemostasis but also contribute to the development of microvascular disease in diabetic patients. While lncRNAs governing this early phase of diabetic wound healing has not been clearly defined, several lncRNAs implicated in thrombus formation may be involved.
Regulatory effects of certain common oxidative stress-associated lncRNAs on the pathology of diabetic wounds.
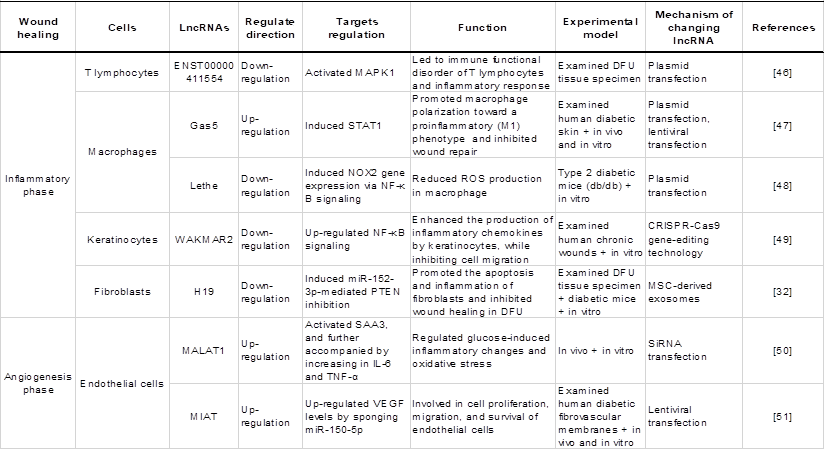
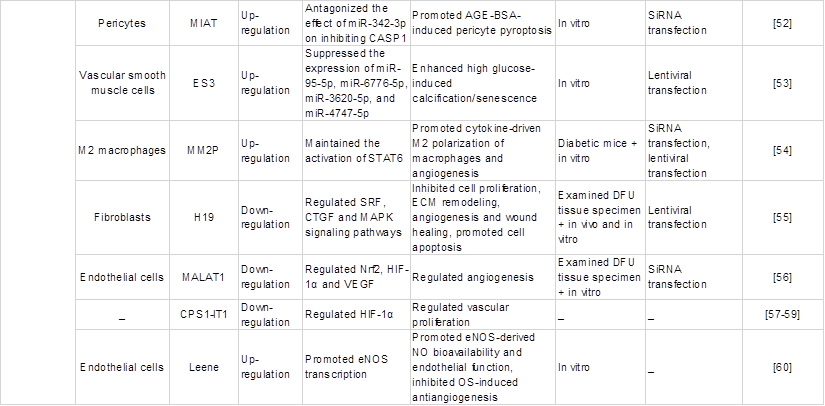
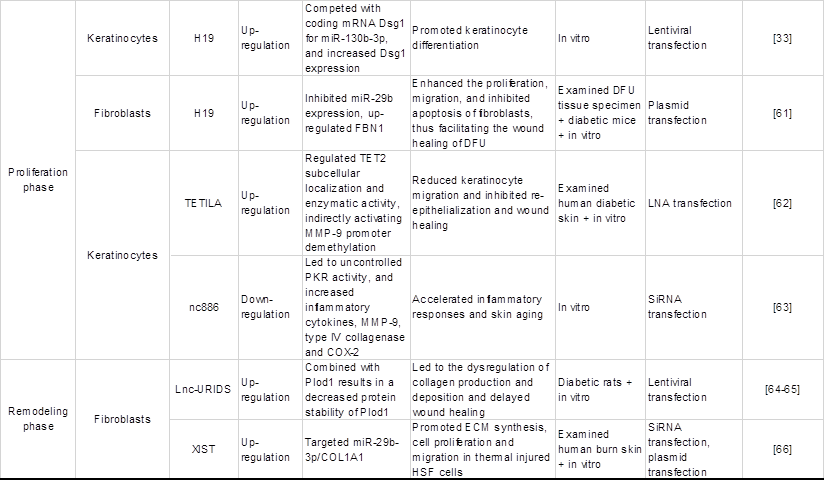
Inflammation
Neutrophils are the main cell types involved in the early stages of inflammation [71]. In diabetic wounds, a state of sustained high blood sugar can shorten the lifespan and increase the clearance rate of neutrophils, resulting in them not being able to reach the base of the wound in a timely manner. Instead, they scatter around the wound surface and combine with advanced glycation end products outside the vascular tissue to release a large number of inflammatory cytokines, aggravating the inflammatory response and oxidative stress and hindering wound healing [72, 73]. In addition, MФs persistently remain in the proinflammatory M1 phenotype in diabetic wounds, which further promotes inflammation and inhibits the initiation of the tissue proliferation period, leading to difficult wound healing [74, 75]. A large number of studies have revealed that lncRNAs also play regulatory roles in ROS production and inflammation by controlling the functions of immune cells, including neutrophils, MФ, and immune cells, which are essential in diabetic wound inflammation.
The biogenesis and regulatory mechanism of lncRNA function is associated with oxidative stress. The diabetic wound microenvironment, which includes hyperglycaemia, OS and AGEs, induces the abnormal expression of lncRNAs, thereby regulating the expression of downstream genes and oxidative stress levels, further affecting the functions of immune cells, endothelial cells, keratinocytes, and fibroblasts. LncRNAs participate in various stages of diabetic wound healing, such as inflammation, angiogenesis, proliferation and remodelling, to delay or accelerate the repair process of wound healing. Red and blue arrows represent up- and downregulation, respectively.
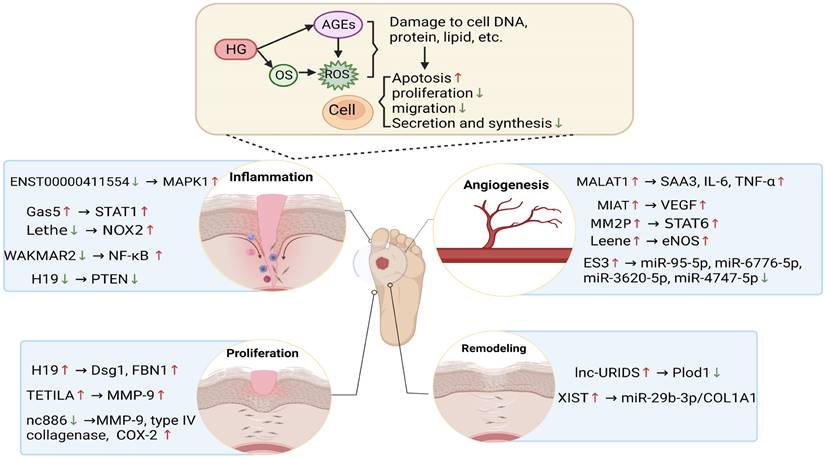
Therefore, the oxidative stress of immune cells caused by lncRNAs through the regulation of ROS production may be an important mechanism underlying diabetic wound inflammation. For example, the existence of immunoregulation‑associated lncRNAs mediated by T lymphocytes in the wound surfaces of diabetic foot ulcers (DFUs) has been demonstrated by Xu et al., who suggested that activation of the MAPK signal transduction pathway mediated by the lncRNA‑ENST00000411554/MAPK1 axis affected the DFU immune regulatory imbalance [46]. A number of studies have confirmed that the activation of the MAPK signalling pathway is closely related to ROS and NO synthesis induced by oxides, and the inflammatory response caused by abnormal lncRNA expression in diabetic wound healing may be related to T lymphocyte immune regulation and redox reactions. Additionally, lncRNAs can also affect the inflammatory response by affecting the transformation of macrophage phenotypes. For example, lncRNA-Gas5 promoted the polarization of MФs to the proinflammatory M1 phenotype by inducing the expression of the downstream target gene STAT1 to inhibit wound healing in diabetic patients [47]. Importantly, another study demonstrated that the lncRNA Lethe regulates oxidative stress in macrophages cultured under high glucose conditions through the regulation of NOX2 expression, which may affect the inflammatory state in diabetic wounds [48]. Specifically, under high glucose conditions, the expression of Lethe is downregulated, resulting in the increased availability of free p65-NFkB to translocate to the nucleus and upregulate NOX2 expression. These results suggest that the abnormal expression of lncRNAs in wounds leads to oxidative stress and immune cell dysfunction, resulting in the abnormal inflammation of diabetic wounds. Although keratinocytes are epidermal cells, they also play an important role in the inflammatory stage of wound healing. Previous studies have shown that the hyperglycaemic environment in diabetic wounds can enhance OS and increase the inflammatory response of keratinocytes, thus stagnating the wound healing process in the inflammatory phase and slowing the healing process [76]. Previous studies have shown that the lncRNA wound and keratinocyte migration-associated long noncoding RNA 2 (WAKMAR2) inhibits the production of inflammatory chemokines in keratinocytes by regulating the TGF-b-WAKMAR2-p65-NF-kB signalling axis [49]. As previously mentioned, NF-kB is closely related to ROS production. Given their relationship, it can be speculated that lncRNAs are associated with ROS production in keratinocytes at the inflammatory stage. In addition, fibroblasts distributed in the dermis can secrete inflammatory factors and affect wound healing [77]. Moreover, the regulation of inflammatory stress by H19 on fibroblasts in diabetic wound healing was validated [32]. The results revealed that overexpressed H19 can suppress fibroblast apoptosis and inflammation and stimulate the wound-healing process in mice suffering from DFUs, which involves an interaction with miR-152-3p via the PTEN-mediated PI3K/AKT signalling pathway. A number of studies have shown that the PI3K/AKT pathway is a key signalling pathway that alleviates cellular oxidative stress damage. That is, lncRNAs act on fibroblasts and may regulate oxidative stress-related signalling pathways to regulate wound inflammation.
Angiogenesis
Angiogenesis is an essential part of granulation tissue, and another key process in wound healing, mainly involving endothelial cell migration and capillary formation [78]. The level of tissue oxygenation is one of the initial microenvironmental cues that activate new blood vessel formation [79]. As the injured tissues are cleared, the progression of healing relies upon a new supply of oxygen and nutrients [80]. ECs, PCs and VSMCs are closely related to angiogenesis, and the functional impairment of these cells is associated with lncRNAs and oxidative stress. A study reported that the expression of lncRNA MALAT1 was upregulated in the early stage but subsequently downregulated in the late stage under hyperglycaemia, which in turn regulated glucose-induced oxidative stress and the inflammatory response through activation of serum amyloid antigen 3 (SAA3), an inflammatory ligand and target of MALAT1, thus possibly influencing endothelial stability [50]. Similarly, the lncRNA myocardial infarction associated transcript (MIAT), a significantly upregulated lncRNA in endothelial cells cultured in high glucose (GH) medium, modulates VEGF levels by sponging miR-150-5p in retinal endothelial cells, thereby promoting angiogenesis [51]. Glucose-induced oxidative stress is a key alteration in endothelial cells and is a mediator of most, if not all, other downstream effects [81]. Hence, the regulation of MIAT on endothelial angiogenesis under high glucose may also be related to the regulation of oxidative stress. Interestingly, it has been reported recently that MIAT can antagonize the effect of miR-342-3p on inhibiting CASP1 and promote advanced glycation end product-modified bovine serum albumin (AGE-BSA)-induced pericyte pyroptosis [52]. AGEs induce pericyte death due to the activation of intracellular oxidative stress and inflammatory responses by binding to the receptor for AGEs (RAGE) on the cell surface [82]. Another example is lncRNA-ES3, which triggers the gene silencing of multiple miRNAs by binding to basic helix-loop-helix family member e40 (Bhlhe40), which promotes the HG-induced calcification/senescence of VSMCs [53]. With the ageing of VSMCs, vascular atrophy occurs and neovascularization cannot be formed. Notably, the production of high levels of ROS, which is associated with aging, has been demonstrated to be critical for the induction and maintenance of cellular senescence [83]. Thus, HG-induced high levels of ROS may have an effect on lncRNA-regulated angiogenesis. Moreover, several lncRNAs have been reported to be involved in macrophage proangiogenic functions. For example, Cao et al. [54] found that lncRNA-MM2P modulates M2 polarization by regulating STAT6 phosphorylation, and MM2P knockout inhibited macrophage-promoted angiogenesis both in vitro and in vivo. Another study showed that M2 macrophage-induced upregulation of prostate cancer-associated transcript 6 (PCAT6) facilitates tumour angiogenesis through modulation of VEGFR2 expression via ceRNA and deubiquitination patterns [84]. Overall, manipulation of lncRNAs can regulate the promotion of macrophage-mediated angiogenesis and even improve inflammation, the induced hypoxic environment and abnormal angiogenesis.
Other lncRNAs implicated in angiogenesis and diabetic wound healing include lncRNA-MALAT1, lncRNA-CPS1-IT1 and lncRNA-Leene, which can regulate related cytokines. Jayasuriya R et al. [56] reported a significant reduction in the expression level of lncRNA MALAT1 in DFU patients, which was positively correlated with the expression of angiogenic factors such as nuclear factor erythroid-2-related Factor 2 (Nrf2), hypoxia-inducible factor-1α (HIF-1α) and VEGF. Nrf2 knockout downregulated the expression of HIF-1α and VEGF-A, suggesting that Nrf2 plays a vital role in the regulation of angiogenesis through the MALAT1/HIF-1α loop. In addition, previous studies have found that MALAT1 protects HUVECs against oxidative stress and further promotes angiogenesis by activating the Nrf2 signalling pathway, suggesting that MALAT1 may have a protective effect against vascular endothelial oxidative stress during the angiogenesis stage [85]. Another genome-wide analysis of gene expression changes in skin from patients with type 2 diabetes (DM2) found that CPS1-IT1 was significantly downregulated and involved in DM2 [57]. Moreover, high glucose-induced high oxygen inhibited hypoxia-inducible factor-1α (HIF-1α) expression, which was controlled by CPS-IT1, and inhibited neovascularization [59]. Therefore, it is speculated that the management of CPS1-IT1 in the process of diabetic wound healing may occur through HIF-1α, which further promotes angiogenesis through the effect of antioxidative stress. Furthermore, eNOS is one of the key regulators of endothelial homeostasis and vascular function [86]. This decoupling not only fails to produce enough NO, but also interferes with the dysfunction of endothelial progenitor cells (EPCs), which interferes with neovascularization and wound healing [87]. Studies have shown that an enhancer-associated lncRNA that enhances eNOS expression (LEENE) promotes eNOS transcription, eNOS-derived NO bioavailability, and endothelial function, which inhibits OS-induced antiangiogenesis [60]. Collectively, lncRNAs can promote angiogenesis and accelerate wound healing in diabetic wounds by improving oxidative stress and regulating the production of various cytokines involved in initiating and maintaining the healing process.
Re-epithelialization (proliferation)
The epidermis is the first barrier against the external environment, and epidermal cells are one of the main repair cells in wound healing [88, 89]. Keratinocytes are important cells for the epidermis, and dysregulated lncRNAs mainly regulate wound epithelialization by affecting keratinocyte functions. Cross-talk between fibroblasts and keratinocytes is crucial to stimulate the proliferation of keratinocytes which regenerates epithelium integrity. It was reported that lncRNA H19 directly binds to miR-130b-3p and inhibits keratinocyte differentiation by targeting Dsg1 [33]. Subsequently, Li et al. [61] constructed a DFU model in mice and found that the expression of miR-29b and its target gene FBN1 involved in wound healing was regulated by H19, which enhances fibroblast proliferation and migration and accelerates epidermal epithelialization of wounds, thus facilitating wound healing through activation of the TGF-β/Smad pathway. Similarly, MMPs play an indispensable role in the migration of keratinocytes. Excess MMP levels can lead to the degradation of the ECM and various growth factors, including TGF-β1 and VEGF, affecting the re-epithelialization and remodelling of wounds [90]. Studies have shown that MMP-9 is present in more than 50% of chronic wounds, and its downregulated expression contributes to diabetic wound healing [91, 92].
Recent studies identified a ten-eleven translocation 2 (TET2)-interacting long noncoding RNA (TETILA) that can regulate MMP-9 expression and is upregulated in human diabetic skin tissues. Specifically, TETILA recruits thymine-DNA glycosylase (TDG), which simultaneously interacts with TET2, for base excision repair-mediated MMP-9 promoter demethylation and MMP-9 transcriptional activation, ultimately reducing keratinocyte migration and inhibiting re-epithelialization and wound healing [62]. Conversely, lncRNA-nc886 inhibits MMP-9 expression in human keratinocytes [63]. In addition, intracellular ROS can be generated and play a considerable role as signalling molecules under hyperglycaemic conditions and can regulate the expression of MMPs [93, 94]. Overall, the results suggested that lncRNA interference with keratinocyte function through targeted regulation of MMP may be mediated by ROS produced by high glucose, thus promoting wound re-epithelialization.
Remodelling phase
An emerging handful of lncRNAs have been implicated in fibroblasts and participate in the remodeling phase. Hu et al. [64] explored a novel lncRNA, MRAK052872 (named lnc-URIDS), which was upregulated in diabetic skin and dermal fibroblasts. Lnc-URIDS knockdown promoted the migration of dermal fibroblasts treated with advanced glycation end products (AGEs) in vitro and accelerated diabetic wound healing in vivo. Mechanistically, lnc-URIDS targets procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1 (Plod1), a critical enzyme responsible for collagen cross-linking, resulting in the decreased protein stability of Plod1, which leads to the dysregulation of collagen production and deposition and delays wound healing. Another study found that adipose-derived stem cell (ASC)-short-hair interfering RNA PLOD1 (shPLOD1) reduced fibrosis in injured rat regions, likely through the suppression of ROS levels [65]. Moreover, ROS are critical for fibrogenesis proliferation and ECM secretion and may affect collagen production and deposition regulated by the binding of lnc-URIDS to Plod1. In addition, lncRNA X-inactive specific transcript (XIST) promoted ECM synthesis and human skin fibroblast (HSF) proliferation and migration by sponging miR-29b-3p and targeting collagen 1 alpha 1 (COL1A1) to promote wound healing [66]. In summary, several lncRNAs have been studied as regulators of tissue remodelling, especially under the mediation of oxidative stress. Therefore, further research at all stages of wound healing is needed to identify additional lncRNAs involved in the regulation of wound healing.
The mechanism of lncRNAs in signalling pathways involved in both oxidative stress and diabetic wound healing
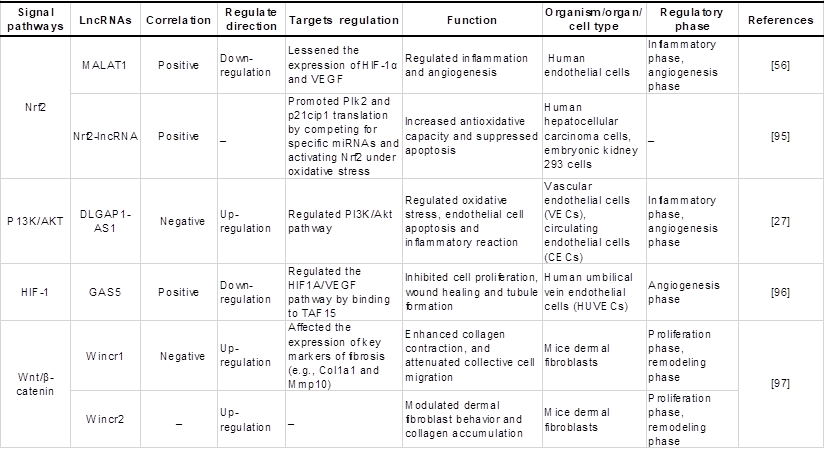
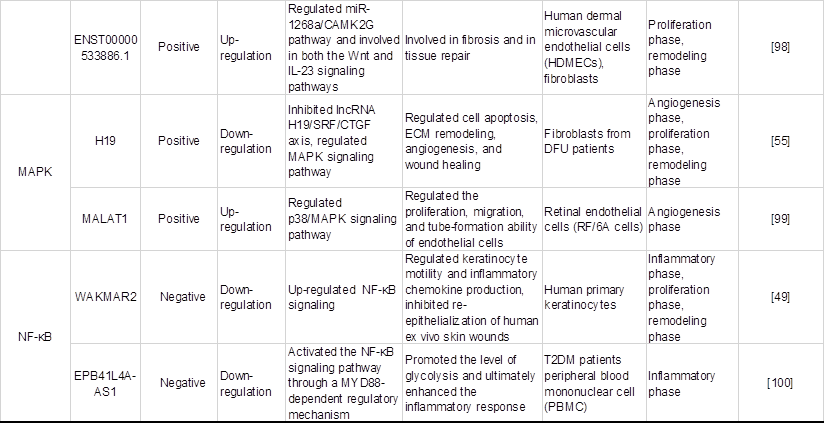
Chronic hyperglycaemia can activate various signalling pathways in the mitochondrial reactive oxygen species system to activate and regulate various transcription factors and induce gene transcription and the expression of related functional proteins, which in turn participate in cell proliferation and migration, ultimately affecting the process of diabetic wound healing. Studies have found that lncRNAs regulate oxidative stress-related signalling pathways such as Nrf2, P13K/Akt and HIF-1, which are closely related to diabetic wound healing, as shown in Table 3 and Figure 4, which exhibit the regulatory mechanisms of oxidative stress-associated lncRNAs associated with key genes and signal pathways in diabetic wound healing.
Regulatory effects of oxidative stress-associated lncRNA pathways on diabetic wound healing.
Regulatory effects of oxidative stress-associated lncRNA pathways on diabetic wound healing. Nrf2-lncRNA acts as a competing endogenous RNA (ceRNA) sponge for miRNAs 128 and 224. miR-224 and miR-128 inhibit PIK2 and p21cip1, which are involved in p53-dependent stress, thereby enhancing the transcription of Nrf2 and cell survival. LncRNA MALAT1 is regulated by Nrf2, which has a positive correlation with Nrf2 expression in wild-type cells. MALAT1 downregulates the expression of TNF-α and IL-6 to promote angiogenesis and upregulates the expression of HIF-1α and VEGF to reduce inflammation. LncRNA DLGAP1-AS1 silencing activates the PI3K/Akt pathway, causing the levels of TNF-α and VCAM-1 to be decreased and the MDA levels and CK activity to be decreased. DLGAP1-AS1 silencing suppresses oxidative stress, endothelial cell apoptosis and inflammatory reactions. LncRNA GAS5 activates the HIF1A/VEGF pathway by binding to TAF15, and promotes cell proliferation, wound healing and tubule formation via the HIF1A/VEGF pathway in HUVECs. Therefore, when lncRNAs activate Nrf2, PI3K/AKT, HIF-1, and other signalling pathways, they can regulate the inflammatory response, cell proliferation, migration, and angiogenesis during the wound healing process, thus promoting wound healing.
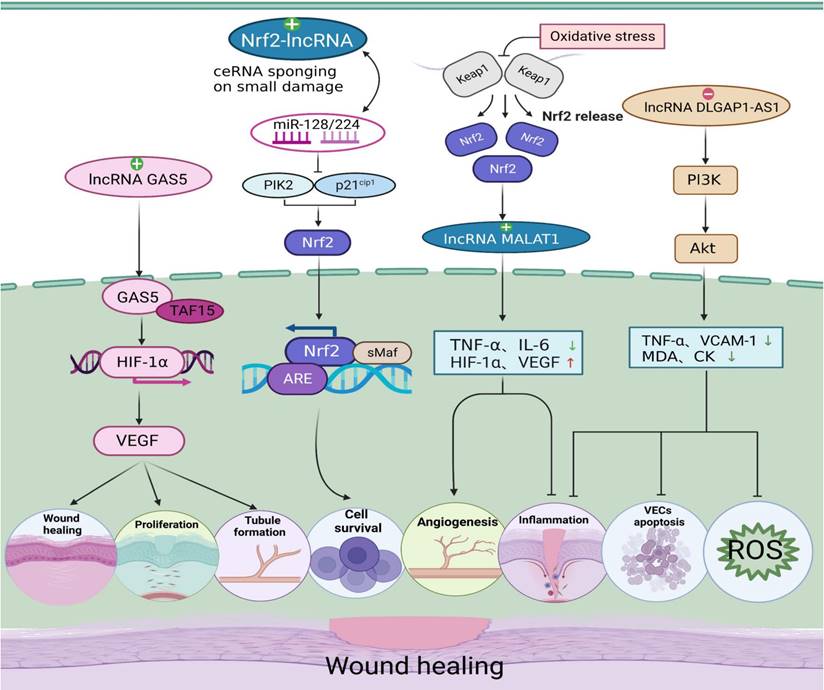
Nrf2 signalling pathway
As a redox-sensitive transcription factor, Nrf2 eliminates excessive ROS by upregulating antioxidant response elements, thus regulating ROS levels in organisms [101]. Li et al. [102] demonstrated that Nrf2 overexpression increased the protective effect of adipose-derived stem cells (ADSCs) on EPC proliferation and angiogenesis in a high glucose environment and was beneficial to wound healing, during which inflammation and oxidative stress-related protein levels were reduced. More importantly, in the angiogenesis stage of chronic wound healing, lncRNA MALAT1 positively regulates Nrf2 and may protect HUVEC from oxidative damage by activating Nrf2 signaling pathway, which is beneficial to wound healing [56]. Separately, another study identified a novel lncRNA named Nrf2-activating lncRNA (Nrf2-lncRNA) transcribed from an upstream region of the microRNA 122 gene (MIR122), which controls cell fate by modulating p53-dependent Nrf2 activation as a miRNA sponge for polo-like kinase (Plk)2 and cyclin-dependent kinase inhibitor 1 (p21cip1) [95]. Although this study did not demonstrate a direct causal relationship between Nrf2-lncRNA and diabetic wound healing, it may contribute to further research on the antioxidant capacity of lncRNAs and their regulation of diabetic wound healing under oxidative stress conditions.
P13K/AKT signalling pathway
Following the activation of PI3K, a cascade of AKT phosphorylation and the second messenger phosphatidylinositol 3 phosphate (GSK-3β) inhibits oxidative damage [103]. Yu et al. [104] demonstrated that hyperglycemia-impaired PI3K-Akt signaling may lead to migration, proliferation and angiogenesis dysfunction of endothelial cells in diabetes patients, which is likely to cause slow healing of diabetic wounds. Additionally, Nishikai-Yan Shen T et al. [105] found that IL-6 promoted the migration of nondiabetic fibroblasts during wound healing through the PI3K/Akt signalling pathway, which was dysfunctional in diabetic wounds. The proliferation and migration of fibroblasts can activate collagen production and promote the growth of new granulation, which is an important part of wound healing, further confirming the key role of the PI3K/Akt pathway in diabetic wound healing. Moreover, lncRNA DLGAP1-AS1 activates the phosphoinositol 3-kinase (PI3K)/Akt pathway to control oxidative stress, and endothelial cell apoptosis has also been previously mentioned [27]. Hence, lncRNA protects against oxidative damage to endothelial cells induced by high glucose and promotes fibroblast migration through the P13K/AKT signalling pathway, which may be another possible mechanism of lncRNAs in diabetic wound healing.
HIF-1 signalling pathway
HIF-1 is an important transcription factor that maintains oxygen homeostasis in hypoxic cells [106]. Once the two subunits of HIF-1 (HIF-1α and HIF-1β) are recognized and bound, they can activate the transcription of downstream target genes [107]. In the case of hypoxia, the function of HIF-1α is activated and can promote the activation of NADH oxidase (NOX), resulting in ROS overproduction. The level of this enzyme will increase during oxidative stress and eventually exacerbate the oxidative environment [108]. Recently, it has been reported that HIF-1α can promote wound healing. β-N-Oxalyl-L-α, β-diaminopropionic acid (L-ODAP) is a natural nonprotein amino acid that can be metabolized in the human body and is also a safe wound healing agent. Sharma et al. [109] conducted an in vitro experiment on the wound healing activity of L-ODAP and confirmed that L-ODAP can upregulate the expression of VEGF, matrix metalloproteinase-2 (MMP-2) and MMP-9 by increasing HIF-1α levels, thus accelerating wound healing. Noncoding gene lncRNAs are also involved in the regulation of the HIF-1 signalling pathway and the healing of chronic skin ulcer wounds. For example, Peng et al. [96] found that in the skin tissues of DFU patients, lncRNA-GAS5 and HIF-1α were downregulated, and GAS5 induced HIF-1α expression by interacting with TAF15. High GAS5 expression positively regulates the HIF-1α/VEGF pathway, which promotes the proliferation of HUVECs and accelerates wound repair and healing.
Other signalling pathways
In addition to the above main signaling pathways, several lncRNAs are reported to be involved in the regulation of the Wnt/β-catenin signalling pathway in wound healing, including lncRNA-Wincr1, -Wincr2, and -ENST00000533886.1 [97, 98]. Moreover, several lncRNAs are involved in the regulation of the MAPK signalling pathway, including lncRNA-H19 and lncRNA-MALAT1 [55, 99]. Additionally, other lncRNAs are involved in the regulation of the NF-κB pathway, including WAKMAR2 and EPB41L4A-AS1 [49, 100]. However, to date, few studies have reported that lncRNAs participate both in oxidative stress and diabetic wound repair. Further evidence is needed to establish the specific mechanisms of lncRNAs in other signaling pathways related to oxidative stress.
Progress and Prospects of lncRNAs in diagnosis and treatment
LncRNA as a diagnostic biomarker
Deregulated lncRNA expression is found in diabetic patients and serves as a diagnostic biomarker to predict diabetes and its complications, including diabetic wounds. For example, Li X et al. [110] showed that the expression levels of 3 lncRNAs in the peripheral blood increased gradually from the control group to the prediabetes group to the T2DM group, especially lncRNA ENST00000550337.1, suggesting that ENST00000550337.1 may potentially distinguish the severity of diabetes and act as a novel reference index for clinical classification. Another study also found that the expression of GAS5 in the wound surface of patients with diabetes was significantly higher and mainly located in the M1 macrophages, which may be a biomarker for diabetic wounds in the early inflammation phase [47]. In addition, the significant increase in the expression level of lncRNA URIDS in the skin and serum samples of DFU patients mentioned above suggests that it may be the key to delayed diabetic wound healing, further providing a basis for diabetic ulcer diagnosis [64]. Overall, we draw a speculation that, lncRNAs isolated from body fluids or tissues may be adopted as an accurate biomarker for the clinical evaluation of the severity of diabetic wounds.
Prospective application of lncRNAs in the treatment of diabetic wounds. (a) Stem cell therapy promotes diabetic wound healing. LncRNA MALAT1 acts as a sponge for miR-205-5p to promote the repairing effect of epidermal stem cells (ESCs) on DFUs. Exosomes secreted from different sources (ADSCs) (MSCs) contain lncRNA H19 to accelerate wound healing through two different pathways. lncRNA ANRIL is involved in the induction of tissue epithelial-mesenchymal transition (EMT). (b) Autologous blood transfusion. H19 accelerates fibroblast activation by recruiting ezh2-mediated histone methylation and regulating the HIF-1α signalling pathway, thereby enhancing autologous blood transfusion and promoting postoperative wound healing in diabetic mice. (c) Clinical studies of lncRNA-based drugs. Most drugs have potential lncRNA binding sites. Melatonin attenuates diabetic cardiomyopathy (DCM) by inhibiting lncRNA malat1-mediated NLRP3 inflammatory vesicles and the TGF-β1/Smad signalling pathway. (d) LncRNA drug delivery system. The use of different nanoparticles and extracellular vesicles (EVs) as carriers of lncRNA complexes for exogenous supplementation or inhibition of related lncRNAs to restore normal expression levels and accelerate diabetic wound healing.
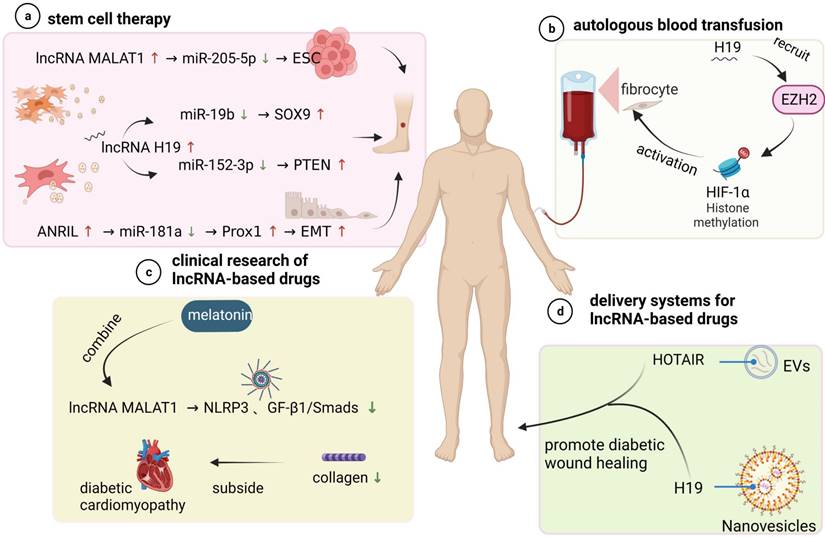
LncRNAs as targets for wound-repair treatment
LncRNAs play a non-negligible role in the regulation of diabetic wounds and can, therefore, be used as alternative therapeutic targets. Many lncRNAs have been applied to advanced treatment methods in different forms, along with many relevant experiments in vitro and in vivo, as shown in Figure 5, which provides drugs directly or indirectly regulating lncRNAs to promote the healing of diabetic wound healing.
Stem cell therapy
Stem cell transplantation can directly promote local cell wound repair and skin wound healing [111]. In diabetic wounds, stem cells not only differentiate into specific cells to replace dysfunctional cells and repair damaged tissue but also promote the migration of fibroblasts, the thickening of granulation tissue, and the secretion of various cytokines to promote angiogenesis [112-117]. Interestingly, studies have demonstrated that lncRNAs play an important role in stem cell therapy of diabetic wound healing. For instance, lncRNA MALAT1 functions as a sponge RNA for miR-205-5p to increase therapeutic effects of epidermal stem cells (ESCs) on DF, which was shown to be associated with improved vascularization on the disease site [118]. In another study, it was shown that the high expression of lncRNA H19 in exosomes derived from adipose mesenchymal stem cells (ADSCs) may upregulate SOX9 expression via miR-19b to accelerate the wound healing of skin tissues [119]. The role of mesenchymal stem cell (MSC)-derived exosomal H19 in promoting DFU wound healing was also proven in a similar study [32]. These studies suggest that lncRNAs may function as promising adjunctive agents to enhance stem cell therapy for DFU and provide support for future clinical trials of stem cell therapy.
In addition, other lncRNAs are reported to be involved in tissue epithelial-mesenchymal transition (EMT) formation, which may serve as a repair system in vivo to renew cells and achieve tissue regeneration and homeostasis. For example, He et al. [120] showed that long noncoding RNA-antisense noncoding RNA in the INK4 locus (ANRIL) could sponge miR-181a to enhance Prox1 expression and rescue the impairments of EMT formation of lymphatic endothelial cells (LECs) caused by HG. Therefore, regulating EMT formation by using lncRNAs to promote wound healing is another feasible method that may provide insights for the design of new therapies to improve wound healing efficacy in diabetes.
Autologous blood transfusion
Autologous blood transfusion (ABT) is the collection and reinfusion or transfusion of one's own blood or blood components before, during or after surgical procedures and may be another promising direction in lncRNA-based therapy [121]. Clinical treatment with ABT can prevent further damage to red blood cell function in diabetic patients and thus improve the function of fibroblasts function, which play a role in wound healing [122]. Apart from these findings, the function of lncRNAs in wound healing impaired by DM through modified preservative fluid-preserved ABT was demonstrated. Guo et al. [123] indicated that modified preservative fluid-preserved autologous blood upregulated lncRNA H19 expression in fibroblasts and maintained better oxygen-carrying and oxygen release capacities as well as coagulation function. Correspondingly, H19 upregulation accelerated fibroblast activation by recruiting EZH2-mediated histone methylation and modulating the HIF-1α signalling pathway, thereby augmenting the process of modified preservative fluid-preserved autologous blood and enhancing postoperative wound healing in diabetic mice.
Clinical Advancements of lncRNA-Based Drugs
It is well established that lncRNAs may affect normal gene expression and disease progression, and most drugs currently have potential lncRNA binding sites. This notion provides a basis for the development of drugs that use lncRNAs as therapeutic targets. For example, Che et al. [124] reported that melatonin alleviates diabetic cardiomyopathy (DCM) by inhibiting MALAT1-mediated NLRP3 inflammasome and TGF-β1/Smad signalling. In related experiments, it reduced collagen production and was beneficial to HG-treated cardiac fibroblasts (CFs). In addition, similar effects on collagen and fibroblasts were consistently observed in diabetic wounds, which provides ideas for developing wound healing drugs.
Delivery Systems for lncRNA-Based Drugs
Since lncRNAs are abnormally expressed diabetic skin ulcer patients, exogenous supplementation or inhibition of the related lncRNAs to restore the expression levels may be an ideal method to speed the healing of diabetic wounds. Different nanoparticles are currently being investigated as vehicles to deliver lncRNA modulation complexes to specifically targeted cells and tissues in an effective, noncytotoxic manner. The use of extracellular vesicles (EVs), nanoscale lipid membrane-bound vesicles that are secreted by cells of both prokaryotes and eukaryotes and carry bioactive cargos, including proteins, nucleic acids and lipids from source cells, may be an promising approach well suited to wound repair [125]. For example, Born L J et al. [126] examined the therapeutic potential of EVs from mesenchymal stem/stromal cells (MSCs) transfected to overexpress long noncoding RNA HOX transcript antisense RNA (HOTAIR). The results showed that MSC EVs containing significantly increased levels of HOTAIR were effective in promoting wound bed vascularization and enhancing wound repair in diabetic mice, which supports the findings of the small number of other studies on EV-mediated lncRNA therapeutic effects. Similarly, Tao et al. [127] delivered lncRNA-H19 to wounds using extracellular vesicle-mimetic nanovesicles (EMNVs) as an effective nanodrug delivery system and observed significant stimulation of angiogenesis. These recent advances in EV-related research have provided feasible approaches for developing emerging therapeutic nanoplatforms using EVs.
Conclusions and perspective
The common factors that promote diabetic wound healing are increased fibroblast proliferation, enhanced angiogenesis, the accelerated rate of regenerative epithelialization, and decreased apoptosis. Among them, ROS levels increased by ROS generators or decreased by antioxidant systems are recognized by ROS sensors that trigger signals that affect lncRNAs, which in turn influence ROS generators or antioxidant systems, thereby affecting key cellular functions. Considering the link between lncRNAs and oxidative stress, their common potential driving role in diabetic wound healing can be inferred. Therefore, in terms of mechanism, lncRNAs may regulate gene expression of immune cells, endothelial cells, vascular smooth muscle cells, pericytes and other cells through oxidative stress, thus affecting cell function. In addition, a variety of oxidative stress signalling pathways and influencing factors are involved in the regulation of lncRNAs in diabetic wound healing, including Nrf2, P13K/Akt, HIF-1, Wnt/β-catenin, MAPK, and NF-κB. The participation of a variety of cells, cytokines, ROS, genes, and other factors, which together constitute a complex biological network of regulation, and ultimately affect the repair and healing of diabetic wounds.
In recent years, the traditional treatment methods for diabetic wounds mostly use debridement, drainage, dressing and other treatments on the basis of blood glucose control and circulation improvement, which may have poor curative effects. Moreover, long-term treatment of the disease may allow the drug to accumulate in the body, leading to increased adverse reactions and increased economic burden. It is critical to find safe and effective treatment for the repair and healing of diabetic wounds. The application of non-coding RNAs such as the lncRNAs presents an attractive breakthrough point for the treatment of diabetic wound healing, and treatment strategies based on lncRNAs have unique advantages as the lncRNAs constitute a powerful class of gene regulators. Usually, the same lncRNA gene is associated with multiple target genes, and one target gene is regulated by multiple lncRNAs. As a result, targeted therapy may exhibit a higher therapeutic efficacy, making the development of new drugs and new therapies for diabetic wounds more promising. For example, nanocomplexes can effectively deliver lncRNAs with optimal targeting specificity. LncRNA loaded on oxygen-deficient molybdenum-based nanodots (MoO3- X) to prepare nanocomplexes may be an emerging therapeutic strategy, thus improving the ROS scavenging effect and bactericidal performance of MoO3- X nanodots and ultimately accelerating wound healing [128]. Notably, MoO3- X nanodots have a singular composition and are simple to prepare, which is more conducive to their clinical transformation. Similarly, Zhao et al. [129] developed a ROS-scavenging hydrogel loaded with different therapeutic contents for effective wound regeneration under various complex conditions. Accordingly, it is speculated that ROS-scavenging hydrogels containing lncRNA-binding site drugs have greater efficacy in treating difficult-to-heal wounds, such as infected diabetic wounds. In addition to controlling the expression level of lncRNAs and ROS level in wounds through the delivery system, damage resulting from abnormal lncRNA expression and oxidative stress can also be reversed by directly using lncRNA mimics or inhibitors. As a potentially important class of therapeutic molecules, lncRNA mimics and inhibitors can be extensively modified, which can promote their high stability in vivo and reduce immunogenicity, as well as be easily labelled with organ-targeted peptides for tissue-specific distribution. Moreover, lncRNAs can be applied in different forms to advanced treatment methods, including stem cell therapy and autologous blood transfusion, to improve the healing of diabetic wounds.
Overall, it is expected that the role of lncRNAs in diabetic wound healing under oxidative stress will be useful to confirm or even discover novel potential targets and cellular pathways, which not only provides accurate diagnostic biomarkers for the clinical evaluation of the severity of diabetic wounds but also provides new insights for the treatment of diabetic wounds. However, the current research on lncRNAs has just started, still facing some of the following challenges, hence further research into their clinical application is needed. First, use of lncRNAs as diagnostic markers due to their instability, low sensitivity, and individual differences further limits application as therapeutic targets. LncRNAs may participate in several important processes of wound healing; however, their roles, expressions and modes of action may differ at each stage. In addition, differences in the level of inflammation in each patient will also lead to differences in the activities of lncRNAs, giving it limited specificity. On the other hand, in addition to dynamic and individual differences, the interactions between lncRNAs are complex. As mentioned above, lncRNAs can have multiple downstream target genes, regulate different signaling pathways, and participate in different physiological processes of various cells and tissues, so their actions differ across cells, tissues, and target sites. In addition, the use of lncRNAs in disease diagnosis and treatment is based on changes in their expression levels or restoration of expression through artificial supplementation for therapeutic purposes, requiring more information about patients. At the same time, due to the individual differences of lncRNAs and patients, the question of whether reversing expression levels will cause serious side effects must also be taken into account. Therefore, the consideration of the above issues further increase the challenge of clinical application of lncRNAs. Besides, the translation of all in vitro, in silico, and animal model discoveries to the clinical treatment of human diabetes is also a huge challenge. Specifically, most of these studies are mainly basic research; hence, preclinical and clinical studies are required to verify clinical applications. Furthermore, the transformation of lncRNAs into drugs is another difficulty. We need not only reliable gene-modification technology but also more efficient drug delivery systems to ensure specific targeting and accurate transmission of target drugs. In a word, a deeper understanding of lncRNA-dependent oxidative stress responses involved in diabetic wound is needed to screen for more specific lncRNAs. This will open up a wide range of biomarkers and therapeutic targets in new areas, to the benefit of numerous patients with diabetic wound healing disorder.
Abbreviations
LncRNAs: long noncoding RNAs; DM: diabetes mellitus; DCU: diabetic cutaneous ulcers; OS: oxidative stress; MФ: macrophages; ECs: endothelial cells; VSMCs: vascular smooth muscle cells; AGPG: actin gamma 1 pseudogene; OXPHOS: oxidative phosphorylation; NOX: NADPH oxidase; ROS: reactive oxygen species; PCs: pericytes; AngII: Angiotensin II; RVSMC: rat VSMC; Egr1: early growth response factor-1; GAS5: growth-arrest specific transcript 5; HCY: homocysteine; CMECs: cardiac microvascular endothelial cells; DLGAP1-AS1: DLGAP1 antisense RNA 1; MDA: malondialdehyde; eNOS: endothelial nitric oxide synthase; ER: endoplasmic reticulum; DR: diabetic retinopathy; USP14: ubiquitin-specific peptidase 14; HG: high glucose; RA: rheumatoid arthritis; FLSs: Fibroblast-like synoviocytes; DFUs: diabetic foot ulcers; WAKMAR2: wound and keratinocyte migration-associated long noncoding RNA 2; SAA3: serum amyloid antigen 3; MIAT: myocardial infarction associated transcript; AGE-BSA: advanced glycation end product modified bovine serum albumin; RAGE: receptor for AGEs; Bhlhe40: basic helix-loop-helix family member e40; PCAT6: prostate cancer-associated transcript 6; Nrf2: nuclear factor erythroid-2-related factor 2; HIF-1α: hypoxia-inducible factor-1α; DM2: type 2 diabetes; EPCs: endothelial progenitor cells; LEENE: enhances eNOS expression; TET2: ten-eleven translocation 2; TETILA: ten-eleven translocation 2-interacting long noncoding RNA; TDG: thymine-DNA glycosylase; AGEs: advanced glycation end products; Plod1: procollagen-lysine 2-oxoglutarate 5-dioxygenase 1; ASCs: adipose-derived stem cells; shPLOD1: short-hair interfering RNA PLOD1; XIST: X-inactive specific transcript; HSF: human skin fibroblasts; COL1A1: collagen 1 alpha 1; ADSCs: adipose-derived stem cells; Nrf2-lncRNA: Nrf2-activating lncRNA; MIR122: microRNA 122 gene; Plk: polo-like kinase; p21cip1: cyclin-dependent kinase inhibitor 1; GSK-3β: phosphatidylinositol 3 phosphate; PI3K: phosphoinositol 3-kinase; NOX: NADH oxidase; L-ODAP, β-N-oxalyl-L-α: β-diaminopropionic acid; MMP-2: matrix metalloproteinase-2; ESCs: epidermal stem cells; ADSCs: adipose mesenchymal stem cells; MSCs: mesenchymal stem/stroma cells; EMT: epithelial-mesenchymal transition; ANRIL: antisense noncoding RNA in the INK4 locus; LECs: lymphatic endothelial cells; ABT: autologous blood transfusion; DCM: diabetic cardiomyopathy; CFs: cardiac fibroblasts; EVs: extracellular vesicles; HOTAIR: HOX transcript antisense RNA; EMNVs: extracellular vesicle-mimetic nanovesicles; MoO3- X: molybdenum-based nanodots.
Acknowledgements
Figures were created with BioRender software, ©biorender.com.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81800434], Grant of Sichuan Province Science and Technology Agency Grant [2019YJ0487], Foundation of Luzhou Municipal Science and Technology Bureau [2021-RCM-113], Supported by Sichuan Science and Technology Program [2022YFS0627].
Author contributions
QY and DF conceived the idea, analysis of literature, and writing of the manuscript. JC, SH, NC, and JJ collected and read the literature and revised the article. MZ and ML read through and corrected the manuscript. All authors contributed to the article and approved the submitted version.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377-390
2. Nie X, Zhao J, Ling H, Deng Y, Li X, He Y. Exploring microRNAs in diabetic chronic cutaneous ulcers: Regulatory mechanisms and therapeutic potential. Br J Pharmacol. 2020;177(18):4077-4095
3. Awasthi A, Singh SK, Kumar B, Gulati M, Kumar R, Wadhwa S. et al. Treatment Strategies Against Diabetic Foot Ulcer: Success so Far and the Road Ahead. Curr Diabetes Rev. 2021;17(4):421-436
4. Li L, Chen D, Wang C, Yuan N, Wang Y, He L. et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen. 2015;23(4):495-505
5. Deng L, Du C, Song P, Chen T, Rui S, Armstrong DG. et al. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev. 2021;2021:8852759
6. Feng J, Wang J, Wang Y, Huang X, Shao T, Deng X. et al. Oxidative Stress and Lipid Peroxidation: Prospective Associations Between Ferroptosis and Delayed Wound Healing in Diabetic Ulcers. Front Cell Dev Biol. 2022;10:898657
7. Van Dam PS, Cotter MA, Bravenboer B, Cameron NE. Pathogenesis of diabetic neuropathy: focus on neurovascular mechanisms. Eur J Pharmacol. 2013;719(1-3):180-186
8. Shi M, Du Z, Qi Y, Li W, Hu H, Lin X. et al. Wound microenvironment-responsive glucose consumption and hydrogen peroxide generation synergistic with azithromycin for diabetic wounds healing. Theranostics. 2022;12(6):2658-2673
9. Gao D, Zhang Y, Bowers DT, Liu W, Ma M. Functional hydrogels for diabetic wound management. APL Bioeng. 2021;5(3):031503
10. Tu C, Lu H, Zhou T, Zhang W, Deng L, Cao W. et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597
11. Choi SW, Kim HW, Nam JW. The small peptide world in long noncoding RNAs. Brief Bioinform. 2019;20(5):1853-1864
12. Li J, Wei M, Liu X, Xiao S, Cai Y, Li F. et al. The progress, prospects, and challenges of the use of non-coding RNA for diabetic wounds. Mol Ther Nucleic Acids. 2021;24:554-578
13. Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z. et al. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int J Mol Sci. 2019;20(22):5573
14. Yang J, Liu F, Wang Y, Qu L, Lin A. LncRNAs in tumor metabolic reprogramming and immune microenvironment remodeling. Cancer Lett. 2022;543:215798
15. Yan P, Luo S, Lu JY, Shen X. Cis- and trans-acting lncRNAs in pluripotency and reprogramming. Curr Opin Genet Dev. 2017;46:170-178
16. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298-1307
17. Borkiewicz L, Kalafut J, Dudziak K, Przybyszewska-Podstawka A, Telejko I. Decoding LncRNAs. Cancers (Basel). 2021;13(11):2643
18. Ji E, Kim C, Kim W, Lee EK. Role of long non-coding RNAs in metabolic control. Biochim Biophys Acta Gene Regul Mech. 2020. 1863(4):194348
19. Gast M, Rauch BH, Haghikia A, Nakagawa S, Haas J, Stroux A. et al. Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovasc Res. 2019;115(13):1886-1906
20. Hu X, Ma R, Fu W, Zhang C, Du X. LncRNA UCA1 sponges miR-206 to exacerbate oxidative stress and apoptosis induced by ox-LDL in human macrophages. J Cell Physiol. 2019;234(8):14154-14160
21. Li X, Wang W, Shao Y, Zhou J, Huang J, Xu F. et al. LncTRPM2-AS inhibits TRIM21-mediated TRPM2 ubiquitination and prevents autophagy-induced apoptosis of macrophages in asthma. Cell Death Dis. 2021;12(12):1153
22. Thakuri BKC, Zhang J, Zhao J, Nguyen LN, Nguyen LNT, Schank M. et al. HCV-Associated Exosomes Upregulate RUNXOR and RUNX1 Expressions to Promote MDSC Expansion and Suppressive Functions through STAT3-miR124 Axis. Cells. 2020;9(12):2715
23. Gao H, Wang X, Lin C, An Z, Yu J, Cao H. et al. Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol Chem. 2020;401(3):367-376
24. Das S, Zhang E, Senapati P, Amaram V, Reddy MA, Stapleton K. et al. A Novel Angiotensin II-Induced Long Noncoding RNA Giver Regulates Oxidative Stress, Inflammation, and Proliferation in Vascular Smooth Muscle Cells. Circ Res. 2018;123(12):1298-1312
25. Lin H, You B, Lin X, Wang X, Zhou D, Chen Z. et al. Silencing of long non-coding RNA Sox2ot inhibits oxidative stress and inflammation of vascular smooth muscle cells in abdominal aortic aneurysm via microRNA-145-mediated Egr1 inhibition. Aging (Albany NY). 2020;12(13):12684-12702
26. Diao L, Bai L, Jiang X, Li J, Zhang Q. Long-chain noncoding RNA GAS5 mediates oxidative stress in cardiac microvascular endothelial cells injury. J Cell Physiol. 2019;234(10):17649-17662
27. Shen GH, Song Y, Yao Y, Sun QF, Jing B, Wu J. et al. Downregulation of DLGAP1-Antisense RNA 1 Alleviates Vascular Endothelial Cell Injury Via Activation of the Phosphoinositide 3-kinase/Akt Pathway Results from an Acute Limb Ischemia Rat Model. Eur J Vasc Endovasc Surg. 2020;59(1):98-107
28. Lv L, Qi H, Guo X, Ni Q, Yan Z, Zhang L. Long Noncoding RNA uc001pwg.1 Is Downregulated in Neointima in Arteriovenous Fistulas and Mediates the Function of Endothelial Cells Derived from Pluripotent Stem Cells. Stem Cells Int. 2017;2017:4252974
29. Bischoff FC, Werner A, John D, Boeckel JN, Melissari MT, Grote P. et al. Identification and Functional Characterization of Hypoxia-Induced Endoplasmic Reticulum Stress Regulating lncRNA (HypERlnc) in Pericytes. Circ Res. 2017;121(4):368-375
30. Fu S, Zheng Y, Sun Y, Lai M, Qiu J, Gui F. et al. Suppressing long noncoding RNA OGRU ameliorates diabetic retinopathy by inhibition of oxidative stress and inflammation via miR-320/USP14 axis. Free Radic Biol Med. 2021;169:361-381
31. Bi X, Guo XH, Mo BY, Wang ML, Luo XQ, Chen YX. et al. LncRNA PICSAR promotes cell proliferation, migration and invasion of fibroblast-like synoviocytes by sponging miRNA-4701-5p in rheumatoid arthritis. EBioMedicine. 2019;50:408-420
32. Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z. et al. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814-826
33. Li CX, Li HG, Huang LT, Kong YW, Chen FY, Liang JY. et al. H19 lncRNA regulates keratinocyte differentiation by targeting miR-130b-3p. Cell Death Dis. 2017;8(11):e3174
34. Han YB, Tian M, Wang XX, Fan DH, Li WZ, Wu F. et al. Berberine ameliorates obesity-induced chronic inflammation through suppression of ER stress and promotion of macrophage M2 polarization at least partly via downregulating lncRNA Gomafu. Int Immunopharmacol. 2020;86:106741
35. Shook BA, Wasko RR, Mano O, Rutenberg-Schoenberg M, Rudolph MC, Zirak B. et al. Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell. 2020;26(6):880-895.e6
36. Wang J, Zhou T, Wang T, Wang B. Suppression of lncRNA-ATB prevents amyloid-β-induced neurotoxicity in PC12 cells via regulating miR-200/ZNF217 axis. Biomed Pharmacother. 2018;108:707-715
37. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633-643
38. Hochrein SM, Wu H, Eckstein M, Arrigoni L, Herman JS, Schumacher F. et al. The glucose transporter GLUT3 controls T helper 17 cell responses through glycolytic-epigenetic reprogramming. Cell Metab. 2022;34(4):516-532.e11
39. Wang C, Li Y, Yan S, Wang H, Shao X, Xiao M. et al. Interactome analysis reveals that lncRNA HULC promotes aerobic glycolysis through LDHA and PKM2. Nat Commun. 2020;11(1):3162
40. Liu J, Liu ZX, Wu QN, Lu YX, Wong CW, Miao L. et al. Long noncoding RNA AGPG regulates PFKFB3-mediated tumor glycolytic reprogramming. Nat Commun. 2020;11(1):1507
41. Kopp F, Elguindy MM, Yalvac ME, Zhang H, Chen B, Gillett FA. et al. PUMILIO hyperactivity drives premature aging of Norad-deficient mice. Elife. 2019;8:e42650
42. Wculek SK, Dunphy G, Heras-Murillo I, Mastrangelo A, Sancho D. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol. 2022;19(3):384-408
43. Amir Aslani B, Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016;146:163-73
44. Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512-523
45. Ridiandries A, Tan JTM, Bursill CA. The Role of Chemokines in Wound Healing. Int J Mol Sci. 2018;19(10):3217
46. Xu S, Weng X, Wang Y, Lv D, Zeng M, Zhao F. et al. Screening and preliminary validation of T lymphocyte immunoregulation-associated long non-coding RNAs in diabetic foot ulcers. Mol Med Rep. 2019;19(3):2368-2376
47. Hu J, Zhang L, Liechty C, Zgheib C, Hodges MM, Liechty KW. et al. Long Noncoding RNA GAS5 Regulates Macrophage Polarization and Diabetic Wound Healing. J Invest Dermatol. 2020;140(8):1629-1638
48. Zgheib C, Hodges MM, Hu J, Liechty KW, Xu J. Long non-coding RNA Lethe regulates hyperglycemia-induced reactive oxygen species production in macrophages. PLoS One. 2017;12(5):e0177453
49. Herter EK, Li D, Toma MA, Vij M, Li X, Visscher D. et al. WAKMAR2, a Long Noncoding RNA Downregulated in Human Chronic Wounds, Modulates Keratinocyte Motility and Production of Inflammatory Chemokines. J Invest Dermatol. 2019;139(6):1373-1384
50. Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med. 2015;19(6):1418-1425
51. Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ. et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116(7):1143-1156
52. Yu X, Ma X, Lin W, Xu Q, Zhou H, Kuang H. Long noncoding RNA MIAT regulates primary human retinal pericyte pyroptosis by modulating miR-342-3p targeting of CASP1 in diabetic retinopathy. Exp Eye Res. 2021;202:108300
53. Zhong JY, Cui XJ, Zhan JK, Wang YJ, Li S, Lin X. et al. LncRNA-ES3 inhibition by Bhlhe40 is involved in high glucose-induced calcification/senescence of vascular smooth muscle cells. Ann N Y Acad Sci. 2020;1474(1):61-72
54. Cao J, Dong R, Jiang L, Gong Y, Yuan M, You J. et al. LncRNA-MM2P Identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol Res. 2019;7(2):292-305
55. Li B, Zhou Y, Chen J, Wang T, Li Z, Fu Y. et al. Long non-coding RNA H19 contributes to wound healing of diabetic foot ulcer. J Mol Endocrinol. 2020 JME-19-0242.R1
56. Jayasuriya R, Dhamodharan U, Karan AN, Anandharaj A, Rajesh K, Ramkumar KM. Role of Nrf2 in MALAT1/ HIF-1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic Biol Med. 2020;156:168-175
57. Takematsu E, Spencer A, Auster J, Chen PC, Graham A, Martin P. et al. Genome wide analysis of gene expression changes in skin from patients with type 2 diabetes. PLoS One. 2020;15(2):e0225267
58. Thangarajah H, Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M. et al. HIF-1alpha dysfunction in diabetes. Cell Cycle. 2010;9(1):75-79
59. Wang TH, Yu CC, Lin YS, Chen TC, Yeh CT, Liang KH. et al. Long noncoding RNA CPS1-IT1 suppresses the metastasis of hepatocellular carcinoma by regulating HIF-1α activity and inhibiting epithelial-mesenchymal transition. Oncotarget. 2016;7(28):43588-43603
60. Miao Y, Ajami NE, Huang TS, Lin FM, Lou CH, Wang YT. et al. Enhancer-associated long non-coding RNA LEENE regulates endothelial nitric oxide synthase and endothelial function. Nat Commun. 2018;9(1):292
61. Li B, Zhou Y, Chen J, Wang T, Li Z, Fu Y. et al. Long noncoding RNA H19 acts as a miR-29b sponge to promote wound healing in diabetic foot ulcer. FASEB J. 2021;35:e20526
62. Zhou L, Ren M, Zeng T, Wang W, Wang X, Hu M. et al. TET2-interacting long noncoding RNA promotes active DNA demethylation of the MMP-9 promoter in diabetic wound healing. Cell Death Dis. 2019;10(11):813
63. Lee KS, Shin S, Cho E, Im WK, Jeon SH, Kim Y. et al. nc886, a non-coding RNA, inhibits UVB-induced MMP-9 and COX-2 expression via the PKR pathway in human keratinocytes. Biochem Biophys Res Commun. 2019;512(4):647-652
64. Hu M, Wu Y, Yang C, Wang X, Wang W, Zhou L. et al. Novel Long Noncoding RNA lnc-URIDS Delays Diabetic Wound Healing by Targeting Plod1. Diabetes. 2020;69(10):2144-2156
65. Tan J, Xu Y, Han F, Ye X. Genetical modification on adipose-derived stem cells facilitates facial nerve regeneration. Aging (Albany NY). 2019;11(3):908-920
66. Cao W, Feng Y. LncRNA XIST promotes extracellular matrix synthesis, proliferation and migration by targeting miR-29b-3p/COL1A1 in human skin fibroblasts after thermal injury. Biol Res. 2019;52(1):52
67. Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36(2):195-198
68. Barale C, Russo I. Influence of Cardiometabolic Risk Factors on Platelet Function. Int J Mol Sci. 2020;21(2):623
69. Sekhon UDS, Sen Gupta A. Platelets and Platelet-Inspired Biomaterials Technologies in Wound Healing Applications. ACS Biomater Sci Eng. 2018;4(4):1176-1192
70. den Dekker A, Davis FM, Kunkel SL, Gallagher KA. Targeting epigenetic mechanisms in diabetic wound healing. Transl Res. 2019;204:39-50
71. Rawat K, Shrivastava A. Neutrophils as emerging protagonists and targets in chronic inflammatory diseases. Inflamm Res. 2022;71(12):1477-1488
72. Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB. et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21(7):815-819
73. Vatankhah N, Jahangiri Y, Landry GJ, McLafferty RB, Alkayed NJ, Moneta GL. et al. Predictive value of neutrophil-to-lymphocyte ratio in diabetic wound healing. J Vasc Surg. 2017;65(2):478-483
74. Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals (Basel). 2020;13(4):60
75. Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv Sci (Weinh). 2019;6(20):1900513
76. Rizwan H, Pal S, Sabnam S, Pal A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020;241:117148
77. Mahmoudi S, Mancini E, Xu L, Moore A, Jahanbani F, Hebestreit K. et al. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature. 2019;574(7749):553-558
78. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60(1):107-114
79. Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70(4):478-490
80. Bi H, Li H, Zhang C, Mao Y, Nie F, Xing Y. et al. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res Ther. 2019;10(1):302
81. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058-1070
82. Maeda S, Matsui T, Ojima A, Takeuchi M, Yamagishi S. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr Res. 2014;34(9):807-813
83. Davalli P, Mitic T, Caporali A, Lauriola A, D'Arca D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med Cell Longev. 2016;2016:3565127
84. Dong F, Ruan S, Wang J, Xia Y, Le K, Xiao X. et al. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020;11(9):728
85. Sireesh D, Dhamodharan U, Ezhilarasi K, Vijay V, Ramkumar KM. Association of NF-E2 Related Factor 2 (Nrf2) and inflammatory cytokines in recent onset Type 2 Diabetes Mellitus. Sci Rep. 2018;8(1):5126
86. Heiss C, Rodriguez-Mateos A, Kelm M. Central role of eNOS in the maintenance of endothelial homeostasis. Antioxid Redox Signal. 2015;22(14):1230-1242
87. Duda DG, Fukumura D, Jain RK. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol Med. 2004;10(4):143-145
88. Chambers ES, Vukmanovic-Stejic M. Skin barrier immunity and ageing. Immunology. 2020;160(2):116-125
89. Yang R, Liu F, Wang J, Chen X, Xie J, Xiong K. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res Ther. 2019;10(1):229
90. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17(2):153-162
91. Tardáguila-García A, García-Morales E, García-Alamino JM, Álvaro-Afonso FJ, Molines-Barroso RJ, Lázaro-Martínez JL. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair Regen. 2019;27(4):415-420
92. Li G, Zou X, Zhu Y, Zhang J, Zhou L, Wang D. et al. Expression and Influence of Matrix Metalloproteinase-9/Tissue Inhibitor of Metalloproteinase-1 and Vascular Endothelial Growth Factor in Diabetic Foot Ulcers. Int J Low Extrem Wounds. 2017;16(1):6-13
93. Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799
94. Almeida DATd, Braga CP, Novelli ELB, Fernandes AAH. Evaluation of lipid profile and oxidative stress in STZ-induced rats treated with antioxidant vitamin. BRAZ ARCH BIOL TECHN. 2012;55(4):527-536
95. Joo MS, Shin SB, Kim EJ, Koo JH, Yim H, Kim SG. Nrf2-lncRNA controls cell fate by modulating p53-dependent Nrf2 activation as an miRNA sponge for Plk2 and p21cip1. FASEB J. 2019;33(7):7953-7969
96. Peng WX, He PX, Liu LJ, Zhu T, Zhong YQ, Xiang L. et al. LncRNA GAS5 activates the HIF1A/VEGF pathway by binding to TAF15 to promote wound healing in diabetic foot ulcers. Lab Invest. 2021;101(8):1071-1083
97. Mullin NK, Mallipeddi NV, Hamburg-Shields E, Ibarra B, Khalil AM, Atit RP. Wnt/β-catenin Signaling Pathway Regulates Specific lncRNAs That Impact Dermal Fibroblasts and Skin Fibrosis. Front Genet. 2017;8:183
98. Li L, Zuo X, Liu D, Luo H, Zhu H. The profiles of miRNAs and lncRNAs in peripheral blood neutrophils exosomes of diffuse cutaneous systemic sclerosis. J Dermatol Sci. 2020;98(2):88-97
99. Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ. et al. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5(10):e1506
100. Wang Z, Liao W, Liu F, Yang T, Xie W, Liao M. et al. Downregulation of lncRNA EPB41L4A-AS1 Mediates Activation of MYD88-Dependent NF-κB Pathway in Diabetes-Related Inflammation. Diabetes Metab Syndr Obes. 2021;14:265-277
101. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291-13295
102. Li X, Xie X, Lian W, Shi R, Han S, Zhang H. et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1-14
103. Wang X, Zhao L. Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3β signaling pathway. Biochem Biophys Res Commun. 2016;473(2):428-434
104. Yu M, Liu W, Li J, Lu J, Lu H, Jia W. et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350
105. Nishikai-Yan Shen T, Kanazawa S, Kado M, Okada K, Luo L, Hayashi A. et al. Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice. PLoS One. 2017;12(5):e0178232
106. Liu M, Galli G, Wang Y, Fan Q, Wang Z, Wang X. et al. Novel Therapeutic Targets for Hypoxia-Related Cardiovascular Diseases: The Role of HIF-1. Front Physiol. 2020;11:774
107. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70(5):1469-1480
108. Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol. 2011;226(11):2925-2933
109. Sharma D, Singh P, Singh SS. β-N-oxalyl-L-α,β-diaminopropionic acid induces wound healing by stabilizing HIF-1α and modulating associated protein expression. Phytomedicine. 2018;44:9-19
110. Li X, Zhao Z, Gao C, Rao L, Hao P, Jian D. et al. The Diagnostic Value of Whole Blood lncRNA ENST00000550337.1 for Pre-Diabetes and Type 2 Diabetes Mellitus. Exp Clin Endocrinol Diabetes. 2017;125(6):377-383
111. Stoff A, Rivera AA, Sanjib Banerjee N, Moore ST, Michael Numnum T, Espinosa-de-Los-Monteros A. et al. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Exp Dermatol. 2009;18(4):362-369
112. Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015;24(14):1635-1647
113. Zhou Z, Zhu X, Huang H, Xu Z, Jiang J, Chen B. et al. Recent Progress of Research Regarding the Applications of Stem Cells for Treating Diabetes Mellitus. Stem Cells Dev. 2022;31(5-6):102-110
114. Gadelkarim M, Abushouk AI, Ghanem E, Hamaad AM, Saad AM, Abdel-Daim MM. Adipose-derived stem cells: Effectiveness and advances in delivery in diabetic wound healing. Biomed Pharmacother. 2018;107:625-633
115. Roncalli JG, Tongers J, Renault MA, Losordo DW. Endothelial progenitor cells in regenerative medicine and cancer: a decade of research. Trends Biotechnol. 2008;26(5):276-283
116. An Y, Liu WJ, Xue P, Ma Y, Zhang LQ, Zhu B. et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018;9(2):58
117. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648-2659
118. Zhu L, Zhong Q, Yang T, Xiao X. Improved therapeutic effects on diabetic foot by human mesenchymal stem cells expressing MALAT1 as a sponge for microRNA-205-5p. Aging (Albany NY). 2019;11(24):12236-12245
119. Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021;101(9):1254-1266
120. He ZY, Wei TH, Zhang PH, Zhou J, Huang XY. Long noncoding RNA-antisense noncoding RNA in the INK4 locus accelerates wound healing in diabetes by promoting lymphangiogenesis via regulating miR-181a/Prox1 axis. J Cell Physiol. 2019;234(4):4627-4640
121. Veikutiene A, Sirvinskas E, Adukauskiene D. [Transfusion of autologous blood]. Medicina (Kaunas). 2008;44(6):482-488
122. Zhu NN, Lu MJ, Chen YQ, Jin XJ, Zhou X, Wei HW. et al. Autologous blood transfusion stimulates wound healing in diabetic mice through activation of the HIF-1α pathway by improving the blood preservation solution. FASEB J. 2020;34(5):6038-6054
123. Guo JR, Yin L, Chen YQ, Jin XJ, Zhou X, Zhu NN. et al. Autologous blood transfusion augments impaired wound healing in diabetic mice by enhancing lncRNA H19 expression via the HIF-1α signaling pathway. Cell Commun Signal. 2018;16(1):84
124. Che H, Wang Y, Li H, Li Y, Sahil A, Lv J. et al. Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-β1/Smads signaling in diabetic cardiomyopathy. FASEB J. 2020;34(4):5282-5298
125. Xing Y, Cheng Z, Wang R, Lv C, James TD, Yu F. Analysis of extracellular vesicles as emerging theranostic nanoplatforms. Coordination Chemistry Reviews. 2020;424:213506
126. Born LJ, Chang KH, Shoureshi P, Lay F, Bengali S, Hsu ATW. et al. HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Adv Healthc Mater. 2022;11(5):e2002070
127. Tao SC, Rui BY, Wang QY, Zhou D, Zhang Y, Guo SC. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25(1):241-255
128. Duan G, Wen L, Sun X, Wei Z, Duan R, Zeng J. et al. Healing Diabetic Ulcers with MoO(3-)(X) Nanodots Possessing Intrinsic ROS-Scavenging and Bacteria-Killing Capacities. Small. 2022;18(10):e2107137
129. Zhao H, Huang J, Li Y, Lv X, Zhou H, Wang H. et al. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials. 2020;258:120286
Author contact
![]() Corresponding authors: Mao Luo and Min Zeng, Postal address: Key Laboratory of Medical Electrophysiology, Ministry of Education, Drug Discovery Research Center of Southwest Medical University and Department of Pharmacy of the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China. E-mail addresses: luomao20050908com (M. LUO), zengminlzcom (M. Zeng).
Corresponding authors: Mao Luo and Min Zeng, Postal address: Key Laboratory of Medical Electrophysiology, Ministry of Education, Drug Discovery Research Center of Southwest Medical University and Department of Pharmacy of the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China. E-mail addresses: luomao20050908com (M. LUO), zengminlzcom (M. Zeng).
 Global reach, higher impact
Global reach, higher impact