13.3
Impact Factor
Theranostics 2023; 13(9):2979-2992. doi:10.7150/thno.86007 This issue Cite
Research Paper
Dual targeting PET tracer [68Ga]Ga-FAPI-RGD in patients with lung neoplasms: a pilot exploratory study
1. Department of Nuclear Medicine, State Key Laboratory of Complex Severe and Rare Diseases, Beijing Key Laboratory of Molecular Targeted Diagnosis and Therapy in Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China.
2. Departments of Diagnostic Radiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 119074, Singapore.
3. Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117599, Singapore.
4. Nanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore.
5. Eight-Year Program of Clinical Medicine, Peking Union Medical College Hospital (PUMCH), Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing 100730, China.
6. Department of Thoracic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
7. Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
8. Departments of Chemical and Biomolecular Engineering and Biomedical Engineering, Faculty of Engineering, National University of Singapore, Singapore 117597, Singapore.
Abstract
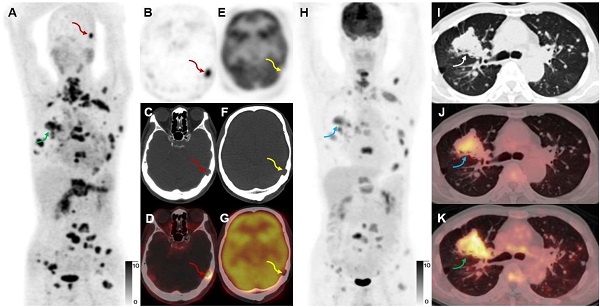
Rationale: Early discovery, accurate diagnosis, and staging of lung cancer is essential for patients to receive appropriate treatment. PET/CT has become increasingly recognized as a valuable imaging modality for these patients, but there remains room for improvement in PET tracers. We aimed to evaluate the feasibility of using [68Ga]Ga-FAPI-RGD, a dual-targeting heterodimeric PET tracer that recognizes both fibroblast activation protein (FAP) and integrin αvβ3 for detecting lung neoplasms, by comparing it with [18F]FDG and single-targeting tracers [68Ga]Ga-RGD and [68Ga]Ga-FAPI.
Methods: This was a pilot exploratory study of patients with suspected lung malignancies. All 51 participants underwent [68Ga]Ga-FAPI-RGD PET/CT, of which: 9 participants received dynamic scans, 44 participants also underwent [18F]FDG PET/CT scan within two weeks, 9 participants underwent [68Ga]Ga-FAPI PET/CT scan and 10 participants underwent [68Ga]Ga-RGD PET/CT scan. The final diagnosis was made based on histopathological analyses and clinical follow-up reports.
Results: Among those who underwent dynamic scans, the uptake of pulmonary lesions increased over time. The optimal timepoint for a PET/CT scan was identified to be 2 h post-injection. [68Ga]Ga-FAPI-RGD had a higher detection rate of primary lesions than [18F]FDG (91.4% vs. 77.1%, p < 0.05), higher tumor uptake (SUVmax, 6.9 ± 5.3 vs. 5.3 ± 5.4, p < 0.001) and higher tumor-to-background ratio (10.0 ± 8.4 vs. 9.0 ± 9.1, p < 0.05), demonstrated better accuracy in mediastinal lymph node evaluation (99.7% vs. 90.9%, p < 0.001), and identified more metastases (254 vs. 220). There was also a significant difference between the uptake of [68Ga]Ga-FAPI-RGD and [68Ga]Ga-RGD of primary lesions (SUVmax, 5.8 ± 4.4 vs. 2.3 ± 1.3, p < 0.001).
Conclusion: In our small scale cohort study, [68Ga]Ga-FAPI-RGD PET/CT gave a higher primary tumor detection rate, higher tracer uptake, and improved detection of metastases compared with [18F]FDG PET/CT, and [68Ga]Ga-FAPI-RGD also had advantages over [68Ga]Ga-RGD and was non-inferior to [68Ga]Ga-FAPI. We thus provide proof-of-concept for using [68Ga]Ga-FAPI-RGD PET/CT for diagnosing lung cancer. With the stated advantages, the dual-targeting FAPI-RGD should also be explored for therapeutic use in future studies.
Keywords: [68Ga]Ga-FAPI-RGD, [18F]FDG, Lung neoplasm, FAP, Integrin αvβ3
 Global reach, higher impact
Global reach, higher impact