13.3
Impact Factor
Theranostics 2023; 13(9):2843-2862. doi:10.7150/thno.83942 This issue Cite
Research Paper
Mesenchymal stromal cells and alpha-1 antitrypsin have a strong synergy in modulating inflammation and its resolution
1. Department of Biomedical Engineering, Pennsylvania State University; University Park, PA, 16802, USA.
2. Huck Institutes of the Life Sciences, Pennsylvania State University; University Park, PA, 16802, USA.
3. Department of Chemical and Biomolecular Engineering, University of Nebraska-Lincoln; Lincoln, NE, 68588, USA.
4. Biomedical Center of Qingdao University; Qingdao, Shandong, 266000, China.
5. Department of Orthopedics Surgery, Pennsylvania State University College of Medicine; Hershey, PA, 17033, USA.
6. Division of Pediatric Critical Care Medicine, Department of Pediatrics, Pennsylvania State Milton S Hershey Medical Center; Hershey, PA, 17033, USA.
7. Division of Critical Care Medicine, Department of Anesthesiology and Perioperative Medicine, Pennsylvania State Milton S Hershey Medical Center; Hershey, PA, 17033, USA.
8. Department of Neurosurgery, Pennsylvania State Milton S Hershey Medical Center; Hershey, PA, 17033, USA.
9. Department of Emergency Medicine, University of Nebraska Medical Center; Omaha, NE, 68105, USA.
Abstract
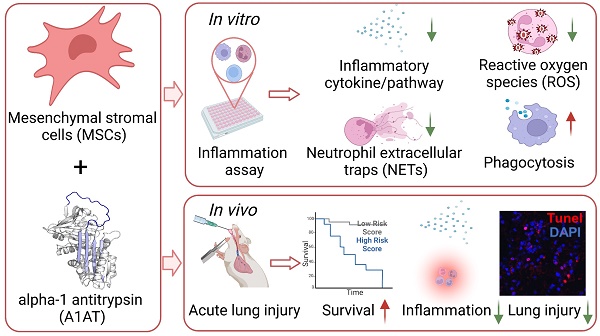
Rationale: Trauma, surgery, and infection can cause severe inflammation. Both dysregulated inflammation intensity and duration can lead to significant tissue injuries, organ dysfunction, mortality, and morbidity. Anti-inflammatory drugs such as steroids and immunosuppressants can dampen inflammation intensity, but they derail inflammation resolution, compromise normal immunity, and have significant adverse effects. The natural inflammation regulator mesenchymal stromal cells (MSCs) have high therapeutic potential because of their unique capabilities to mitigate inflammation intensity, enhance normal immunity, and accelerate inflammation resolution and tissue healing. Furthermore, clinical studies have shown that MSCs are safe and effective. However, they are not potent enough, alone, to completely resolve severe inflammation and injuries. One approach to boost the potency of MSCs is to combine them with synergistic agents. We hypothesized that alpha-1 antitrypsin (A1AT), a plasma protein used clinically and has an excellent safety profile, was a promising candidate for synergism.
Methods: This investigation examined the efficacy and synergy of MSCs and A1AT to mitigate inflammation and promote resolution, using in vitro inflammatory assay and in vivo mouse acute lung injury model. The in vitro assay measured cytokine releases, inflammatory pathways, reactive oxygen species (ROS), and neutrophil extracellular traps (NETs) production by neutrophils and phagocytosis in different immune cell lines. The in vivo model monitored inflammation resolution, tissue healing, and animal survival.
Results: We found that the combination of MSCs and A1AT was much more effective than each component alone in i) modulating cytokine releases and inflammatory pathways, ii) inhibiting ROS and NETs production by neutrophils, iii) enhancing phagocytosis and, iv) promoting inflammation resolution, tissue healing, and animal survival.
Conclusion: These results support the combined use of MSCs, and A1AT is a promising approach for managing severe, acute inflammation.
Keywords: inflammation, mesenchymal stromal cells, alpha-1 antitrypsin, combination therapy
 Global reach, higher impact
Global reach, higher impact