13.3
Impact Factor
Theranostics 2023; 13(9):2734-2756. doi:10.7150/thno.82005 This issue Cite
Review
Current advances in temozolomide encapsulation for the enhancement of glioblastoma treatment
1. Cellular Oncology group, Biodonostia Health Research Institute, San Sebastian, Spain.
2. IKERBASQUE, Basque Foundation for Science, Bilbao, Spain.
3. Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento (CIBERfes), Carlos III Institute, Madrid, Spain.
Received 2022-12-20; Accepted 2023-4-20; Published 2023-5-8
Abstract
Glioblastoma is the most common and lethal brain tumor in adults. The incorporation of temozolomide (TMZ) into the standard treatment has increased the overall survival rate of glioblastoma patients. Since then, significant advances have been made in understanding the benefits and limitations of TMZ. Among the latter, the unspecific toxicity of TMZ, poor solubility, and hydrolyzation are intrinsic characteristics, whereas the presence of the blood-brain barrier and some tumor properties, such as molecular and cellular heterogeneity and therapy resistance, have limited the therapeutic effects of TMZ in treating glioblastoma. Several reports have revealed that different strategies for TMZ encapsulation in nanocarriers overcome those limitations and have shown that they increase TMZ stability, half-life, biodistribution, and efficacy, offering the promise for future nanomedicine therapies in handling glioblastoma. In this review, we analyze the different nanomaterials used for the encapsulation of TMZ to improve its stability, blood half-life and efficacy, paying special attention to polymer- and lipid-based nanosystems. To improve TMZ drug resistance, present in up to 50% of patients, we detail TMZ combined therapeutic with i) other chemotherapies, ii) inhibitors, iii) nucleic acids, iv) photosensitizers and other nanomaterials for photodynamic therapy, photothermal therapy, and magnetic hyperthermia, v) immunotherapy, and vi) other less explored molecules. Moreover, we describe targeting strategies, such as passive targeting, active targeting to BBB endothelial cells, glioma cells, and glioma cancer stem cells, and local delivery, where TMZ has demonstrated an improved outcome. To finish our study, we include possible future research directions that could help decrease the time needed to move from bench to bedside.
Keywords: glioblastoma, temozolomide, encapsulation, nanosystems, targeted delivery
Current standard of care and treatment of glioblastoma
Glioblastoma (GBM), considered as grade 4 glioma attending to the 2021 CNS WHO classification [1], is the most common and lethal brain tumor in adults accounting for almost 50% of all cases. The annual incidence is around 1-5 per 100,000 population, and it presents a devastating prognosis with a median survival of 15-18 months and a five-year survival rate of less than 5% [2-4].
Surgery remains the best therapeutic option. Indeed, a greater extent of surgical resection is associated with better clinical outcomes. However, this is not always possible because usually the tumor affects areas in the eloquent cortex that are crucial for speech control, motor function, and the senses [5]. Moreover, as GBM is a highly invasive tumor, radical resection of the tumor mass is not curative and the infiltrating tumor cells that remain within the surrounding area lead to later disease progression or recurrence [6]. To enhance surgical resection, advances in the imaging techniques have been developed, including intraoperative MRI, diffusion tensor imaging, awake craniotomy, cortical mapping, stereotactic guidance, and fluorescent‑guided resection. For instance, studies have shown that fluorescent-guided resection enables patients to be progression-free for six months, by achieving more complete resections of contrast-enhancing tumors [7-9]. However, even if maximal surgical resection remains important for the progression of patients, it seems that GBM is not completely cured with just a surgical answer [6]. After surgery, the next step in the current standard treatment is the administration of radiation and chemotherapy. Indeed, the gold standard treatment for newly diagnosed patients consists of maximal surgical resection of the tumor (if possible), followed by the alkylating agent temozolomide (TMZ) in combination with 60 Gy of X-ray irradiation, fractionated in 30 sessions of 2 Gy each during six weeks, and six more cycles of TMZ for maintenance [10,11].
TMZ is an oral alkylating agent that transfers alkyl groups to guanine bases, causing DNA damage, which, if not repaired, causes apoptosis. This chemotherapeutic agent was added to the standard of care for GBM, when the clinical trial carried out in 2005 [10] and additional trials [12,13] demonstrated that concurrent radiotherapy and TMZ followed by adjuvant TMZ significantly increased the median survival of the patients from 12.1 months (just radiotherapy) up to 14.6 months. In addition, studies revealed that the two-year survival rate was raised from 10.4% up to 26.5%.
Several omic studies [5,14-17] have revealed the existence of molecular, genetic, and epigenetic heterogeneity in GBM that have served as potential biomarkers with prognostic and diagnostic potential. These biomarkers include i) the expression of mutated isocitrate dehydrogenase (IDH) which is usually correlated with a better prognosis, ii) amplification of EGFR, iii) mutations in genes such as p53 and NF1, and iv) telomerase reverse transcriptase promoter mutations. Although this knowledge has served to better understand the disease, its translation into treatment efficacy has not yet been successful. In the case of TMZ, it has been described that TMZ response could depend on O6-methylguanine-DNA methyltransferase (MGMT) methylation status [10]. MGMT is a DNA repair protein that removes alkyl groups from the O6 position of guanine in DNA. Methylation in MGMT causes its silencing and, thus, increases TMZ sensitivity, whereas no methylation in the MGMT promoter activates the gene expression, triggering enzyme repair and TMZ chemotherapy resistance [18-20].
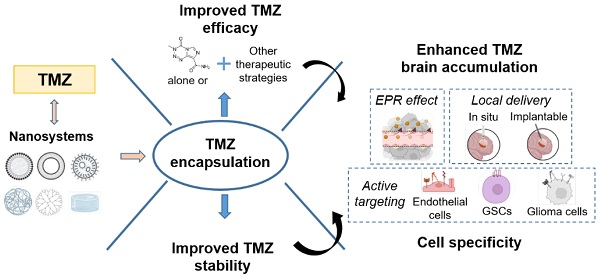
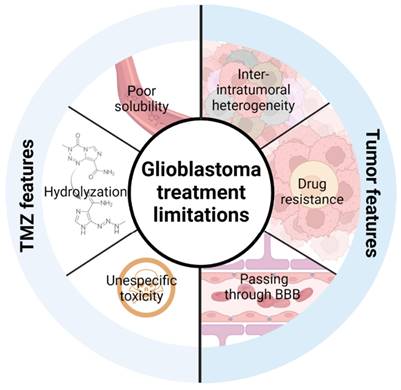
Taking into account that TMZ is the only treatment that has demonstrated an advance in patient survival in clinical studies, strategies to improve its efficacy are promising to extend patient survival and increase their quality of life. In this review, we will describe the recent advances of TMZ encapsulation and how this strategy can improve TMZ limitations (its solubility, hydrolyzation and unspecific toxicity) and limitations due to the intrinsic features of the tumor including the blood-brain barrier (BBB), high inter- and intratumor cell and molecular heterogeneity and the development of therapy resistance [21,22] (Figure 1).
Limitations of current TMZ treatment that need to be overcome to improve its efficacy. TMZ features include its poor solubility, its hydrolyzation in contact with physiological medium and its unspecific toxicity. Tumor intrinsic features include inter- and intratumoral cell and molecular heterogeneity, drug resistance, and the blood-brain barrier.
Improving TMZ limitations
TMZ mechanism of action
The metabolic pathway of TMZ is a pH-dependent reaction that begins when it comes into contact with a physiological medium. TMZ is hydrolyzed to 5(-3methyltriazen-1-yl) imidazole-4-carboxamide) (MTIC). This polar molecule is then degraded to AIC and the methyldiazonium cation. While AIC is secreted through the kidneys, the methyldiazonium cation is the responsible agent that transfers the methyl group to the DNA causing DNA damage followed by apoptosis when cellular repair mechanisms are unable to adjust the methylated bases [23].
MTIC has a short half-life (~ 2 h) and poor penetration through the BBB [24], so to achieve the desired therapeutic effect, patients receive high doses of TMZ for a long period, which can lead to serious systemic toxicity, drug resistance, and side effects [25,26]. Nanomedicine can help overcome these problems: the encapsulation of TMZ offers different advantages including i) the improvement of TMZ's solubility and stability, ii) better TMZ brain accumulation, iii) reduction of high dosage administration, and subsequently, iv) a decrease of TMZ's side effects.
Improving the solubility and stability of TMZ
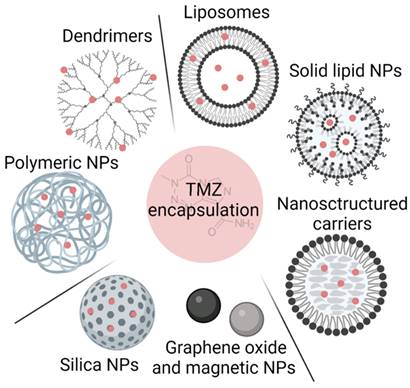
In this section, we analyze the current status of nanomaterials that have been used to encapsulate TMZ and improve its solubility and stability, and consequently its blood circulation time and efficacy. We detail the advantages and disadvantages of lipid- and polymer-based nanosystems and comment on newer systems based on other types of nanomaterials (summarized in Figure 2 and Table 1).
Summary of different possibilities for TMZ encapsulation
Polymer-based nanosystems
Polymer-based nanosystems have been studied for biomedical applications due to their advantages, such as stability and long blood circulation time, significant water solubility or dispersibility, controlled size (10-200 nm), biodegradability, modifiable surface, and controlled drug release [27]. Different nanosystems based on polymers have been used for TMZ encapsulation, among which polymeric nanoparticles (polymeric NPs) and dendrimers have been the most studied.
Polymeric nanoparticles are solid, colloidal nano-sized (100-200 nm) particles that entrap or adsorb therapeutic agents into the polymer matrix or in the surface. The first polymeric NP for drug delivery to the brain was developed in 1995 [28]; however, it was not until 2011 that the first polymeric NP was described encapsulating TMZ [29]. In that study, the researchers used polybutylcyanoacrylate nanoparticles, a minimally toxic, biocompatible, and biodegradable polymer with an easy synthesis and industrial scalable production [29]. They tested and demonstrated for the first time in healthy Wistar rats that encapsulated TMZ was highly accumulated in the brain compared to free TMZ. Moreover, the study showed that TMZ's concentration was lower in the heart and kidney compared to free TMZ, suggesting that this system accumulated lower toxicity of TMZ in these organs [29]. After this study, different researchers have performed additional approaches encapsulating TMZ in other polymeric NPs and analyzed the drug release profile in vitro. In particular, poly(lactic-co-glycolic acid) (PLGA) has been the most studied polymer. The first approach performed by Jain et al., described an enhanced release pattern of TMZ from PLGA nanoparticles. Free TMZ was dissolved in ~2h, while encapsulated TMZ had a sustained release pattern up to 120h, thus increasing TMZ availability 60 times [30]. Additional reports have shown that the sustained release pattern could be from over 3 days [31] up to 9 [32] and even 10 days [33].
Although PLGA nanoparticles increase the stability of TMZ in physiological media, their loading capacity and encapsulation efficacy (EE) are usually poor at just 2-4% and 17-50%, respectively. To overcome these disadvantages, NPs composed of different polymers have been studied. For example, PLGA and chitosan nanoparticles achieved an encapsulation efficiency of 27.3% [34] and PLGA nanoparticles with polyethylene glycol (PEG) increased their EE to 64.5% [35]. Moreover, nanoparticles based on polymers such as chitosan [36-38], polysaccharides [39], PLA [40] or albumin [41,42] have confirmed the same release pattern, thus improving TMZ stability.
In addition to polymeric NPs, other polymeric structures such as dendrimers are being studied for TMZ encapsulation. Dendrimers are branched polymeric molecules that consist of an internal core and repeated external branching units (Figure 2). Their structure allows i) conjugation of active drugs or functional groups in the peripheral branching framework by covalent binding, hydrogen bonding, or electrostatic adsorption and ii) entrapping drugs between the segmented cavities of the polymer blocks. Sharma et al. developed a PAMAM-chitosan conjugate and tested them in vitro in U251 and T98G cell lines showing that dendrimers caused higher cell death in both cell lines [43]. They also performed pharmacokinetic analysis in vivo and demonstrated that the formulation had a sustained release with a TMZ half-life one and a half times higher, increasing TMZ brain concentration by almost twofold when it was administrated encapsulated [43].
In general, polymer-based nanosystems have demonstrated the ability to increase the stability of TMZ and control its release, which could be translated into an increase in the blood circulation time in vivo, and hence, an improvement in TMZ brain accumulation.
Lipid-based nanosystems
Lipid-based nanosystems have been widely studied for TMZ encapsulation. They have different advantages, including i) encapsulation of both hydrophobic and hydrophilic molecules, ii) improvement of drug solubility and blood half-life time, iii) low toxicity and safe biodegradation, and iv) the possibility of controlled drug release. The most characterized nanosystems for TMZ encapsulation in GBM are liposomes and nanostructured carriers (NSCs) (Table 1).
Liposomes are small spherical vesicles composed of a phospholipid bilayer in an aqueous medium, with a structure and composition very similar to the phospholipids of the cell membrane; thus, they are nontoxic and biodegradable. The first articles reporting TMZ encapsulation in liposomes were carried out in 2009 [44] and 2010 [45]. However, the first study reporting a liposomal formulation encapsulating TMZ for GBM treatment was in 2015 [46]. In this initial approach, the authors studied the pharmacokinetics and biodistribution of administrated liposomes in healthy rabbits and mice, showing that liposomal encapsulation of TMZ prolonged its half-life in plasma. Moreover, TMZ brain concentration was higher in animals administrated with the liposomal formulation. They also observed that less liposomal TMZ accumulated in the heart and lungs, which could indicate fewer TMZ side effects in these tissues. However, the liposomal TMZ concentration was higher in liver, kidney, and spleen, highlighting a note of caution regarding this strategy. In this case, the high level of TMZ in the liver and spleen could be explained by the fact that macrophages in these organs tend to take up nanoparticles. Plasma proteins bind to the surface of nanoparticles and are recognized by the reticuloendothelial system, clearing them from the liver and spleen [47].
Advantages and disadvantages of the different nanosystems that have been used to improve TMZ limitations based on polymers, lipids, and other nanomaterials.
| Advantages | Disadvantages | ||
|---|---|---|---|
| Polymer-based nanosystems | Polymeric nanoparticles | Scalable production, improved TMZ stability, co-encapsulation | Low encapsulation efficiency |
| Dendrimers | Improved TMZ stability, controlled release, multiple encapsulation, co-encapsulation | Reduced number of studies | |
| Lipid-based nanosystems | Liposomes | More studied nanosystem, excellent biocompatible, sustained release, multiple encapsulation, co-encapsulation | Rapid clearance, low stability, immune response, not studied with other drugs |
| Solid lipid nanoparticles | Sustained release, stability, scalable industrial production | Reduced number of studies, not studied with other drugs | |
| Nanostructured carriers | Biocompatible, encapsulation efficiency, controlled release, co-encapsulation | Reduced number of studies | |
| Other nanomaterials | Mesoporous silica nanoparticles | Biocompatible controlled release, co-encapsulation, combination with other nanomaterials | Reduced number of just in vitro studies |
| Graphene oxide nanoparticles | Improved TMZ efficacy | There is only one in vitro study | |
| Magnetic nanoparticles | Improved TMZ stability and cell uptake, improved TMZ efficacy, co-encapsulation | There is only one in vitro study |
Therefore, although the blood circulation time was increased compared to that of the free drug, it was still low due to their rapid elimination by macrophages from the reticuloendothelial system. To solve this problem, different researchers have incorporated PEG on the surface of liposomes, by a process called PEGylation [47]. This polymer is hydrophilic and inert and provides a steric barrier on the surface of liposomes, minimizing plasma protein binding and enhancing nanoformulation stability. A study performed by Hu et al. [48] not only demonstrated that PEGylated liposomes increased the plasma concentration of TMZ and were more concentrated in the brain, but they were also able to delay the liposomes' clearance, suggesting that PEGylation is a good strategy for overcoming the reticuloendothelial system.
However, it has been reported that repeated intravenous injections of PEGylated liposomes led to a phenomenon called “accelerated blood clearance,” where the induction of anti-PEG antibodies in the first injection triggers the removal of the formulation from the body [49,50]. Thus, solid lipid nanoparticles (SLNPs) were designed to improve the stability of nanoformulations. SLNPs are a new generation of colloidal lipid carriers composed of physiological lipids that are in the solid phase at room and at physiological temperatures [51,52]. Huang et al. demonstrated that, similar to liposomes, the release pattern of TMZ from this nanosystem was slow, increasing the plasma concentration of TMZ and brain accumulation from 6.7% up tp 13.25% [53].
SLNPs present some advantages over liposomes such as better stability and scalable industrial production. However, they still have some drawbacks to overcome. They present a moderate drug-loading capacity and can expulse the load due to the crystallization process under storage conditions [52]. For this reason, nanostructured carriers are being developed. Nanostructured carriers are a second generation of lipid-based nanosystems that combine in their structure solid and liquid lipids at room and at physiological temperatures. The use of both types of lipids improves the encapsulation capacity and avoids drug expulsion [52]. In 2016, Qu et al. published an article where they compared the best nanosystems to carry and deliver TMZ between NSCs, SLNPs, and polymeric NPs [54]. They concluded that NSCs had the best anti-tumor activity in vitro and in vivo. Although all nanoformulations showed significantly higher efficacy than the free drug, the IC50 value of NSCs was 4 and 7 times lower than those of SLNPs and polymeric nanoparticles, respectively. Moreover, U87 solid tumors in mice were inhibited by 85% in the case of NSCs, while it was by 59% and by 45% in the cases of SLNPs and polymeric NPs, respectively. These differences might be attributable to the better capacity of NSCs to enter the tumor and release the drug into cancer cells. Moreover, when comparing the efficacy of TMZ loaded in SLNPs with that of NSCs, Wu et al. demonstrated that NSCs had a significantly better tumor inhibition efficiency both in vitro and in vivo [55]. While TMZ loaded NSCs displayed a tumor inhibition rate of 70% compared with control animals, SLNPs had a tumor inhibition rate of 43%.
Other nanomaterials
Lipid-based and polymeric-based nanosystems have been widely studied for the encapsulation of TMZ for GBM. Indeed, clinical studies examining the efficacy of other drugs, such as doxorubicin or irinotecan, encapsulated in liposomal formulations suggest that these nanosystems may still be a feasible approach against GBM in the near future. However, different nanomaterials are also being studied to develop new strategies, including mesoporous silica nanoparticles, graphene oxide nanoparticles, and magnetic nanoparticles (Table 1).
Mesoporous silica nanoparticles are silicon oxide porous structures that exhibit good biocompatibility and nontoxicity [56,57]. Preliminary studies in vitro have demonstrated that TMZ is efficiently encapsulated in silica nanoparticles, having a better antitumoral effect than free TMZ [58-60]. However, more studies are needed in this new field, especially with in vivo models.
In addition to silica nanoparticles, other nanomaterials have been studied for the delivery of TMZ, among which, there has been one study reported that used graphene oxide nanoparticles. It was demonstrated that this strategy increased cell inhibition in rat glioma cells [61]. In addition, magnetic nanoparticles are another nanomaterial that has been used for the delivery of TMZ. Indeed, Dilnawaz et al. demonstrated in T98G cell line 2D cultures and 3D spheroids that TMZ loaded in these nanoparticles triggered cell death induction [62].
Combinations of nanomaterials
Different studies have proposed that the combination of different nanomaterials, such as silica nanoparticles with polymers, improves the nanoformulation in different diseases including COVID-19 and breast cancer [63-65]. Moreover, Mazarei et al. [66] designed TMZ-loaded selenium nanoparticles functionalized with chitosan and Eudragit®, a pH-dependent polymer that is dissolved in a medium with pH > 5.5, to enhance site-specific drug delivery. They measured the effect of their nanoformulation in vitro, demonstrating that i) the stability of TMZ improved at acidic and neutral pH by reducing the IC50, ii) increased the cellular uptake, and iii) treated cells decreased the expression of important genes such as MGMT and induced more efficiently apoptosis with higher cytotoxicity.
Furthermore, other nanomaterials such as superparamagnetic nanoparticles [67-71] have been used to design TMZ-loading nanosystems to add new functionalities [51]. The use of superparamagnetic nanoparticles offers various advantages, including the possibility of i) actively targeting them under an external magnetic field, ii) gaining visibility in magnetic resonance imaging (MRI) by reducing both T1 and T2/T2* relaxation times, and iii) magnetic hyperthermia (discussed in the Section "TMZ with photodynamic therapy, photothermal therapy, and magnetic hyperthermia). Hence, adding superparamagnetic nanoparticles to the nanosystems might offer these advantages. For instance, Ling et al. combined superparamagnetic nanoparticles with PLGA and Tween 80 for TMZ encapsulation [67]. In addition to an excellent sustained release profile and an inhibitory cell proliferation effect in vitro, this study demonstrated that the combination of superparamagnetic nanoparticles with polymeric nanoparticles could be used as contrast agents. Bernal et al. [68] tested their nanosystem composed of polymeric (PEG-PLA-PCL) superparamagnetic-bearing nanoparticles in vitro and in vivo with local delivery (convection-enhanced delivery, CED). They showed by MRI, due to the magnetic nanoparticles, that CED could effectively distribute their nanosystem with just some local edema but with no parenchymal changes. Moreover, glioma cells trapped the nanosystem and the TMZ release prolonged the survival of the animals compared to the control ones. More recently, the same group demonstrated similar results in an in vivo dog model [69], showing that the encapsulation of superparamagnetic nanoparticles adds the advantage of image guidance.
Improving TMZ resistance: Combined therapy
The encapsulation of TMZ in nanosystems improves the stability and brain accumulation of the drug compared with free TMZ administration. However, it has been reported that up to 50% of patients do not respond favorably to TMZ therapy. As other studies have demonstrated that the combination of therapies achieved a synergic effect between them [72,73], researchers are studying the co-delivery of TMZ with other therapeutic agents. In fact, nanosystems are being developed to be encapsulated together with TMZ. These agents include other chemotherapeutic agents (paclitaxel [74], doxorubicin [37], and 5-Fluoracil [39]); inhibitors [75,76]; nucleic acids [58,77-80]; photosensitizers for photodynamic therapy [81,82]; other nanomaterials for photothermal therapy [83,84] and magnetic hyperthermia [85-87] and other molecules [59,62,88] (summarized in Table 2 and Figure 3).
Nanosystems that have been studied for TMZ co-delivery with other molecules.
| Nanosystem | Co-delivery | Therapeutic strategies | Studies | Ref | |
|---|---|---|---|---|---|
| Polymer-based nanosystems | Polymeric nanoparticles | Doxorubicin | Chemotherapy | Characterization studies | [37] |
| 5-Fluoracil | Chemotherapy | Characterization studies | [39] | ||
| Idasanutlin | MDM2 inhibitor | In vitro (GSCs) | [75] | ||
| si-EGFR | Nucleic acids | In vivo | [77] | ||
| Super-paramagnetic nanoparticles | Magnetic hyperthermia | In vitro | [85] | ||
| Cy5-dye | Imaging | In vivo | [34] | ||
| Dendrimers | Paclitaxel | Chemotherapy | In vitro | [74] | |
| Polymeric micelles | si-Polo like kinase 1 | Nucleic acids | In vivo | [78] | |
| Si-Bcl2 | Nucleic acids | In vivo | [79] | ||
| Verteporfin | Photodynamic therapy | In vitro | [81] | ||
| Lipid-based nanosystems | SLNPs | Vincristine | Chemotherapy | In vitro | [55] |
| Magnetic nanoparticles | Magnetic hyperthermia | In vitro (spheroids) | [86] | ||
| Liposomes | Bromodomain inhibitor | Inhibitors | In vivo | [76] | |
| Super-paramagnetic nanoparticles | Magnetic hyperthermia | In vitro | [87] | ||
| NSCs | Vincristine | Chemotherapy | In vitro | [55] | |
| GFP gene | Nucleic acids | In vivo | [80] | ||
| Curcumin | Other molecules | In vivo | [88] | ||
| Other nanomaterials | Silica-based nanoparticles | anti-miR221 | Nucleic acids | In vitro | [58] |
| Porous silicon NPs | Photothermal therapy | In vitro | [83] | ||
| DNAzyme | Other molecules | In vitro | [59] | ||
| Magnetic nanoparticles | Indocyanine green | Photothermal and photodynamic therapy | In vitro | [82] | |
| Curcumin | Other molecules | In vitro | [62] | ||
| Copper-based nanoparticles | Photothermal and photodynamic therapy | In vitro | [84] | ||
Schematic illustration of combined therapeutical approaches with TMZ for GBM treatment, including i) other chemotherapies, ii) inhibitors, iii) nucleic acids, iv) alternative treatments, such as photodynamic therapy, photothermal therapy, and magnetic hyperthermia, v) immunotherapy, and vi) other less-explored molecules.
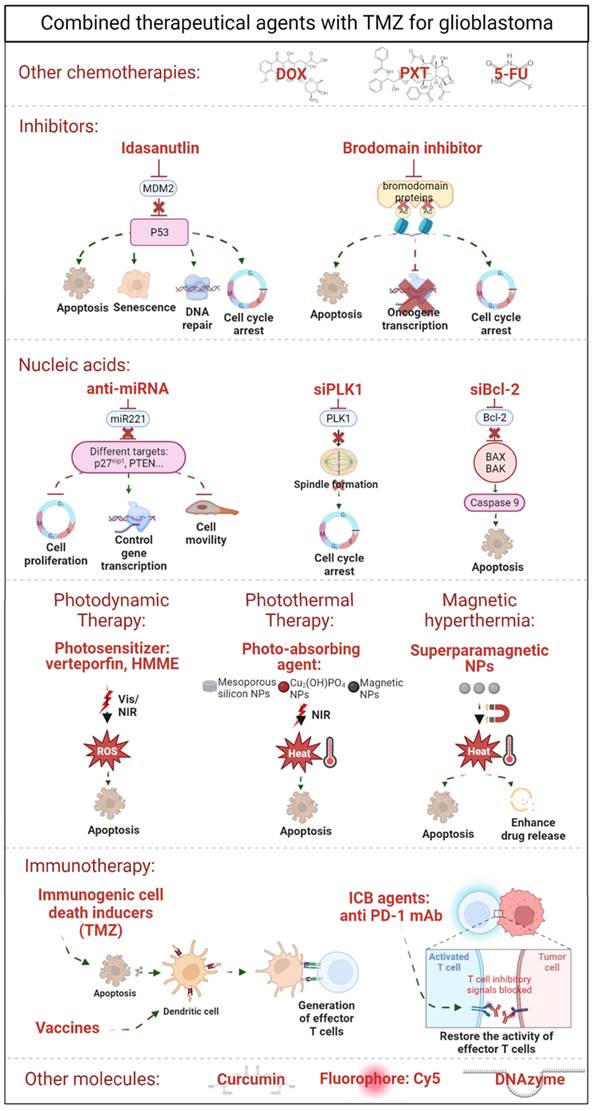
TMZ with other chemotherapies
Several studies have reported that polymer-based nanosystems could be used to co-encapsulate TMZ with other chemotherapies including doxorubicin [37], paclitaxel [74] and 5-Fluoracil [39]. In studies carried out by Di Martino [37,39], both doxorubicin and 5-Fluoracil were co-encapsulated with TMZ in polymeric NPs. Behrooz et al., on the other hand, used dendrimers composed of PAMAM to deliver TMZ with paclitaxel to U87 CD133+ and CD44+ stem cells [74]. They demonstrated that this nanosystem was able to increase the early apoptosis from 28.2% in the case of the administration of the free drug, up to 73.3% with the encapsulated co-delivery. In addition to polymer-based nanosystems, studies reporting the co-encapsulation of TMZ with other chemotherapies, such as vincristine in SLNPs and NSCs, suggesting that lipid-based nanosystems could also be used for the co-delivery of TMZ with other chemotherapies [55].
TMZ with inhibitors
To try diverse therapeutical strategies, researchers have encapsulated TMZ with specific inhibitors. For instance, polymeric NPs, such as PLGA and poly(styrene-b-ethylene oxide) (PS-b-PEO) nanoparticles have been used to encapsulate TMZ together with an inhibitor of the oncoprotein MDM2, called idasanutlin [75]. Idasanutlin has been described as a MDM2 inhibitor of the nutlin class that binds specifically to MDM2 with > 100-fold selectivity for GBM in various cell lines [89]. The administration of both therapies was performed in glioma stem cells (GSCs) and they proved that both treatments had a synergic effect increasing the cytotoxicity percentage from ~ 10% in the case of TMZ-loaded NP administration compared to ~ 75% when they were exposed to polymeric NPs with both therapies [75]. Moreover, PEGylated liposomes were studied for the co-delivery of TMZ with a brodomain inhibitor [76], a molecule that binds competitively to acetyl-lysine binding motifs, triggering DNA damage and apoptosis [90]. They showed that both treatments were more efficient together than the administration of TMZ and the inhibitor separately in U87 and GL261 tumor-bearing mice.
TMZ with nucleic acids
Nucleic acid-based therapies have emerged as a powerful strategy for the treatment of brain tumors due to their direct, effective, and lasting therapeutic effect [91]. However, due to their instability, their difficulty traversing biological barriers and their off-target effect, they are perfect candidates to be encapsulated together with TMZ.
As a proof of concept, Chen et al. co-loaded TMZ with an enhanced green fluorescent protein gene in NSCs, confirming that NSCs could efficiently achieve stable gene and drug delivery, reduce the tumor growth in mice, and extend their overall survival due to the effect of TMZ [80]. In addition to NSCs, polymer-based structures, such as polymeric micelles, have been shown to be effective in the co-delivery of TMZ with nucleic acids. Polymeric micelles (10-200 nm) are colloidal systems composed of polymeric molecules dispersed in liquid. They have a core-shell structure, with an inner core composed of a hydrophobic region, and a corona composed of a hydrophilic part for the micelle stabilization [27]. Shi et al. co-delivered TMZ with a polo-like kinase-1 siRNA (PLK-1) [78], which is involved in the spindle formation and chromosome segregation during mitosis and is overexpressed in glioma tissues and whose inhibition causes cell cycle arrest and cell apoptosis [92]. Shi et al. demonstrated this not only in cell cultures, but also in a U87 orthotopic mouse model. They showed that, in addition to the tumor size reduction, the median survival of the mice and their weight were 1.2 and 1.17 times higher, respectively, compared to animals administrated with only TMZ [78]. In addition, Peng et al. encapsulated TMZ and a Bcl-2 siRNA that promotes cell apoptosis in a triblock copolymer micelle using poly(ε-caprolactone) (PCL), poly(ethylenimine) (PEI), and PEG [79]. The three polymers self-assembled via hydrophobic interaction encapsulating the drug in the hydrophobic core. Subsequently, the siRNA was complexed with the micelles through electrostatic interaction. They demonstrated that in rats bearing an orthotopic glioma model, micelles containing both strategies reduced the tumors from ~ 200 mm3 and ~ 170 mm3 in the case of the administration of siRNA and TMZ, respectively, to ~ 75 mm3 with both therapies [79]. Moreover, in a recent study, Wang et al. co-delivered TMZ with an EGFR-siRNA in polymeric nanoparticles and observed that while in mice treated with TMZ, tumors grew rapidly with a glioma inhibition rate of 164.3, the tumor inhibition rate for animals treated with both drugs was 15.3, suggesting a high antitumor efficacy and synergic effect [77].
The work carried out by Bertucci et al. combined TMZ with an anti-microRNA in silica nanoparticles. Various studies have reported that the abnormal expression profile of miRNAs is correlated with the pathogenesis of cancer cells, tumor progression, and drug resistance [93]. Indeed, in glioma, one of the upregulated miRNAs is the miR221 [94], which is responsible for the regulation of several key target genes of cell-cycle progression [95]. Interestingly, different reports have also shown that the downregulation of miR221 sensitized glioma cells to temozolomide [96,97]. Thus, Bertucci et al. co-delivered an anti-mR221 and TMZ in vitro and demonstrated that their nanosystem induced apoptosis by combining a synergic effect of both strategies [58].
TMZ with photodynamic therapy, photothermal therapy, and magnetic hyperthermia
In addition to chemotherapy, inhibitors, and nucleic acids, researchers are studying other therapeutic approaches, including photodynamic therapy (PDT), photothermal therapy (PTT), and magnetic hyperthermia.
Photodynamic therapy consists of the use of a photosensitizer (PS), which after light stimulation (usually with visible or NIR light), forms highly reactive oxygen species that interact with cellular structures and lead to cell damage and possible cell death (Figure 3). The FDA recently approved 5-aminolevulinic acid (ALA) for fluorescence-guided surgery on high-grade gliomas [7,98]. Indeed, ALA and its derivatives are one of the most studied compounds as PSs for PDT in GBM. Although studies have proved the beneficial effect of PDT in GBM (summarized in [99,100]), in this review, we are focusing on works that have studied the synergic effect of TMZ with PDT. To our knowledge, the first article reporting combined therapy of TMZ with PDT was performed by Zhang et al. [101], who administrated both treatments (hematoporphyrin monomethyl ether, or HMME, as a second-generation PS and TMZ) in glioma-bearing rats, demonstrating that their combination increased glioma cell apoptosis and also prolonged the overall survival of rats from approximately 21 days, 37 days, and 33 days in control rats and rats treated with PDT or TMZ alone, respectively, up to 51.55 days in the case of PDT-TMZ treated animals. A more recent study has demonstrated that the use of HMME as a PS for PDT-TMZ combined therapy inhibits cell migration and invasion, and more specifically induces mitochondrial-associated apoptosis [102]. Most second-generation PSs are lipophilic with a lack of solubility in aqueous media; thus, their encapsulation in nanocarriers has been proposed to avoid their aggregation and improve their solubility in aqueous media [103]. For example, in a study performed by Pellosi et al. polymeric micelles were used for the co-delivery of TMZ in combination with verteporfin, an FDA-approved PS [81]. Micelles were prepared with Pluronic®, a tri-block copolymer made of PEO, poly(propylene oxide) (PPO) and PEO, where the Pluronic PEO block is hydrophilic and water soluble and PPO is hydrophobic and water insoluble. They showed the synergic response between the two therapies in three different cell lines (U87, T98G, U343), including a TMZ-resistant cell line [81].
Photothermal therapy is a noninvasive therapy that requires the use of an external near-infrared laser to irradiate the tumor and a photo-absorbing agent that will convert the light energy into heat, triggering localized hyperthermia. Cancer cells are more sensitive to temperature than normal cells [104]. Thus, increasing therapeutic temperature (42-47 °C) in the tumor region by photothermal therapy might be a feasible strategy for irreversible cellular damage and cellular apoptosis (Figure 3). Different nanomaterials have been used as photo-absorbing agents for GBM, including Cu2(OH)PO4 NPs [105], porous silicon NPs [83], and iron oxide NPs [82]. Chen et al. demonstrated that under NIR irradiation, Cu2(OH)PO4 nanoparticles increased the temperature of the tumor region, which consequently diminished the tumors even completely [105]. As the next step, Zeng et al. [83] combined PTT with TMZ using porous silicon NPs. In fact, it has been reported that heating the tumors at 40-42 °C increases the blood flow and the partial pressure of oxygen, enhancing the delivery of chemotherapies to tumor cells [106]. Hence, this study showed that while the inhibition rates of plain NPs, TMZ, irradiated NPs, and encapsulated TMZ were 16.5%, 37.2%, 24.2%, and 57.2%, respectively, the combination of encapsulated TMZ plus PTT increased the rate up to 71.5% [83]. In addition to porous silicon NPs, iron oxide NPs have also been studied for TMZ-PTT treatments [82]. Kwon et al. loaded in Fe3O4 NPs TMZ together with indocyanine green, a photothermal agent and photodynamic photosensitizer that under NIR laser irradiation can convert absorbed NIR light to thermal energy and reactive oxygen species (ROS). Indeed, Fe3O4 magnetic NPs are known to have good photothermal conversion efficacy in the NIR region and low toxicity. Hence, this study has merged chemo-photothermal-photodynamic therapy and demonstrated synergic U87 cytotoxicity, inducing ROS generation and cell death [82]. In a more recent study, Cao et al. combined hollow mesoporous copper sulfide nanoparticles with TMZ for PDT and mild PTT [84]. Under a tumor acidic microenvironment and NIR irradiation, copper NPs dissociated Cu2+ to consume glutathione by redox reaction, generating Cu+ that converts H2O2 into highly toxic OH for PDT. Moreover, the mild photothermal effect produced (rise of temperature to 40-44 °C), increased blood flow and, hence, TMZ delivery, showing a decrease of the relative tumor volume (V/V0) from almost 5 to almost 0 [84].
Magnetic hyperthermia is another therapeutic strategy that has been combined with TMZ in GBM. Actually, superparamagnetic nanoparticles heat up (preferably to 42-45 °C) under an external alternating magnetic field, which causes changes in the functions of the cell membrane, proteins, and the synthesis of nucleic acids, triggering apoptosis (Figure 3) [107]. Indeed, Minaei et al. designed polymeric nanoparticles containing both superparamagnetic nanoparticles and TMZ. They showed in vitro in C6 cancer cells that localized heating-triggered TMZ release from nanoparticles resulted in a synchronized effect of both chemotherapy and hyperthermia [85]. In addition to polymer-based nanosystems, Yao et al. encapsulated TMZ and ferroferric oxide (Fe3O4) in temperature-sensitive liposomes to enhance the drug release and combine both TMZ and magnetic hyperthermia effects in vitro [87]. Marino et al. embedded magnetic NPs and TMZ in SLNPs and showed in U87 spheroids that both treatments, hyperthermia and TMZ, were able to decrease their size from ~ 550 µm in control spheroids to ~ 150 µm [86], demonstrating the possible use of lipid-based nanosystems for magnetic NPs loading systems.
TMZ as immunogenic cell-death (ICD) inducer and immunotherapy
Immunotherapy is based on re-educating and harnessing the patient's immune response against tumors. This method includes immune checkpoint blockade (ICB) agents, therapeutic vaccines, adoptive cell therapy, monoclonal antibodies (mAbs), and oncolytic viruses [108]. Although ICB agents have demonstrated success in patients with melanomas and other tumors [109,110], most patients with solid tumors do not respond to immunotherapy. Indeed, less than 15% of cancer patients do respond to ICB agents [111]. In the case of glioblastoma patients, results from phase III clinical trials with ICB agents and vaccine therapies have not demonstrated a major benefit in terms of patient survival or immune modulation [112]. However, two recent studies have raised hope for the immunotherapy in GBM: in fact, the administration of PD-1 mAbs prior to tumor resection increased local and systemic antitumor immune responses [113]. Moreover, a phase II clinical trial has shown that patients' survival increased after the administration of a combination of different immunotherapies [114]. Thus, it seems that stimulating the immune system at different times and locations or combining it with other treatments could be possible and effective. It is thought that one possibility to increase the immune response against GBM is by stimulating antigen release from dying tumor cells and their presentation to dendritic cells by immunogenic cell-death (ICD) inducers, such as radiotherapy or TMZ (Figure 3) [115]. It has been demonstrated that modifying the dose (metronomic doses) [116] or the administration route (local delivery) [117] of TMZ together with ICB agents has increased the efficacy of anti-PD-1 antitumor efficacy and vaccine therapies [118].
It is true that the administration of TMZ in all these examples was done freely, and, as it has been shown that the route of administration can play a key role in improving the efficacy of immunotherapies, the use of drug delivery systems could improve the penetration of TMZ and, hence, its role as an ICD inducer. In fact, there are examples with other chemotherapies such as DOX [119] and MIT [120] encapsulated in polymersomes and hydrogels, respectively, that have been used as ICD inducers together with radiotherapy and a siRNA against an immunosuppressive mediator, respectively, showing promising results in vivo in glioblastoma.
TMZ with other molecules
In addition to all the approaches featuring co-delivery of TMZ with different therapeutic choices, other less-explored molecules are also being studied, including curcumin, dyes, and DNAzyme.
Different nanomaterials, such as magnetic nanoparticles [62] and NSCs [88], have been used for the co-delivery of TMZ with curcumin, a natural compound that has demonstrated anticancer activity inducing differentiation, apoptosis, and inhibition of cell growth [121]. The nanosystem proposed by Xu et al. [88] exhibited enhanced inhibitory effects on glioma cells due to a combination of S-phase cell-cycle arrest and induced apoptosis. They also tested the formulation in a C6-bearing mouse model and concluded that, while drugs accumulated better in the brain and in tumor sites when they were encapsulated, the co-delivery performed a significant synergic effect between TMZ and curcumin, decreasing the tumor weight from ~ 0.8 and 0.75 g in animals with just curcumin or TMZ administration, respectively, to < 0.5 g with both treatments [88].
A recent study carried out in 2020 by Nie et al. confirmed that silica nanoparticles could be used for co-delivery of TMZ with a DNAzyme (for the gene silencing of MGMT). They were able to cleavage MGMT mRNA, knock down the MGMT protein, and improve the therapeutic effect of TMZ in vitro in T98G glioma cells [59].
Dual encapsulation of TMZ with other therapeutic agents seems to be a good strategy to increase the sensitivity of cells to TMZ and improve the antitumoral effect of both molecules. In addition to the co-delivery of two therapeutic agents, novel approaches have been developed to extend the use of nanosystems. Indeed, Schmitt et al., co-encapsulated TMZ with a dye (Cy5) as a potential diagnostic probe in chitosan nanoparticles. Moreover, they showed that the co-encapsulation of the Cy5 dye could provide a marker for real-time monitoring of the delivery and accumulation of the nanoparticles while showing TMZ efficacy [34].
Improving GBM limitations
As mentioned in the previous section, the encapsulation of TMZ not only improves its stability and protection but also enables the possibility of dual encapsulation with other therapeutic strategies, achieving a synergic effect. In addition to these advantages, drug encapsulation can improve the delivery of the treatment into tumoral tissue and cells via passive targeting, active targeting, or local delivery.
Passive targeting
Tumor vessels differ from normal vessels in several ways. Most tumor vessels have an irregular diameter and an abnormal branching pattern, making it difficult to classify them as arterioles, capillaries, or veins [122,123]. Moreover, they do not have a smooth muscle coating, and large vessels usually have thin walls. Besides, the same vessels have an incomplete basement membrane, an abnormal pericyte coat and are unusually leaky [124,125]. This leakiness can lead to extravasation of proteins and might facilitate the circulation into the blood stream of tumor cells—thus forming metastases—as well as other molecules such as radioisotopes [124,126,127]. Indeed, it is thought that this leakiness is due to a defective endothelial monolayer, which has spaces or pores between poorly connected, branched lining cells [123].
Illustration of local delivery for GBM including implantable wafers, convection-enhanced delivery and hydrogels, where implantable or in situ-formed hydrogels can be distinguished.
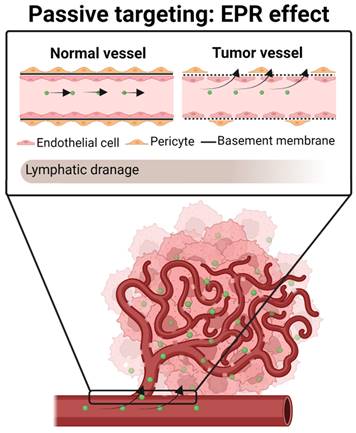
It has been shown that the pore size between new tumor blood vessels can range from 10 up to 2000 nm [123,128]. In contrast, blood vessels in healthy tissues have a pore size of approximately 10 nm. Consequently, when a drug is systemically administrated, while free chemotherapeutic molecules (< 1 nm) reach not only tumors but also normal cells, nanocarriers (~ 100-200 nm) may preferentially pass through the pores of tumor vessels [129].
Matsumura and Maeda were the first to demonstrate that nanoparticles can extravasate through leaky blood vessels to reach the tumor space and be retained there, due to the poor lymphatic drainage of tumors [130]. This phenomenon is known as the enhanced permeability and retention effect (EPR effect) (Figure 4). It is based on several pathophysiological characteristics of solid tumors, such as i) the massive irregular neovascularization of tumors, ii) the elevated expression of inflammatory factors that sustain the EPR effect, including prostaglandins, VEGF, interleukin 2 and 6, and iii) inefficient lymphatic drainage, which retains the extravasated nanosystems [130-132].
In the case of TMZ-loaded nanosystems, studies have demonstrated that its encapsulation in lipid-based nanosystems, including liposomes, PEGylated liposomes, SLNPs, and NSCs [46,53,55,88], have enhanced the brain accumulation of the TMZ in vivo, increasing the therapeutic effect of TMZ and reducing its toxicity, compared with the administration of the free drug.
Active targeting
GBM neovasculature is highly permeable and the EPR effect has been demonstrated in vivo. However, disruption of the BBB remains a local event that is more evident in the tumor core but absent in the growing margins. In conclusion, it has been reported that with the ERP effect there is an accumulation of therapeutic agents in necrotic tumor areas with an almost undetectable concentration of drug in the peritumoral regions [133,134]. Therefore, other strategies capable of optimizing the BBB crossing and the specific interaction between TMZ and targeted cells, including endothelial cells, glioma cancer cells, and glioma stem cells, are needed.
Most studied targeting strategies to improve TMZ delivery to the cells of interest.
| Ligand | Target | Nanosystem | Moiety | Advantages | Ref |
|---|---|---|---|---|---|
| Tf | BBB Glioma cells | Liposome PEGylated liposomes SLNPs Polymeric NPs | Antibody Aptamers Peptides | In vitro, in vivo studies Dual targeting Co-delivery Improve TMZ resistance | [32,76,135-140] |
| Integrin | BBB Glioma cells | NSC | Penetrating peptide | In vitro, in vivo studies Dual targeting | [141] [138] |
| LDL | BBB | Liposomes Polymeric micelles | Peptides Protein | In vitro, in vivo studies Co-delivery | [78,142,143] |
| Transporters | BBB | Liposomes | Glucose Peptides | In vitro, in vivo studies Co-delivery | [144,145] |
| Folate | Glioma cells | Polymeric NPs Polymeric micelles | Folic acid | In vitro, in vivo studies | [79,146] |
| EGFR | Glioma cells | Polymeric NP | Antibody Small molecule | In vitro studies | [33] |
| CD133 | GSCs | Polymeric NPs Liposomes | Aptamer Antibody | In vitro, in vivo studies Dual targeting Co-delivery | [74,75,142,147] |
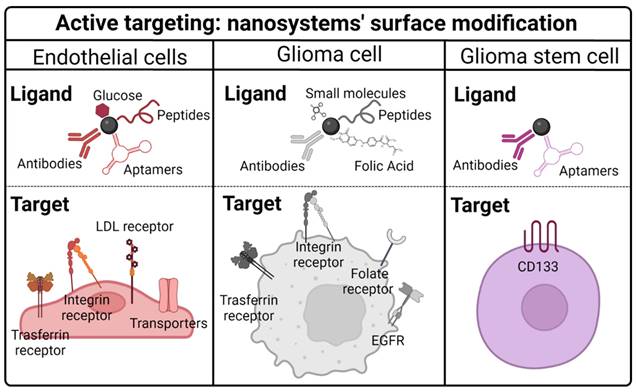
Modification of nanosystem surfaces with ligands could be a possible strategy for this active targeting. Indeed, nanomaterials have a high surface/volume ratio and a highly reactive surface; thus, they have been functionalized with different ligands to target TMZ to different cell types [20] (Table 3, Figure 5).
Targeting BBB endothelial cells
It has been described that transferrin (Tf), integrin, and low-density lipoprotein (LDL) receptors are overexpressed in BBB endothelial cells. To our knowledge, the first approach using transferrin receptor as a target molecule was reported in 2015 [135], where researchers designed a TMZ-loading liposome functionalized with an anti-Tf receptor antibody. First, they demonstrated in vitro in U87 and U251 and in two TMZ-resistant cell lines (U87R and T98G) that encapsulation of TMZ improved the antitumor activity of TMZ in both TMZ-sensitive and TMZ-resistant cell lines [135]. In addition, they analyzed the in vivo efficacy in intracranial U87 and subcutaneous T98G tumors. Systemic administration of the liposomal formulation triggered a substantial inhibition of tumor growth, as well as an increase in cell apoptosis and animal survival compared with animals with free TMZ administration. Moreover, they demonstrated a decrease in the toxic effects of encapsulated TMZ [135]. A design composed of PEGylated liposomes with a Tf receptor-targeting moiety was also studied to co-deliver TMZ and a brodomain inhibitor [76]. Mice bearing U87 and GL261 intracranial orthotopic tumors presented more prolonged survival, smaller tumor size, and lessened toxic effect when they were administrated with both encapsulated treatments [76]. In addition to liposomes, SLNPs have been modified to target a Tf receptor. A study carried out by Jain et al. showed that Tf-TMZ-SLNPs were accumulated more in the brain of rats compared to unconjugated SLNPs and control animals (free TMZ) [136]. Besides, they revealed a 70% lower hemolytic toxicity in Tf-TMZ-SLNPs animals, indicating good biocompatibility of the system. Furthermore, Tf-TMZ-SLNPs had a better tumor-inhibitory effect than free TMZ or plain SLNPs. Similar results in terms of active targeting were also achieved when Fu et al. targeted the Tf receptor by using an aptamer [137], a short single strand of DNA or RNA that has a specific 3D structure capable of binding with high affinity to proteins [148]. They showed that ten minutes after the nanosystem was injected into the tail vein, part of it was already distributed in the brain area and maintained for at least 1h.
As these reports have shown, targeting the Tf receptor has been demonstrated to be a good strategy to pass TMZ or other treatments through endothelial BBB cells and improve the accumulation in brain tissues [136], healthy animals' brains [76], and in U87 orthotopic model tumors as well as in TMZ-resistant mouse tumors [135].
Apart from Tf receptors, integrin receptors are also overexpressed in endothelial cells. Song et al. modified the surface of an NSC with an arginine-glycine-aspartic acid peptide (RGD): a penetrating peptide that facilitates intracellular delivery through integrin receptors [141]. They showed that the IC50 of cells treated with RGD-functionalized TMZ-NSCs was 10 times lower than cells treated with free TMZ. In addition, the in vivo antitumor therapeutic effect was also higher with the nanosystem: mice treated with RGD-TMZ-NSCs had an inhibition rate of 83% compared to control animals, while the rate in animals with nonfunctionalized NSCs was 66% and the one with free TMZ was 21%.
Additionally, a dual-targeting strategy has been reported as a further improvement to TMZ accumulation. A recent study used RGD peptide and lactoferrin to target both integrin and transferrin receptors. This NSC was used to co-deliver TMZ and vincristine [138]. They demonstrated better targeting efficacy in the dual-ligand nanosystem than in NSCs targeted with only one ligand. Moreover, tumor inhibition was higher in dual-targeting and both chemotherapies (~ 80%) than in animals treated with only one chemotherapy (around 40%) or one ligand (around 60%) [138].
Targeting Low density lipoprotein receptor (LDL) has also been demonstrated to be a good strategy for delivering TMZ through BBB [78,142]. Seok et al. used liposomes functionalized with the angiopep-2 peptide, an LDL receptor-targeting ligand [142]. They showed that mice bearing U87-stem cell tumors had a decreased tumor size; their life span was increased (from 0 in untreated animals up to 211.2%), as was the median survival time (from 23.3 days up to 49.2 days). Recently, Ismail et al. used liposomes functionalized with another LDL receptor-targeting ligand named Apolipoprotein E [143]. In this study, they tested liposomes loaded with TMZ and artemisinin, a drug that induces dysregulation of the Wnt/β-catenin pathway, interfering with MGMT and inducing ROS. Hence, they demonstrated in TMZ-resistant U251-bearing mice that the targeting ligand was effective, increasing drug accumulation in the brain. Moreover, they observed that both treatments were efficient in TMZ-resistant tumors, decreasing the tumor size with no observable aggressive tumor growth as happened in control animals. In addition to liposomes, the surface of polymeric micelles has also been functionalized with angiopep-2, demonstrating a higher accumulation of TMZ in the brain and an improved efficacy of the drug, decreasing the tumor size of mice bearing an orthotopic U87 model and improving their survival [78].
As targeting receptors overexpressed in endothelial cells has been demonstrated to be a good strategy for enhancing TMZ brain accumulation, researchers are studying more alternatives. Zhang et al. used glucose attached to liposomes to deliver TMZ and a pro-apoptotic peptide [144]. Studies in a highly aggressive intracranial tumor mouse model showed that the system could easily penetrate the BBB (using the glucose-GLU1 pathway), release the two drugs, exhibit a better antitumor effect, and improve the total survival of the animals. Other BBB endothelial-specific transporters have been targeted by short peptides [145]. Gabay et al. developed liposomes with these moieties loaded with TMZ, curcumin, and doxorubicin. They demonstrated in vivo that functionalized liposomes could cross the BBB and penetrate the brain 35% more than nontargeted liposomes. Furthermore, they showed that tumor growth was delayed and mice survival was increased in animals treated with targeted liposomes plus the three drugs, compared to animals treated with nontargeted liposomes or free drugs [145].
To further know which ligand could be used, Arcella et al. incubated TMZ-loaded liposomes in human plasma and administrated them to human umbilical vein endothelial cells. They studied which proteins attached to liposomes were more concentrated in those cells, observing that liposomes with a higher concentration of molecules, such as apolipoproteins, vitronectin, or vitamin K-dependent proteins, on their surface were accumulating better. Indeed, when the liposomes were added to U87 cell cultures, they showed an enhanced TMZ anti-tumoral effect [149]. This preliminary in vitro study may serve as the basis for the development of new therapeutic targets.
Targeting glioma cells
Tumors are composed of several cell populations, including malignant cancer cells, supportive cells, tumor-infiltrating cells, and cancer stem cells. As mentioned in the previous section, the functionalization of nanosystem surfaces with ligands that target overexpressed receptors of BBB endothelial cells enhances the accumulation of TMZ in the brain tissue. Hence, nanosystem surfaces can be modified to improve TMZ targeting to other types of cells, such as malignant cancer cells or glioma stem cells.
Studies have demonstrated that the active targeting of cancer cells by nanosystem surface modification enhances the efficacy of TMZ. It has been described that there are some receptors overexpressed in cancer cells, including transferrin, integrin, folate, EGFR, CD13, monocarboxylate transporter-1 and biotin (Figure 5).
The expression of the transferrin receptor is increased not only in endothelial cells but also in proliferating cells and cells that have undergone malignant transformation [150,151]. Indeed, researchers have modified some nanosystems to target Tf receptors. In vitro studies carried out by Ramalho et al. showed that polymeric nanoparticles functionalized with an antibody against the transferrin receptor enhanced TMZ accumulation and efficacy in U251 and U87 cell lines [32]. Patil et al. also used anti-transferrin receptor antibodies to target TMZ in cell lines, demonstrating that encapsulated TMZ had a higher efficacy in cell lines resistant to TMZ [139,140]. Moreover, Kim et al. designed a liposome functionalized with an antibody anti-Tf receptor and demonstrated a higher uptake of the nanosystem not only in vitro in U87, U251, and two TMZ-resistant cell lines, but also confirmed a better TMZ efficacy in mice bearing orthotopic U87 tumors and TMZ-resistant tumors [135].
Schematic illustration of the most ligands and targets used in TMZ encapsulation systems.
Another receptor overexpressed not only in endothelial cells but also in glioma cells is the integrin receptor. Studies have demonstrated that mice bearing U87 subcutaneous tumors and treated with NSCs functionalized with RGD peptides efficiently delivered TMZ not only through the BBB but also into glioma cells [138,141].
Folic acid, a molecule responsible for the initiation and propagation of cancer due to its role in cell division and proliferation, has also been attached to nanosystems to deliver TMZ to cancer cells [152]. An in vitro study showed that polymeric nanoparticles functionalized with folic acid enabled the internalization of TMZ in glioma cells compared to cells that did not have folate receptors overexpressed [146]. Moreover, TMZ efficacy was enhanced showing a higher cytotoxic effect in cells overexpressing the receptor [146]. Peng et al. functionalized polymeric micelles with folic acid to co-deliver TMZ and an anti-Bcl-2 siRNA [79]. They first demonstrated in C6 cells that the functionalization with folic acid improved the delivery of TMZ and the siRNA. Moreover, in vivo experiments with rats bearing intracranial C6 cells showed that functionalized micelles decreased by 1.6 times the tumor volume compared to nonfunctionalized micelles [79].
It has also been described that the EGF receptor is overexpressed in glioma cells and approximately 50% of GBM patients present EGFR amplification. Banstola et al. used an anti-EGFR antibody to target polymeric nanoparticles containing TMZ [33]. They showed an increase in the cellular uptake of functionalized nanoparticles, together with a higher TMZ efficacy [33]. Moreover, Schmitt et al. functionalized chitosan-PLGA polymeric nanoparticles with a small molecule that binds to EGFR, demonstrating that functionalization enhances the TMZ effect in U87 cells [34].
Other less explored targets that have been used to deliver TMZ to GBM cells are the CD13 [60,153], the monocarboxylate transporter-1 [154] (a transporter that regulates the activity of signaling pathways and controls the exchange of monocarboxylate in aerobic glycolysis [155]), and biotin [81] (a molecule whose levels and receptors are highly expressed in tumors [156]).
Targeting glioma stem cells
Lapidot et al. discovered the first experimental evidence that proved the activity of cancer stem cells in hematological malignancies. They showed that the CD34+/CD38- cell subpopulation from acute myeloid leukemia could form leukemia after transplantation into NOD.SCID mice [157]. After this study, different researchers have demonstrated the presence of cancer stem cells in other types of cancers, such as breast [158], prostate [159], colorectal [160,161], pancreatic [162] and brain cancers [163-166].
Glioma stem cells, also known as glioma initiating cells, are a small subpopulation of slow-growing cells that exhibit stem cell properties. They have self-renewal and differentiation abilities, which leads to tumor formation and progression [167-169]. They present neural stem cell molecular signatures such as Wnt/β-catenin, bone morphogenetic protein, and Sonic Hedgehog, Notch, STAT3, or EGFR pathways [170]. Moreover, GSCs are quiescent and have infiltrative capacity, can modulate the microenvironment for their maintenance [171], and are responsible for therapy resistance [172,173]. Therefore, the crucial role of GSCs in GBM and their characteristics make them a promising therapeutic target.
It is already known that CD133 is an important cell surface marker for cancer stem cells and also for GSCs [164]. Moreover, Singh et al. demonstrated that only a small number of CD133+ patient-derived cells were able to initiate tumors in NOD-SCID mouse brains. Indeed, they showed that this CD133+ xenograft resembled the original patient tumor. They further observed that these CD133+ cells had the capability of self-renewal by isolating those cells from primary tumors and reinjecting them into a secondary mouse. After five weeks they observed that these tumors obtained the phenotype of both the original patient tumor and the primary mouse tumor [165].
Taking this information into account, researchers have recently targeted TMZ specifically to the GSC subpopulation using CD133 markers to improve drug outcomes and drug resistance (Table 3). Studies carried out by Smiley [75,147] encapsulated TMZ and idasanutlin in polymeric nanoparticles and conjugated them with an aptamer against CD133. Although they did not observe statistical differences, they noticed a trend of higher killing capacity of CD133-labeled nanoparticles compared to that of nonlabeled nanoparticles. Moreover, the co-delivery of both TMZ and idasanutlin effectively increased the cytotoxicity percentage from ~ 10% with TMZ-loaded nanoparticles up to 80% with TMZ and idasanutlin targeted nanoparticles. A more recent study carried out by Behrooz et al. [74] demonstrated a higher killing capacity of CD133 targeted polymeric nanoparticles together with higher nanoparticle accumulation in U87 stem cells. In this case, they also conjugated an aptamer against CD133 to polymeric nanoparticles, and they co-loaded TMZ with paclitaxel [74]. Seok et al. went one step further and demonstrated in vivo using U87 GSC-bearing mice that their nanoformulation, composed of liposomes functionalized with an anti-CD133 monoclonal antibody and the angiopep-2 peptide, was better accumulated in the brain, demonstrating an optimal targeting ability and an improved efficacy of TMZ [142]. Indeed, the median survival rate of mice was doubled in animals treated with dual-targeted liposomes compared to animals treated with free TMZ. Besides, the anti-tumor efficacy was studied visually by IVIS and the bioluminescence intensity (photon flux) of tumors treated with the nanosystem was 1.000-10.000 times lower than that of free TMZ or nontargeted liposomes [142].
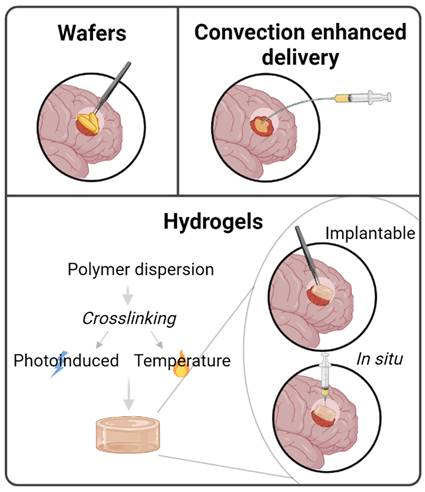
Local delivery
The use of implants in the case of GBM is minimally invasive because surgery is part of the patients' treatment (65-75% of patients undergo surgical resection [174]). Gliadel wafers (loading carmustine) already approved by the FDA, are placed in resection cavities after surgery for 2-3 weeks to prevent recurrence [175]. However, its use has been restricted due to its side effects and low drug diffusion. In fact, the stiffness of implants is a key feature: materials stiffer than the brain tissue can enhance gliosis and inflammation, but can also modulate GBM proliferation and invasion. On the contrary, materials softer than the brain tissue have worse stability and fixation at the implant site and are less effective [176]. Thus, strategies are being developed for the local delivery of TMZ including hydrogels or convection-enhanced delivery (Figure 6). Hydrogels are composed of a three-dimensional hydrophilic polymeric network formed by the crosslinking of polymers in an aqueous medium. Due to their high-water content they are soft and flexible and, hence, have recently attracted much attention. Crosslinking of polymer chains can be achieved by chemical or physical reactions, generating a chemical or physical gel. Hydrogel formation can occur before the local injection (implantable hydrogels) or in situ [177], offering the possibility of directly injecting it into either the surgical cavity or the tumor [174]. The most studied hydrogels for TMZ encapsulation are those formed in situ. For TMZ, the in situ crosslinking that has been studied is the photoinduced polymerization, forming irreversible covalent bonds, or via self-assembly in response to temperature [178]. Several studies have demonstrated the tolerability and efficacy of photoinduced hydrogels containing TMZ [179,180]. Fourniols et al. [179] designed a hydrogel composed of PEG dimethacrylate (PEG-DMA) that photopolymerized under 400 nm after its injection in the brain cavity. They analyzed the tolerability of the hydrogel by creating a cavity in the cortex of healthy NMRI mice. They evaluated microglial activation and apoptosis in the surrounding brain tissue once the hydrogel was implanted and observed that this activation was induced by brain tissue resection itself, not due to hydrogel implantation. Moreover, microglial activation was not detected inside the hydrogel. Another study carried out by Zhao et al. first encapsulated paclitaxel in PGLA nanoparticles and co-loaded them with TMZ in a PEG-DMA photoinduced hydrogel [180]. The injectable pre-hydrogel mixture was photopolymerized under 400 nm light into the resection cavity before closing the cranial window, and they confirmed that U87-bearing mice showed i) no infiltrative cells inside the co-loaded hydrogel and ii) no tumor recurrence after hydrogel implantation (before 110 days post-tumor inoculation). This study also demonstrated that co-delivery of TMZ with another drug enhances the synergic effect of both drugs. Indeed, although the overall survival of mice with just one drug increased 2 and 2.5 times with TMZ and PTX, respectively, compared to untreated mice, animals treated with both drugs were still alive at the end of the experiment (110 days after tumor implantation).
In addition to photoinduced hydrogels, TMZ has been encapsulated in hydrogels that crosslink in response to temperature changes [181-183]. Adhikari et al. [181] used an amphiphilic diblock copolypeptide hydrogel of 180-poly-lysine and 20-poly-leucine that solidifies at room temperature. They tested its efficacy in an orthotopic glioma xenograft mouse model and demonstrated that the hydrogel drastically reduced the tumor size compared to tumors treated with systemically administrated TMZ. In addition, the overall survival rate was increased almost twofold in animals treated with hydrogels compared to those treated with TMZ alone [181].
In addition to in situ-formed hydrogels, injectable hydrogels containing TMZ are also being studied. For instance, Zhao et al. [182] have used an injectable hydrogel loaded with TMZ in combination with an MGMT inhibitor that was formed after the mixture reached room temperature. In their study, they generated an orthotopic TMZ-resistant glioma model and showed that the hydrogel i) inhibited MGMT expression and sensitized TMZ-resistant cells to the drug, and ii) reduced tumor growth in vivo after surgical resection, proving the efficacy of the hydrogel after partial removal of the tumor. Thermoresponsive hydrogels can also be loaded with nano/microstructures. In fact, Ding et al. [183] encapsulated TMZ in PEG-dipalmitoylphosphatidylethanoiamine calcium phosphate nanoparticles and placed them together with paclitaxel-loaded nanoparticles in a thermoresponsive hydrogel. This system promoted a synergic inhibition effect of the TMZ and PXT nanoparticles-hydrogel increasing twofold the overall survival rate of U87-bearing rats compared to control rats treated with surgery alone. Moreover, they showed that this overall survival increase was accompanied by a higher number of apoptotic cells [183]. Furthermore, Akbar et al. [184] designed a hydrogel composed of TMZ, PLGA, and plasticizers that make the material softer. In this case, the hydrogel was implanted 15 days after the tumor removal. Notably, the hydrogel reduced the tumor weight by 98% and 94% in mice and rats after 35 days, respectively. Puente et al. developed an injectable chitosan hydrogel crosslinked with glutaraldehyde [185]. Apart from TMZ, this hydrogel contained a radioactive isotope agent (iodine) encapsulated in alginate microparticles for local chemo-radiotherapy treatment of GBM [185]. This approach demonstrated for the first time in a GBM subcutaneous mouse model that the tumor size reduction in animals with the hydrogel compared to control animals was due to the localized TMZ and retention of the radioisotope, suggesting that chemo-radio-hydrogel implants might enhance the local control and overall result of GBM. Although in this study the authors did not study the possible effect of the remaining glutaraldehyde, several studies have reported that plain chitosan hydrogels crosslinked with glutaraldehyde and their degradation do not have any toxic effect [186,187], suggesting that the antitumor effect was caused by the cargo.
In addition to the use of hydrogels, convection-enhanced delivery (CED) has also been studied for local delivery of TMZ. The first such study was conducted by Nordling-David et al., who used liposomal TMZ [188]. Although in this study there were no statistically significant differences between animals treated with free TMZ and TMZ encapsulated in liposomes, animals treated with the liposomal formulation had a 22% longer survival time. Moreover, they showed a significant decrease in the volume of edemas [188]. Also, in a study that used PEGylated liposomes administrated via CED showed that the formulation inhibited tumor growth with no systemic toxicity, suggesting that convection-enhanced delivery could be a good method for the intratumoral administration of TMZ [189].
Although local administration has been less explored in GBM, it offers some advantages, including avoidance of crossing the BBB, access to a drug reservoir near the tumor, improvement in the drug efficiency, and reduction of systemic toxicity.
Future perspectives
As pointed out in this review, the use of nanosystems for TMZ encapsulation has improved both TMZ limitations and the limitations associated with GBM in in vivo models. However, the clinical outcomes of these studies are limited. In this section, we analyze different approaches that can be improved to reduce the time from bench to bedside.
Improve in vitro and in vivo models
Most studies presented in this review have been performed in vitro in commercial cell lines such as U87, U252, and T98G. These cell lines present enrichment of cancer stem cells when they grow as spheres. However, these classical 2D cellular models are limited due to intrinsic limitations, including inappropriate cell density, gradients of medium components, non-physiological oxygen levels, disruption of the GBM original spatial context, lack of interactions with the extracellular matrix, and lack of other nontumor cells in the GBM microenvironment. Moreover, successive cell passages can select cells with the greatest proliferative potential, decreasing the genetic heterogeneity, and resulting in genetic drift, accumulation of chromosomal aberrations, and phenotypic alterations in cell lines. To improve some of these drawbacks, cell lines derived from surgical samples of GBM patients that preserve GSC features have been developed. Indeed, it is known that GSCs are vital to maintain tumor heterogeneity, as well as tumor initiation, maintenance, and invasion in vivo due to their self-renewal and differentiation capacity. Patient-derived GSCs are maintained in vitro under floating conditions, as neurospheres, with a serum-free medium supplemented with bFGF and EGF. Nevertheless, neurospheres can be composed of non-homogenous cell populations, the environment can limit stem cell division and induce cell death, and when drugs or treatments are administrated, the distribution can be uneven in the sphere. As GSCs do not need to grow in suspension, GSC laminin-adherent cultures are being used to improve the degree of homogeneity, enhance imaging analysis, and enable clonal propagation. Regardless of the culture conditions (neurosphere or adherent), the GSC model has a major disadvantage: the addition of EGF and bFGF to the culture causes the cells to lose EGFRvIII and EGFR amplification, which is present in approximately 50% of GBM cases. When researchers have tried to grow GSCs without these growth factors, it seems that their survival and maintenance have been affected.
Regarding the in vivo models used in the studies mentioned in this work, they are mainly xenograft models in immunodeficient animals with cell lines (U87, U252, and T98G) injected subcutaneously in the flank (heterotopic implantation) or directly in the brain (orthotopic implantation). To improve the outcome of the model and maintain the histopathologic, genomic, and phenotypic characteristic of the primary tumors, patient-derived xenografts might be implemented. Moreover, GSCs could be used to initiate tumors to preserve the tumor heterogeneity better. These models have advantages, including the possibility to test the efficacy of the nanosystems in single patients, the maintenance of the original tumor characteristics, and their genetic stability. However, all these models are done in immunodeficient mice and, thus, they do not have inflammatory responsive cells or an intact immune system. Consequently, the microenvironment and the stroma of the tumor are not the same, limiting the study of the biology and the tumor resistance.
Illustration of local delivery for GBM including implantable wafers, convection-enhanced delivery and hydrogels, where implantable or in situ-formed hydrogels can be distinguished.
New approaches are being used to study GBM including 3D co-cultures, microfluidics, organoids, and 3D bioprinting [190], which could overcome the disadvantages of above cited models. Hydrogels coated with 3D scaffolds are being used to culture GSCs or pieces of patient-derived cells to create a more realistic tumor environment. These 3D co-cultures that include more than one type of cell, reproduce the cell growth environment of GBM, as well as the soluble signaling and extracellular matrix signaling. Although this model can be useful as a first approach, the substrate stiffness, the selection of the cells across the cell passages, and the constant microenvironment limit this model. Thus, microfluidic devices that can create a changing environment are being developed. However, this technology is still expensive and time-consuming, and although it is an adequate model to study specific targeted drug tests, it takes time to search for appropriate materials to deliver microfluidics depending on the type of study. Although GSCs are the best available in vitro model, there are no physiological and reciprocal interactions between GSC and other cells such as nontumor cells and vascular cells. To improve upon these drawbacks, brain organoids are being developed. Brain organoids are 3D tissues generated from pluripotent stem cells, such as induced pluripotent stem cells and embryonic stem cells, or adult-tissue-resident cells, which, in a controlled environment, grow and differentiate slowly. This approach resembles the cell heterogeneity of the tumor microenvironment in vivo and it is suitable to study the niche microenvironment and cell invasion. Moreover, cell populations can be genetically modified and, hence, it is possible to study the consequences of genome mutation, evaluate the susceptibility of patients to combinations of driver mutations, and test new therapies and treatments. Although this methodology is limited by the lack of vasculature, immune cells, and BBB functions, it could be very useful to study local delivery systems. In fact, it has been proven in metastatic colorectal cancer that patient-derived tumor organoids predict the response of the biopsied lesion in more than 80% of patients treated with irinotecan-based therapies without misclassifying patients who would have benefited from treatment [191]. Thus, brain organoids are a promising new technology for ex vivo study of the molecular and cellular mechanisms of the disease, as well as for treatment.
Synthesis and storage conditions
As shown in section "Improving the solubility and stability of TMZ", the design of nanoformulations is becoming more and more complex, making sample reproducibility and development particularly difficult, which translates into the high cost of nanosystems. To improve reproducibility and decrease the time between preclinical and clinical studies, nanosystems should be designed considering manufacturing constraints in the industry [192]. The design of all nanoformulations must ensure their sterility; thus, finding an appropriate sterilization method that does not compromise their physicochemical properties and stability is critical. Another important aspect to consider is the storage condition of the formula. Understanding the effects of storage conditions on the stability and biocompatibility of nanosystems is vital for their clinical application. Storage in water, PBS, or biological fluids may affect the size, surface charge, drug-release profile, and degradation of the nanoformulation. As Abdelwahed proposed, freeze-drying could be evaluated as a universal storage approach [193].
Study the interaction of the nanosystem with the plasma: Biocorona
Most studies that have designed nanosystems for the encapsulation and delivery of TMZ are administrated systemically. When a nanosystem is administrated through this route, it is covered with plasma proteins and lipids, forming a biocorona. It is already known that the distribution and subsequent pharmacological and toxicological effects depend on this biocorona [194,195]. Thus, studying the biocorona in vitro and in vivo may help predict the biocorona in clinics, thus making it possible to predict the biodistribution and efficacy of the nanosystem in patients. In this sense, Mahmoudi has already determined some strategies that may improve the translation of nanomedicine from preclinical to clinical studies [195]. Incubation of a nanosystem in the same protein source (e.g. human plasma) has significant effects on the composition of the biocorona, depending on the age, gender, ethnicity, or health/pathological condition of the donors. Thus, it is important that studies analyzing biocorona consider all this information to predict the behavior of nanosystems in blood circulation.
Biodegradation of the nanosystems
So as not to limit the use of drug delivery systems, it is important to verify their biodegradation once they have performed their function. In the case of Gliadel wafers, for instance, although they are already approved by the FDA, their use has been restricted due to their side effects. Thus, it is vitally important to verify that the system is biocompatible in the long term and does not generate adverse effects on healthy tissues. Lipid-based nanosystems are probably the most biodegradable and biocompatible nanomaterials because they are usually composed of the same phospholipids as the cell membranes. In the case of polymer-based nanosystems, PLGA nanoparticles might be the most biocompatible. However, as other nanomaterials such as silica nanoparticles or metallic nanoparticles have been less explored, more in vitro and in vivo studies are needed to analyze their biocompatibility so as to further their move to clinics.
Conclusions
The incorporation of TMZ into the standard gold treatment of GBM in 2005 was a relevant step forward in extending the overall survival of patients. However, the poor solubility and hydrolyzation of TMZ in physiological media, together with the intrinsic characteristics of GBM have led to an impedance in the effectiveness of GBM therapy.
To overcome these limitations, several nanomaterials to encapsulate TMZ in nanosystems are being postulated, with polymeric and lipid-based nanocarriers being the most advanced ones. It is already demonstrated that TMZ encapsulation increases its solubility, blood circulation, half-life, and biodistribution. Moreover, due to the high surface/volume ratio of nanomaterials, they can be functionalized with ligands to specifically target endothelial cells and enhance BBB crossing and glioma cells. Besides, as it has been described that glioma stem cells could be responsible for the recurrence and resistance of GBM, TMZ has also been specifically targeted at this population of cells using anti-CD133 targeting moieties. Several studies have already demonstrated that this approach is effective for TMZ-resistant tumors in vivo.
To reduce the time spent moving from pre-clinics to clinics, some important limitations need to be overcome. It is crucial to improve in vitro and in vivo models, paying special attention to GSCs, brain organoids, and patient-derived xenografts. Moreover, a lack of information regarding the physico-chemical characterization of the designed nanosystems is still a factor. This causes problems particularly in sample reproducibility and the development of a robust, scalable, and affordable preparation process; thus, it is crucial to pay special attention to sterility and the storage conditions of the nanoformulations. Another important aspect to consider is the interaction of the nanocarrier with the biological context: first with the blood circulation if the chosen route is the systemic one, and then with the interaction of the nanosystem with the specific cell. Thus, more studies are needed to understand the specific nanosystem/cell interactions, paying special attention to the nanomaterial used (size, surface charge, and shape) and the in vitro or in vivo models.
In summary, some unknowns still remain to be overcome. However, nanosystems can improve the delivery and efficacy of TMZ, suggesting that this strategy is promising in the field of nanomedicine and oncology, particularly in GBM.
Acknowledgements
N.I-R is the recipient of a Sara Borrell postdoctoral contract from the Instituto Salud Carlos III (CD22/00076). This work was supported by grants from Instituto Salud Carlos III co-financed by the European Union (PI19/01355, DTS20/00179, PI22/01905) and the Health Department of the Basque Country (2022333034).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23(8):1231-51
2. Delgado-López PD, Corrales-García EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062-71
3. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C. et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:V1-100
4. Torrisi F, Alberghina C, Aprile SD, Pavone AM, Longhitano L, Giallongo S. et al. The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression. Biomedicines. 2022;10(806):1-22
5. Davis ME. Glioblastoma: Overview of disease and treatment. Clin J Oncol Nurs. 2016;20(5):1-8
6. Wilson TA, Karajannis MA, Harter DH. Glioblastoma multiforme: State of the art and future therapeutics. Surg Neurol Int. 2014;5:64
7. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392-401
8. Pichlmeier U, Bink A, Schackert G, Stummer W. Resection and survival in glioblastoma multiforme: An RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008;10(6):1025-34
9. Hoover JM, Chang SM, Parney IF. Clinical trials in brain tumor surgery. Neuroimaging Clin N Am. 2010;20(3):409-24
10. Stupp R, Mason WP, Bent MJ van den, Weller M, Fisher B, Taphoorn MJB. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma—A critical review. N Engl J Med. 2005;352(10):987-96
11. Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B. et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma a randomized clinical trial. JAMA - J Am Med Assoc. 2017;318(23):2306-16
12. Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88(1):97-103
13. Minniti G, Lanzetta G, Scaringi C, Caporello P, Salvati M, Arcella A. et al. Phase II study of short-course radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2012;83(1):93-9
14. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061-8.
15. Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD. et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98-110
16. Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD. et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157-73
17. Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L. et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell. 2017;32(1):42-56.e6
18. Nakagawachi T, Soejima H, Urano T, Zhao W, Higashimoto K, Satoh Y. et al. Silencing effect of CpG island hypermethylation and histone modifications on O6-methylguanine-DNA methyltransferase (MGMT) gene expression in human cancer. Oncogene. 2003;22:8835-44
19. Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol. 2013;125(5):621-36
20. Yeini E, Ofek P, Albeck N, Rodriguez Ajamil D, Neufeld L, Eldar-Boock A. et al. Targeting Glioblastoma: Advances in Drug Delivery and Novel Therapeutic Approaches. Adv Ther. 2021;4(1):1-33
21. Perrin SL, Samuel MS, Koszyca B, Brown MP, Ebert LM, Oksdath M. et al. Glioblastoma heterogeneity and the tumour microenvironment: Implications for preclinical research and development of new treatments. Biochem Soc Trans. 2019;47(2):625-38
22. Bozzato E, Bastiancich C, Préat V. Nanomedicine: A useful tool against glioma stem cells. Cancers (Basel). 2021;13(9):1-17
23. Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6(7):2585-97
24. Yasaswi PS, Shetty K, Yadav KS. Temozolomide nano enabled medicine: promises made by the nanocarriers in glioblastoma therapy. J Control Release. 2021;336:549-71
25. Fang C, Wang K, Stephen ZR, Mu Q, Kievit FM, Chiu DT. et al. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl Mater Interfaces. 2015;7(12):6674-82
26. Amarandi RM, Ibanescu A, Carasevici E, Marin L, Dragoi B. Liposomal-Based Formulations: A Path from Basic Research to Temozolomide Delivery Inside Glioblastoma Tissue. Pharmaceutics. 2022;14(308):1-40
27. Tang Z, He C, Tian H, Ding J, Hsiao BS, Chu B. et al. Polymeric nanostructured materials for biomedical applications. Prog Polym Sci. 2016;60:86-128
28. Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995;674:171-4
29. Tian XH, Lin XN, Wei F, Feng W, Huang ZC, Wang P. et al. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int J Nanomedicine. 2011;6:445-52
30. Jain DS, Athawale RB, Bajaj AN, Shrikhande SS, Goel PN, Nikam Y. et al. Unraveling the cytotoxic potential of Temozolomide loaded into PLGA nanoparticles. DARU J Pharm Sci. 2014;22(1):1-9
31. Ananta JS, Paulmurugan R, Massoud TF. Temozolomide-loaded PLGA nanoparticles to treat glioblastoma cells: A biophysical and cell culture evaluation. Neurol Res. 2016;38:51-9
32. Ramalho MJ, Sevin E, Gosselet F, Lima J, Coelho MAN, Loureiro JA. et al. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int J Pharm. 2018;545:84-92
33. Banstola A, Duwa R, Emami F, Jeong JH, Yook S. Enhanced caspase-mediated abrogation of autophagy by temozolomide-loaded and panitumumab-conjugated poly(lactic- co-glycolic acid) nanoparticles in epidermal growth factor receptor overexpressing glioblastoma cells. Mol Pharm. 2020;17(11):4386-400
34. Schmitt RR, Mahajan SD, Pliss A, Prasad PN. Small molecule based EGFR targeting of biodegradable nanoparticles containing temozolomide and Cy5 dye for greatly enhanced image-guided glioblastoma therapy. Nanomedicine Nanotechnology, Biol Med. 2022;41:102513
35. Xu Y, Shen M, Li Y, Sun Y, Teng Y, Wang Y. et al. The synergic antitumor effects of paclitaxel and temozolomide co-loaded in mPEG-PLGA nanoparticles on glioblastoma cells. Oncotarget. 2016;7(15):20890-901
36. Abrudan C, Florian IS, Baritchii A, Soritau O, Dreve S, Tomuleasa C. et al. Assessment of temozolomide action encapsulated in chitosan and polymer nanostructures on glioblastoma cell lines. Rom Neurosurg. 2014;21(1):19-30
37. Di Martino A, Sedlarik V. Amphiphilic chitosan-grafted-functionalized polylactic acid based nanoparticles as a delivery system for doxorubicin and temozolomide co-therapy. Int J Pharm. 2014;474(1-2):134-45
38. Irani M, Mir Mohamad Sadeghi G, Haririan I. A novel biocompatible drug delivery system of chitosan/temozolomide nanoparticles loaded PCL-PU nanofibers for sustained delivery of temozolomide. Int J Biol Macromol. 2017;97:744-51
39. Di Martino A, Pavelkova A, Maciulyte S, Budriene S, Sedlarik V. Polysaccharide-based nanocomplexes for co-encapsulation and controlled release of 5-Fluorouracil and Temozolomide. Eur J Pharm Sci. 2016;92:276-86
40. Jain DS, Athawale RB, Bajaj AN, Shrikhande SS, Goel PN, Nikam Y. et al. Poly lactic acid (PLA) nanoparticles sustain the cytotoxic action of temozolomide in C6 Glioma cells. Biomed Aging Pathol. 2013;3:201-8
41. Kudarha RR, Sawant KK. Hyaluronic acid conjugated albumin nanoparticles for efficient receptor mediated brain targeted delivery of temozolomide. J Drug Deliv Sci Technol. 2021;61:102129
42. Kudarha RR, Sawant KK. Chondroitin sulfate conjugation facilitates tumor cell internalization of albumin nanoparticles for brain-targeted delivery of temozolomide via CD44 receptor-mediated targeting. Drug Deliv Transl Res. 2021;11:1994-2008
43. Sharma AK, Gupta L, Sahu H, Qayum A, Singh SK, Nakhate KT. et al. Chitosan Engineered PAMAM Dendrimers as Nanoconstructs for the Enhanced Anti-Cancer Potential and Improved In vivo Brain Pharmacokinetics of Temozolomide. Pharm Res. 2018;35(9):1-14
44. Kong B, Sun Y, Li Y, Hu D. Preparation and the influencing factors of timozolomide liposomes. Artif Cells, Blood Substitutes, Biotechnol. 2009;37:279-82
45. Wauthoz N, Deleuze P, Hecq J, Roland I, Saussez S, Adanja I. et al. In vivo assessment of temozolomide local delivery for lung cancer inhalation therapy. Eur J Pharm Sci. 2010;39:402-11
46. Gao J, Wang Z, Liu H, Wang L, Huang G. Liposome encapsulated of temozolomide for the treatment of glioma tumor: preparation, characterization and evaluation. Drug Discov Ther. 2015;9(3):205-12
47. Li SD, Huang L. Stealth nanoparticles: High density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145(3):178-81
48. Hu J, Wang J, Wang G, Yao Z, Dang X. Pharmacokinetics and antitumor efficacy of DSPE-PEG2000 polymeric liposomes loaded with quercetin and temozolomide: Analysis of their effectiveness in enhancing the chemosensitization of drug-resistant glioma cells. Int J Mol Med. 2016;37:690-702
49. Wang F, Ye X, Wu Y, Wang H, Sheng C, Peng D. et al. Time Interval of Two Injections and First-Dose Dependent of Accelerated Blood Clearance Phenomenon Induced by PEGylated Liposomal Gambogenic Acid: The Contribution of PEG-Specific IgM. J Pharm Sci. 2019;108:641-51
50. McSweeney MD, Price LSL, Wessler T, Ciociola EC, Herity LB, Piscitelli JA. et al. Overcoming anti-PEG antibody mediated accelerated blood clearance of PEGylated liposomes by pre-infusion with high molecular weight free PEG. J Control Release. 2019;311-312:138-46
51. Ramalho MJ, Coelho MAN, Pereira MC. Nanocarriers for the delivery of temozolomide in the treatment of glioblastoma: a review. In: Design and Development of New Nanocarriers. Elsevier Inc. 2018 p. 687-722
52. Iturrioz-Rodríguez N, Bertorelli R, Ciofani G. Lipid-Based Nanocarriers for The Treatment of Glioblastoma. Adv NanoBiomed Res. 2020 2000054
53. Huang G, Zhang N, Bi X, Dou M. Solid lipid nanoparticles of temozolomide: Potential reduction of cardial and nephric toxicity. Int J Pharm. 2008;355:314-20
54. Qu J, Zhang L, Chen Z, Mao G, Gao Z, Lai X. et al. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016;23(9):3408-16
55. Wu M, Fan Y, Lv S, Xiao B, Ye M, Zhu X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: a comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016;23(8):2720-5
56. Liu Z, Ji X, He D, Zhang R, Liu Q, Xin T. Nanoscale Drug Delivery Systems in Glioblastoma. Nanoscale Res Lett. 2022;17(27):1-25
57. Iturrioz-Rodríguez N, Correa-Duarte MA, Fanarraga ML. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int J Nanomedicine. 2019;14:3389-401
58. Bertucci A, Prasetyanto EA, Septiadi D, Manicardi A, Brognara E, Gambari R. et al. Combined Delivery of Temozolomide and Anti-miR221 PNA Using Mesoporous Silica Nanoparticles Induces Apoptosis in Resistant Glioma Cells. Small. 2015;11(42):5687-95
59. Nie C, Chu X, Pan Q, Zhang J, Hu Y, Yi J. et al. Engineering a Biodegradable Nanocarrier for Enhancing the Response of T98G Cells to Temozolomide. ACS Appl Bio Mater. 2020;3(5):3337-44
60. Zhang P, Tang M, Huang Q, Zhao G, Huang N, Zhang X. et al. Combination of 3-methyladenine therapy and Asn-Gly-Arg (NGR)-modified mesoporous silica nanoparticles loaded with temozolomide for glioma therapy in vitro. Biochem Biophys Res Commun. 2019;509:549-56
61. Wang LH, Liu JY, Sui L, Zhao PH, Ma HD, Wei Z. et al. Folate-modified Graphene Oxide as the Drug Delivery System to Load Temozolomide. Curr Pharm Biotechnol. 2020;21(11):1088-98
62. Dilnawaz F, Sahoo SK. Enhanced accumulation of curcumin and temozolomide loaded magnetic nanoparticles executes profound cytotoxic effect in glioblastoma spheroid model. Eur J Pharm Biopharm. 2013;85:452-62
63. Xu L, Chu Z, Zhang J, Cai T, Zhang X, Li Y. et al. Steric Effects in the Deposition Mode and Drug-Delivering Efficiency of Nanocapsule-Based Multilayer Films. ACS Omega. 2022;7(34):30321-32
64. Xu L, Chu Z, Wang H, Cai L, Tu Z, Liu H. et al. Electrostatically Assembled Multilayered Films of Biopolymer Enhanced Nanocapsules for on-Demand Drug Release. ACS Appl Bio Mater. 2019;2(8):3429-38
65. Xu L, Zhang X, Chu Z, Wang H, Li Y, Shen X. et al. Temperature-Responsive Multilayer Films Based on Block Copolymer-Coated Silica Nanoparticles for Long-Term Release of Favipiravir. ACS Appl Nano Mater. 2021;4(12):14014-25
66. Mazarei M, Mohammadi Arvejeh P, Mozafari MR, Khosravian P, Ghasemi S. Anticancer potential of temozolomide-loaded eudragit-chitosan coated selenium nanoparticles: In vitro evaluation of cytotoxicity, apoptosis and gene regulation. Nanomaterials. 2021;11:1-18
67. Ling Y, Wei K, Zou F, Zhong S. Temozolomide loaded PLGA-based superparamagnetic nanoparticles for magnetic resonance imaging and treatment of malignant glioma. Int J Pharm. 2012;430:266-75
68. Bernal GM, LaRiviere MJ, Mansour N, Pytel P, Cahill KE, Voce DJ. et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine Nanotechnology, Biol Med. 2014;10(1):149-57
69. Young JS, Bernal G, Polster SP, Nunez L, Larsen GF, Mansour N. et al. Convection-Enhanced Delivery of Polymeric Nanoparticles Encapsulating Chemotherapy in Canines with Spontaneous Supratentorial Tumors. World Neurosurg. 2018;117:e698-704
70. Prabhu S, Goda JS, Mutalik S, Mohanty BS, Chaudhari P, Rai S. et al. A polymeric temozolomide nanocomposite against orthotopic glioblastoma xenograft: Tumor-specific homing directed by nestin. Nanoscale. 2017;9(30):10919-32
71. Senturk F, Cakmak S, Gumusderelioglu M, Ozturk GG. Hydrolytic instability and low-loading levels of temozolomide to magnetic PLGA nanoparticles remain challenging against glioblastoma therapy. J Drug Deliv Sci Technol. 2022;68:103101
72. Chen G, Chen Z, Wen D, Wang Z, Li H, Zeng Y. et al. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc Natl Acad Sci U S A. 2020;117(7):3687-92
73. López-Camacho E, Trilla-Fuertes L, Gámez-Pozo A, Dapía I, López-Vacas R, Zapater-Moros A. et al. Synergistic effect of antimetabolic and chemotherapy drugs in triple-negative breast cancer. Biomed Pharmacother. 2022 149
74. Behrooz AB, Vazifehmand R, Tajudin AA, Masarudin MJ, Sekawi Z, Masomian M. et al. Tailoring drug co-delivery nanosystem for mitigating U-87 stem cells drug resistance. Drug Deliv Transl Res. 2022;12(5):1253-69
75. Smiley SB, Yun Y, Ayyagari P, Shannon HE, Pollok KE, Vannier MW. et al. Development of CD133 Targeting Multi-Drug Polymer Micellar Nanoparticles for Glioblastoma - In vitro Evaluation in Glioblastoma Stem Cells. Pharm Res. 2021;38:1067-79
76. Lam FC, Morton SW, Wyckoff J, Vu Han TL, Hwang MK, Maffa A. et al. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat Commun. 2018;9(1991):1-11
77. Wang Z, Liu Y, Xiao Y, Xie Y, Wang R, Zhang Y. Intelligent Nanoparticles With pH-Sensitive Co-Delivery of Temozolomide and siEGFR to Ameliorate Glioma Therapy. Front Genet. 2022;13:921051
78. Shi H, Sun S, Xu H, Zhao Z, Han Z, Jia J. et al. Combined delivery of temozolomide and siPLK1 using targeted nanoparticles to enhance temozolomide sensitivity in glioma. Int J Nanomedicine. 2020;15:3347-62
79. Peng Y, Huang J, Xiao H, Wu T, Shuai X. Codelivery of temozolomide and siRNA with polymeric nanocarrier for effective glioma treatment. Int J Nanomedicine. 2018;13:3467-80
80. Chen Z, Lai X, Song S, Zhu X, Zhu J. Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv. 2016;23(4):1369-73
81. Pellosi DS, Paula LB, De Melo MT, Tedesco AC. Targeted and Synergic Glioblastoma Treatment: Multifunctional Nanoparticles Delivering Verteporfin as Adjuvant Therapy for Temozolomide Chemotherapy. Mol Pharm. 2019;16(3):1009-24
82. Kwon YM, Je JY, Cha SH, Oh Y, Cho WH. Synergistic combination of chemo-phototherapy based on temozolomide/ICG-loaded iron oxide nanoparticles for brain cancer treatment. Oncol Rep. 2019;42(5):1709-24
83. Zeng X, Wang Q, Tan X, Jia L, Li Y, Hu M. et al. Mild thermotherapy and hyperbaric oxygen enhance sensitivity of TMZ/PSi nanoparticles via decreasing the stemness in glioma. J Nanobiotechnology. 2019;17(1):1-12
84. Cao Y, Jin L, Zhang S, Lv Z, Yin N, Zhang H. et al. Blood-brain Barrier Permeable and Multi-stimuli Responsive Nanoplatform for Orthotopic Glioma Inhibition by Synergistic Enhanced Chemo-/Chemodynamic/Photothermal/Starvation Therapy. Eur J Pharm Sci. 2023;180:106319
85. Minaei SE, Khoei S, Khoee S, Vafashoar F, Mahabadi VP. In vitro anti-cancer efficacy of multi-functionalized magnetite nanoparticles combining alternating magnetic hyperthermia in glioblastoma cancer cells. Mater Sci Eng C. 2019;101:575-87
86. Marino A, Camponovo A, Degl A, Bartolucci M, Tapeinosa C, Martinellia C. et al. Multifunctional temozolomide-loaded lipid superparamagnetic nanovectors: Dual targeting and disintegration of glioblastoma spheroids by synergic chemotherapy and hyperthermia treatment. Nanoscale. 2019;11(44):21227-48
87. Yao J, Feng X, Dai X, Peng G, Guo Z, Liu Z. et al. TMZ magnetic temperature-sensitive liposomes-mediated magnetothermal chemotherapy induces pyroptosis in glioblastoma. Nanomedicine Nanotechnology, Biol Med. 2022;43:102554
88. Xu M, Li G, Zhang H, Chen X, Li Y, Yao Q. et al. Sequential delivery of dual drugs with nanostructured lipid carriers for improving synergistic tumor treatment effect. Drug Deliv. 2020;27(1):983-95
89. Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM. et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013;56(14):5979-83
90. Pérez-Salvia M, Esteller M. Bromodomain inhibitors and cancer therapy: From structures to applications. Epigenetics. 2017;12(5):323-39
91. Zhang Z, Conniot J, Amorim J, Jin Y, Prasad R, Yan X. et al. Nucleic acid-based therapy for brain cancer: Challenges and strategies. J Control Release. 2022;350:80-92
92. Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6(4):321-30
93. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857-66
94. Brognara E, Fabbri E, Bazzoli E, Montagner G, Ghimenton C, Eccher A. et al. Uptake by human glioma cell lines and biological effects of a peptide-nucleic acids targeting miR-221. J Neurooncol. 2014;118:19-28
95. Borriello A, Cucciolla V, Oliva A, Zappia V, Della Ragione F. p27Kip1 metabolism: A fascinating labyrinth. Cell Cycle. 2007;6(9):1053-61
96. Chen L, Zhang J, Han L, Zhang A, Zhang C, Zheng Y. et al. Downregulation of miR-221/222 sensitizes glioma cells to temozolomide by regulating apoptosis independently of p53 status. Oncol Rep. 2012;27:854-60
97. Hirst TC, Vesterinen HM, Sena ES, Egan KJ, MacLeod MR, Whittle IR. Systematic review and meta-analysis of temozolomide in animal models of glioma: Was clinical efficacy predicted. Br J Cancer. 2013;108:64-71
98. Lakomkin N, Hadjipanayis CG. Fluorescence-guided surgery for high-grade gliomas. J Surg Oncol. 2018;118(2):356-61
99. Miretti M, González Graglia MA, Suárez AI, Prucca GC. Photodynamic therapy for glioblastoma: A light at the end of the tunnel. J Photochem Photobiol J. 2023;13:100161
100. Bartusik-Aebisher D, Żołyniak A, Barnaś E, Machorowska-Pieniążek A, Oleś P, Kawczyk-Krupka A. et al. The Use of Photodynamic Therapy in the Treatment of Brain Tumors—A Review of the Literature. Molecules. 2022;27(20):6847
101. Zhang X, Guo M, Shen L, Hu S. Combination of photodynamic therapy and temozolomide on glioma in a rat C6 glioma model. Photodiagnosis Photodyn Ther. 2014;11(4):603-12
102. Jia Y, Chen L, Chi D, Cong D, Zhou P, Jin J. et al. Photodynamic therapy combined with temozolomide inhibits C6 glioma migration and invasion and promotes mitochondrial-associated apoptosis by inhibiting sodium-hydrogen exchanger isoform 1. Photodiagnosis Photodyn Ther. 2019;26:405-12
103. Miretti M, Prucca CG, Tempesti TC, Baumgartner MT. Current Phthalocyanines Delivery Systems in Photodynamic Therapy: An Updated Review. Curr Med Chem. 2021;28(26):5339-67
104. Suit HD, Gerweck LE. Potential for Hyperthermia and Radiation Therapy. Cancer Res. 1979;39:2290-8
105. Chen Y, Liu P, Sun P, Jiang J, Zhu Y, Dong T. et al. Oncogenic MSH6-CXCR4-TGFB1 feedback loop: A novel therapeutic target of photothermal therapy in Glioblastoma multiforme. Theranostics. 2019;9(5):1453-73
106. Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperth. 2005;21(8):761-7
107. Egea-Benavente D, Ovejero JG, Morales MDP, Barber DF. Understanding MNPs behaviour in response to amf in biological milieus and the effects at the cellular level: Implications for a rational design that drives magnetic hyperthermia therapy toward clinical implementation. Cancers (Basel). 2021 13(18)
108. Bausart M, Préat V, Malfanti A. Immunotherapy for glioblastoma: the promise of combination strategies. J Exp Clin Cancer Res. 2022;41(35):1-22
109. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363(8):711-23
110. Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M. et al. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy. Oncologist. 2016;21(5):634-42
111. Haslam A, Prasad V. Estimation of the percentage of us patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535
112. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A. et al. Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(7):1003-10
113. Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB. et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477-86
114. Bota DA, Chung J, Dandekar M, Carrillo JA, Kong XT, Fu BD. et al. Phase II study of ERC1671 plus bevacizumab versus bevacizumab plus placebo in recurrent glioblastoma: interim results and correlations with CD4+ T-lymphocyte counts. CNS Oncol. 2018;7(3):CNS22
115. Ameratunga M, Coleman N, Welsh L, Saran F, Lopez J. CNS cancer immunity cycle and strategies to target this for glioblastoma. Oncotarget. 2018;9(32):22802-16
116. Karachi A, Yang C, Dastmalchi F, Sayour EJ, Huang J, Azari H. et al. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro Oncol. 2019;21(6):730-41
117. Mathios* D, Kim* JE, Mangraviti A, Jillian Phallen, Park CK, Jackson CM. et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8:370ra180
118. Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW. et al. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med. 2016;8(328):328ra27
119. He C, Ding H, Chen J, Ding Y, Yang R, Hu C. et al. Immunogenic cell death induced by chemoradiotherapy of novel pH-sensitive cargo-loaded polymersomes in glioblastoma. Int J Nanomedicine. 2021;16:7123-35
120. Zhang J, Chen C, Li A, Jing W, Sun P, Huang X. et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat Nanotechnol. 2021;16(5):538-48
121. Kunwar A, Barik A, Mishra B, Rathinasamy K, Pandey R, Priyadarsini KI. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta-General Subj. 2008;1780:673-9
122. BA W. The vascular morphology of tumors. Tumor Blood Circulation: Angiogenesis, Vascular Morphology and Blood Flow of Experimental and Human Tumors. Edited. Peterson H. Boca Raton. 1979:1-48 p
123. Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S. et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363-80
124. Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559-93
125. Steinberg F, Konerding MA, Streffer C. The vascular architecture of human xenotransplanted tumors: histological, morphometrical, and ultrastructural studies. J Cancer Res Clin Oncol. 1990;116(5):517-24
126. Peterson HI, Appelgren L. Tumour vessel permeability and transcapillary exchange of large molecules of different size. Bibl Anat. 1977(15 Pt 1):262-5
127. Rakesh K. Jain. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2012;23:1-7
128. Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP. et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95(8):4607-12
129. Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2010;2(14):1-19
130. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387-92
131. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65:271-84
132. Wu J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J Pers Med. 2021;11(771):1-8
133. Donelli MG, Zucchetti M, D'Incalci M. Do anticancer agents reach the tumor target in the human brain? Cancer Chemother Pharmacol. 1992;30(4):251-60
134. Steiniger SCJ, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS. et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109(5):759-67
135. Kim SS, Rait A, Kim E, DeMarco J, Pirollo KF, Chang EH. Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of glioblastoma. Cancer Lett. 2015;369:250-8
136. Jain A, Singhai P, Gurnany E, Updhayay S, Mody N. Transferrin-tailored solid lipid nanoparticles as vectors for site-specific delivery of temozolomide to brain. J Nanoparticle Res. 2013;15(3):1-9
137. Fu W, You C, Ma L, Li H, Ju Y, Guo X. et al. Enhanced Efficacy of Temozolomide Loaded by a Tetrahedral Framework DNA Nanoparticle in the Therapy for Glioblastoma. ACS Appl Mater Interfaces. 2019;11:39525-33
138. Zhang J, Xiao X, Zhu J, Gao Z, Lai X, Zhu X. et al. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int J Nanomedicine. 2018;13:3039-51
139. Patil R, Portilla-Arias J, Ding H, Inoue S, Konda B, Hu J. et al. Temozolomide Delivery to Tumor Cells by a Multifunctional Nano Vehicle Based on Poly(β-L-malic acid). Pharm Res. 2010;27(11):2317-29
140. Patil R, Holler E, Black KL, Ljubimova JY. Drug delivery of temozolomide for systemic based treatment of cancer. US 8.785.371 B2. 2014
141. Song S, Mao G, Du J, Zhu X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016;23(4):1404-1408
142. Seok J, Hwan D, Kim J seok. Dual-targeting immunoliposomes using angiopep-2 and CD133 antibody for glioblastoma stem cells. J Control Release. 2018;269:245-57
143. Ismail M, Yang W, Li Y, Chai T, Zhang D, Du Q. et al. Targeted liposomes for combined delivery of artesunate and temozolomide to resistant glioblastoma. Biomaterials. 2022;287:121608
144. Zhang Y, Qu H, Xue X. Blood-brain barrier penetrating liposomes with synergistic chemotherapy for glioblastoma treatment. Biomater Sci. 2022;10(2):423-34
145. Gabay M, Weizman A, Zeineh N, Kahana M, Obeid F, Allon N. et al. Liposomal Carrier Conjugated to APP-Derived Peptide for Brain Cancer Treatment. Cell Mol Neurobiol. 2021;41:1019-29
146. Emamgholizadeh Minaei S, Khoei S, Khoee S, Karimi MR. Tri-block copolymer nanoparticles modified with folic acid for temozolomide delivery in glioblastoma. Int J Biochem Cell Biol. 2019;108:72-83
147. Smiley SB, Yun Y, Ayyagari P, Shannon HE, Pollok KE, Vannier MW. et al. Development of Targetable Multi-Drug Nanoparticles for Glioblastoma Treatment and In vitro Evaluation in Glioblastoma Stem Cells. Pharm Res. 2021(9):1-27
148. Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5(12):2957-62
149. Arcella A, Palchetti S, Digiacomo L, Pozzi D, Capriotti AL, Frati L. et al. Brain Targeting by Liposome-Biomolecular Corona Boosts Anticancer Efficacy of Temozolomide in Glioblastoma Cells. ACS Chem Neurosci. 2018;9:3166-74
150. Voth B, Nagasawa DT, Pelargos PE, Chung LK, Ung N, Gopen Q. et al. Transferrin receptors and glioblastoma multiforme: Current findings and potential for treatment. J Clin Neurosci. 2015;22:1071-6
151. Ramalho MJ, Loureiro JA, Coelho MAN, Pereira MC. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Vol. 14, Pharmaceutics. 2022:1-31 p
152. McCord E, Pawar S, Koneru T, Tatiparti K, Sau S, Iyer AK. Folate Receptors' Expression in Gliomas May Possess Potential Nanoparticle-Based Drug Delivery Opportunities. ACS Omega. 2021;6:4111-8
153. Huang N, Cheng S, Zhang X, Tian Q, Pi J, Tang J. et al. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood-brain barrier and targeted fluorescence imaging of glioma and tumor vasculature. Nanomedicine Nanotechnology, Biol Med. 2017;13:83-93
154. Ak G, Ünal A, Karakayalı T, Özel B, Günel NS, Şanlıer ŞH. Brain-targeted, drug-loaded solid lipid nanoparticles against glioblastoma cells in culture. Colloids Surfaces B Biointerfaces. 2021;206:22-4
155. Sun Y, Sun J, He Z, Wang G, Wang Y, Zhao D. et al. Monocarboxylate Transporter 1 in Brain Diseases and Cancers. Curr Drug Metab. 2019;20(11):855-66
156. Yoon J, Grinchuk O V, Kannan S, Ang MJY, Li Z, Tay EXY. et al. A chemical biology approach reveals a dependency of glioblastoma on biotin distribution. Sci Adv. 2021;7(36):1-14
157. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645-8
158. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983-8
159. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946-51
160. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106-10
161. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C. et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111-5
162. Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V. et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030-7
163. Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193-206
164. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T. et al. Identification of a cancer stem cell in human brain tumors. Nature. 2004;432(18):396-401
165. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T. et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396-401
166. Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011-21
167. Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675-99
168. Lathia JD, Gallagher J, Myers JT, Li M, Vasanji A, McLendon RE. et al. Direct In vivo evidence for tumor propagation by glioblastoma cancer stem cells. PLoS One. 2011;6(9):1-9
169. Hu B, Wang Q, Wang YA, Hua S, Sauvé CEG, Ong D. et al. Epigenetic Activation of WNT5A Drives Glioblastoma Stem Cell Differentiation and Invasive Growth. Cell. 2016;167(5):1281-1295.e18
170. Lombard A, Digregorio M, Delcamp C, Rogister B, Piette C, Coppieters N. The Subventricular Zone, a Hideout for Adult and Pediatric High-Grade Glioma Stem Cells. Front Oncol. 2021;10:614930
171. Codrici E, Enciu AM, Popescu ID, Mihai S, Tanase C. Glioma Stem Cells and Their Microenvironments: Providers of Challenging Therapeutic Targets. Stem Cells Int. 2016. 2016
172. Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG. et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522-6
173. Bao S, Wu Q, Mclendon RE, Hao Y, Shi Q, Hjelmeland AB. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-60
174. Alghamdi M, Gumbleton M, Newland B. Local delivery to malignant brain tumors: Potential biomaterial-based therapeutic/adjuvant strategies. Biomater Sci. 2021;9:6037-51
175. Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC. et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2004;5(2):79-88
176. Bastiancich C, Malfanti A, Préat V, Rahman R. Rationally designed drug delivery systems for the local treatment of resected glioblastoma. Adv Drug Deliv Rev. 2021;177:113951
177. Yu J, Wang J, Zhang Y, Chen G, Mao W, Ye Y. et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat Biomed Eng. 2020;4(5):499-506
178. Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355:1-18
179. Fourniols T, Randolph LD, Staub A, Vanvarenberg K, Leprince JG, Préat V. et al. Temozolomide-loaded photopolymerizable PEG-DMA-based hydrogel for the treatment of glioblastoma. J Control Release. 2015;210:95-104
180. Zhao M, Bozzato E, Joudiou N, Ghiassinejad S, Danhier F, Gallez B. et al. Codelivery of paclitaxel and temozolomide through a photopolymerizable hydrogel prevents glioblastoma recurrence after surgical resection. J Control Release. 2019;309:72-81
181. Adhikari B, Li J, Brandel MG, Futalan D, Akers J, Deming T. et al. The use of TMZ embedded hydrogels for the treatment of orthotopic human glioma xenografts. J Clin Neurosci. 2017;45:288-92
182. Zhao Z, Shen J, Zhang L, Wang L, Xu H, Han Y. et al. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater Sci. 2020;8:5306-16
183. Ding L, Wang Q, Shen M, Sun Y, Zhang X, Huang C. et al. Thermoresponsive nanocomposite gel for local drug delivery to suppress the growth of glioma by inducing autophagy. Autophagy. 2017;13(7):1176-90
184. Akbar U, Jones T, Winestone J, Michael M, Shukla A, Sun Y. et al. Delivery of temozolomide to the tumor bed via biodegradable gel matrices in a novel model of intracranial glioma with resection. J Neurooncol. 2009;94:203-12
185. Puente P de la, Fettig N, Luderer MJ, Jin A, Shah S, Muz B. et al. Injectable Hydrogels for Localized Chemotherapy and Radiotherapy in Brain Tumors. J Pharm Sci. 2018;107:922-33
186. Azab AK, Doviner V, Orkin B, Kleinstern J, Srebnik M, Nissan A. et al. Biocompatibility evaluation of crosslinked chitosan hydrogels after subcutaneous and intraperitoneal implantation in the rat. J Biomed Mater Res Part A. 2006;79(4):963-73
187. Azab AK, Kleinstern J, Doviner V, Orkin B, Srebnik M, Nissan A. et al. Prevention of tumor recurrence and distant metastasis formation in a breast cancer mouse model by biodegradable implant of 131I-norcholesterol. J Control Release. 2007;123(2):116-22
188. Nordling-David MM, Yaffe R, Guez D, Meirow H, Last D, Grad E. et al. Liposomal temozolomide drug delivery using convection enhanced delivery. J Control Release. 2017;261:138-46
189. Lin CY, Li RJ, Huang CY, Wei KC, Chen PY. Controlled release of liposome-encapsulated temozolomide for brain tumour treatment by convection-enhanced delivery. J Drug Target. 2018;26:325-32
190. Gómez-Oliva R, Domínguez-García S, Carrascal L, Abalos-Martínez J, Pardillo-Díaz R, Verástegui C. et al. Evolution of Experimental Models in the Study of Glioblastoma: Toward Finding Efficient Treatments. Front Oncol. 2021;10:1-16
191. Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E. et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. 2019;11(513):1-10
192. Agrahari V, Agrahari V. Facilitating the translation of nanomedicines to a clinical product: challenges and opportunities. Drug Discov Today. 2018;23(5):974-91
193. Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688-713
194. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF. et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014
195. Mahmoudi M. Debugging Nano-Bio Interfaces: Systematic Strategies to Accelerate Clinical Translation of Nanotechnologies. Trends Biotechnol. 2018;36(8):755-69
Author contact
![]() Corresponding author: Dr. Ander Matheu. ander.matheuorg
Corresponding author: Dr. Ander Matheu. ander.matheuorg
 Global reach, higher impact
Global reach, higher impact