13.3
Impact Factor
Theranostics 2023; 13(9):2757-2773. doi:10.7150/thno.81406 This issue Cite
Research Paper
The STAT1/HMGB1/NF-κB pathway in chronic inflammation and kidney injury after cisplatin exposure
1. Department of Nephrology, Hunan Key Laboratory of Kidney Disease and Blood Purification, The Second Xiangya Hospital of Central South University, Changsha 410011, China.
2. Department of Cellular Biology and Anatomy, Medical College of Georgia at Augusta University and Charlie Norwood VA Medical Center, Augusta, GA 30912, USA.
Abstract
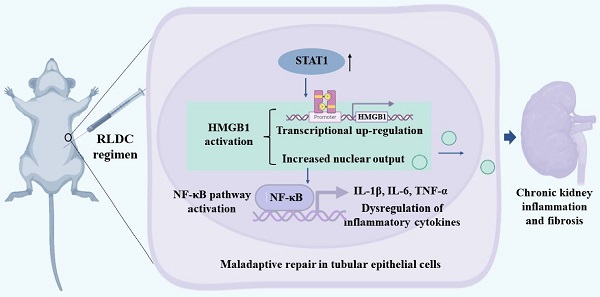
Rationale: Cisplatin, a potent chemotherapeutic drug, induces side effects in normal tissues including the kidney. To reduce the side effects, repeated low-dose cisplatin (RLDC) is commonly used in clinical setting. While RLDC reduces acute nephrotoxicity to certain extents, a significant portion of patients later develop chronic kidney problems, underscoring the need for novel therapeutics to alleviate the long-term sequelae of RLDC therapy.
Methods: In vivo, the role of HMGB1 was examined by testing HMGB1 neutralizing antibodies in RLDC mice. In vitro, the effects of HMGB1 knockdown on RLDC-induced nuclear factor-κB (NF-κB) activation and fibrotic phenotype changes were tested in proximal tubular cells. To study signal transducer and activator of transcription 1 (STAT1), siRNA knockdown and its pharmacological inhibitor Fludarabine were used. We also searched the Gene Expression Omnibus (GEO) database for transcriptional expression profiles and evaluated kidney biopsy samples from CKD patients to verify the STAT1/HMGB1/NF-κB signaling axis.
Results: We found that RLDC induced kidney tubule damage, interstitial inflammation, and fibrosis in mice, accompanied by up-regulation of HMGB1. Blockage of HMGB1with neutralizing antibodies and Glycyrrhizin suppressed NF-κB activation and associated production of pro-inflammatory cytokines, reduced tubular injury and renal fibrosis, and improved renal function after RLDC treatment. Consistently, knockdown of HMGB1 decreased NF-κB activation and prevented the fibrotic phenotype in RLDC-treated renal tubular cells. At the upstream, knockdown of STAT1 suppressed HMGB1 transcription and cytoplasmic accumulation in renal tubular cells, suggesting a critical role of STAT1 in HMGB1 activation. Upregulation of STAT1/HMGB1/NF-κB along with inflammatory cytokines was also verified in kidney tissues of CKD patients.
Conclusion: These results unravel the STAT1/HMGB1/NF-κB pathway that contributes to persistent inflammation and chronic kidney problems after cisplatin nephrotoxicity, suggesting new therapeutic targets for kidney protection in cancer patients receiving cisplatin chemotherapy.
Keywords: cisplatin, inflammation, renal fibrosis, HMGB1, NF-κB
 Global reach, higher impact
Global reach, higher impact