13.3
Impact Factor
Theranostics 2023; 13(8):2721-2733. doi:10.7150/thno.83543 This issue Cite
Research Paper
MOF-derived bimetallic nanozyme to catalyze ROS scavenging for protection of myocardial injury
1. Department of Diagnostic Radiology, Nanjing Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu, China.
2. Department of Diagnostic Radiology, Jinling Hospital, Nanjing Medical University, Nanjing, Jiangsu, China.
3. Heart Center, Department of Cardiovascular Medicine, Zhejiang Provincial People's Hospital, Affiliated People's Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, China.
4. Department of Diagnostic Radiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore.
5. Nanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore.
6. School of Medicine and Health, Key Laboratory of Micro-systems and Micro-structures Manufacturing (Ministry of Education), Harbin Institute of Technology, Harbin, 150001 China.
7. Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117599, Singapore.
#These authors equally contributed to this work.
Abstract
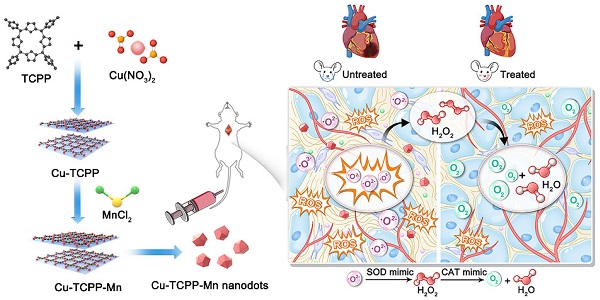
Rationale: Myocardial injury triggers intense oxidative stress, inflammatory response, and cytokine release, which are essential for myocardial repair and remodeling. Excess reactive oxygen species (ROS) scavenging and inflammation elimination have long been considered to reverse myocardial injuries. However, the efficacy of traditional treatments (antioxidant, anti-inflammatory drugs and natural enzymes) is still poor due to their intrinsic defects such as unfavorable pharmacokinetics and bioavailability, low biological stability, and potential side effects. Nanozyme represents a candidate to effectively modulate redox homeostasis for the treatment of ROS related inflammation diseases.
Methods: We develop an integrated bimetallic nanozyme derived from metal-organic framework (MOF) to eliminate ROS and alleviate inflammation. The bimetallic nanozyme (Cu-TCPP-Mn) is synthesized by embedding manganese and copper into the porphyrin followed by sonication, which could mimic the cascade activities of superoxide dismutase (SOD) and catalase (CAT) to transform oxygen radicals to hydrogen peroxide, followed by the catalysis of hydrogen peroxide into oxygen and water. Enzyme kinetic analysis and oxygen-production velocities analysis were performed to evaluate the enzymatic activities of Cu-TCPP-Mn. We also established myocardial infarction (MI) and myocardial ischemia-reperfusion (I/R) injury animal models to verify the ROS scavenging and anti-inflammation effect of Cu-TCPP-Mn.
Results: As demonstrated by kinetic analysis and oxygen-production velocities analysis, Cu-TCPP-Mn nanozyme possesses good performance in both SOD- and CAT-like activities to achieve synergistic ROS scavenging effect and provide protection for myocardial injury. In both MI and I/R injury animal models, this bimetallic nanozyme represents a promising and reliable technology to protect the heart tissue from oxidative stress and inflammation-induced injury, and enables the myocardial function to recover from otherwise severe damage.
Conclusions: This research provides a facile and applicable method to develop a bimetallic MOF nanozyme, which represents a promising alternative to the treatment of myocardial injuries.
Keywords: reactive oxygen species, myocardial injury, nanozyme, metal-organic framework, nanomedicine
 Global reach, higher impact
Global reach, higher impact