13.3
Impact Factor
Theranostics 2023; 13(5):1607-1631. doi:10.7150/thno.82690 This issue Cite
Review
Nasopharyngeal carcinoma ecology theory: cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem
Cancer Research Institute, Department of Pathology, The Second Affiliated Hospital of Southern University of Science and Technology, Shenzhen Third People's Hospital, National Clinical Research Center for Infectious Diseases, Shenzhen, China.
Received 2023-1-16; Accepted 2023-2-26; Published 2023-3-5
Abstract
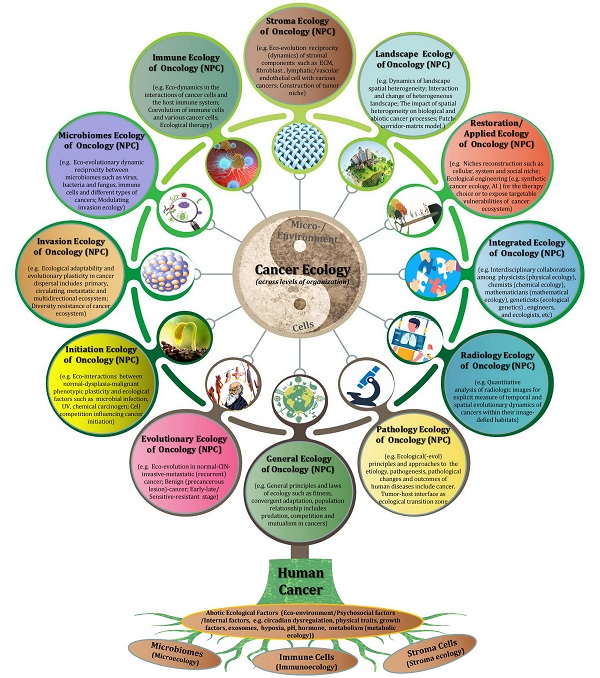
Nasopharyngeal carcinoma (NPC) is a particular entity of head neck cancer that is generally regarded as a genetic disease with diverse intertumor and intratumor heterogeneity. This perspective review mainly outlines the up-to-date knowledge of cancer ecology and NPC progression, and presents a number of conceptual stepping-stones. At the beginning, I explicitly advocate that the nature of NPC (cancer) is not a genetic disease but an ecological disease: a multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. The hallmarks of cancer is proposed to act as ecological factors of population fitness. Subsequently, NPC cells are described as invasive species and its metastasis as a multidirectional ecological dispersal. The foundational ecological principles include intraspecific relationship (e.g. communication) and interspecific relationship (e.g. competition, predation, parasitism and mutualism) are interpreted to understand NPC progression. “Mulberry-fish-ponds” model can well illustrate the dynamic reciprocity of cancer ecosystem. Tumor-host interface is the ecological transition zone of cancer, and tumor buddings should be recognized as ecological islands separated from the mainland. It should be noted that tumor-host interface has a significantly molecular and functional edge effect because of its curvature and irregularity. Selection driving factors and ecological therapy including hyperthermia for NPC patients, and future perspectives in such field as “ecological pathology”, “multidimensional tumoriecology” are also discussed. I advance that “nothing in cancer evolution or ecology makes sense except in the light of the other”. The cancer ecology tree is constructed to comprehensively point out the future research direction. Taken together, the establishment of NPC ecology theory and cancer ecology tree might provide a novel conceptual framework and paradigm for our understanding of cancer complex causal process and potential preventive and therapeutic applications for patients.
Keywords: Nasopharyngeal carcinoma ecology, Unity of ecology and evolution, Pathological ecosystem, Tumor microenvironment, Tumor-host interface, Tumor budding, Ecological therapy, Ecological pathology, Ecological radiology, Synthetic cancer ecology, Multidimensional tumoriecology, Cancer ecology tree
Introduction
Nasopharyngeal carcinoma (NPC) is a disease with remarkably distinctive ethnic and geographic distributions, which is highly prevalent in Southern China and Southeast Asia. The pathogenesis of NPC is closely associated with Epstein-Barr virus (EBV) infection, other pathogen infections such as HPV and bacteria and environmental factors including chemical carcinogens and social practice such as alcohol consumption and smoking also contribute to the increased risk of NPC (1-4). Although a large number of funds, labor and material resources have been spent in the last decades, a brutal truth is that most of patients are still diagnosed at advanced stages, and tend to have local recurrence and distant metastasis after conventional therapy, even emerging immunotherapy such as PD-1 inhibition benefits only a subset of clinical cases (5). Perhaps, it is time for us to rethink this disease viewed as the characteristic cancer in China. But where to move on? This is what this paper is about.
NPC is generally characterized as a genetic disease with diverse extent of intertumor and intratumor heterogeneity (6). During the past several decades, a great deal of attention and achievements have been reached according to the possible mechanisms about the genetic and molecular carcinogenesis and metastasis of this disease, as well as the mutual regulation of the molecules such as DNA and RNA (e.g. miRNA and lncRNA). However, we should be aware that the role of DNA is not for programming cells. As early as in 1962, the radiologist Smithers DW has stated that "cancer is no more of a disease of cells than a traffic jam is a disease of cars" (7). Too much concentration on internal combustion engines would not help us to understand the traffic problems. NPC is a complex social community. It consists of dysfunctional cell populations and a great heterogeneous context that is characterized by highly variable immune cells infiltration such as lymphocytes and plasma cells (some cases with strong desmoplastic stroma) (8,9). Concentrating on what goes on within a tumor is certainly insufficient for elucidating the underlying mechanisms that support malignant spread and metastasis. In case taking a part for the whole, we should acknowledge that the impacts of tumor microenvironment (TME) on NPC have gained much more attention over the past ten years (10). However, it is not difficult to find such a fact that there was a lack of the matrix context and spatial architecture in most in vitro experiments. Additionally, I think that the complex relationship between NPC cells and TME also needs to be generalized essentially.
Su Shi, a famous poet in the Song Dynasty of China, once wrote “it's a range viewed in face and peaks viewed from the side; assuming different shapes viewed from far and wide”. Therefore, it is necessary to turn this disease from reductionism to holism, and ultimately reach out unity of molecular reductionism with quantitative holism. A multidimensional perspective is needed to review the historic research of NPC and its microenvironment, and to clearly elucidate the nature of this disease and move cancer research into a new direction. In this study, I propose that NPC is significantly more complicated than “a genetic disease”, and it could best be conceptualized as a multidimensional spatiotemporal “ecological and evolutionary unity” disease: an evolutionary adaptive pathological ecosystem that consisting of four interdependent parts: primary ecosystem, circulating ecosystem, metastatic ecosystem and multidirectional ecosystem, which was predescribed on 17 October 2022 (https://www.preprints.org/manuscript/202210.0226/v1). The ecological theory of NPC is hoped to provide a novel and fundamental framework about comprehensively understanding of the complicated progression of this disease, and develop more effective treatment strategies for cancer patients.
NPC as pathological ecosystem of “unity of ecology and evolution”
The first use of the word “ecosystem” began with British ecologist Tansley AG in 1935 (11). In the 1950s, Odum EP creatively proposed the change rules of the structure and function characteristics during the ecosystem development as “any unit that includes all of the organisms (the biotic community) in a given area interacting with the physical environment so that a flow of energy leads to clearly defined biotic structures and cycling of materials between living and nonliving components is an ecological system” (12). A nature ecosystem consists of two main parts including populations (animals, plants, virus, bacteria, etc.) and the physical environment they inhabit (oxygen, water, soil, food, etc.). Similarly, cancer tissues including NPC create a complex spatially structured ecosystem made up of various cell types and essential stromal resources. Microscopically, NPC cells encompass squamous cell carcinoma, nonkeratinizing carcinoma (differentiated or undifferentiated) and basaloid squamous cell carcinoma. Among them, differentiated carcinoma (DNKC) and undifferentiated carcinoma (UDC) are the main cell subtypes. It should be noted that either the differentiated or undifferentiated subtype generally has a certain number of spindle cells (sarcoma-like) (8,13), which tend to cause erosion of surrounding tissue (9) and predominantly present in the invasive front or infiltrating the stroma (14,15). On the other side, the NPC microenvironment is highly heterogeneous with tumor stroma reaction (9), biotic components comprise of cancer-associated fibroblasts (CAFs), immune cells and endothelial cells, and abiotic components include extracellular matrix (ECM), cytokines, nutrients (8,12,16). Together, we can postulate that this variety of cancer cell subtypes, cooperating and competing with each other and interacting within their habitats spatiotemporally in the whole ecosystem of a high degree of genetic and phenotypic diversity, that jointly contribute to both malignant progression and clinical practise in NPC patients.
It is well-known that in 1973, Dobzhansky T made a remark as “nothing in biology makes sense except in the light of evolution” (17). It is a truism not just for biology but also for many disciplines of medicine like oncology (18). During the pathological diagnosis of nasopharyngeal biopsy specimens, the changes from low or high dysplasia to carcinoma-in situ (the prevalence varies from 2% to 3.6% of NPC) can be observed in the place where is jointed with normal nasopharyngeal epithelial cells. Histologically, these atypical cells consist of cells with variable loss of polarity, or enlarged oval-shaped pleomorphic nuclei with obvious nucleoli and indistinct cytoplasmic boundaries confined to the surface or crypt epithelium (12,19). There is a meaningful implication of carcinogenesis from the normal nasopharyngeal epithelium. It is generally accepted that nasopharyngeal epithelial transformation and pathogenesis is closely linked with, at first chronic exposure of the nasopharyngeal mucosa to environmental carcinogens such as nitrite amines, alcohol consumption and smoking, and then accelerated by the infection of EBV infection (other pathogens such as HPV and bacteria might also be involved) (1-5). As the early stage of initiating tumorigenic transformation, for example, reports have showed direct infection and transformation of human nasopharyngeal epithelial cells destined to evolve into the carcinoma by EBV through EBVR/CR2 (20). EphA2 is also a critical player for EBV epithelial cell entry (21). In addition, EBV-infection encoded oncoproteins such as LMP1, LMP2 and EBNA1 or their interactions with the TME includes immune cells and rodent fibroblasts have been proved to directly impact morphological and phenotypic alterations in epithelial cells, and the malignant progression or therapy response of NPC (22-25). One of the best models of the roles of EBV infection and genomic changes during NPC development was constructed (5). In fact, as early as the 1980s, Yao KT has advocated the “three strikes” hypothesis to interpret the pathogenesis of NPC (2). In brief, some familial cases often carry germline mutation, also known as reproductive mutation (one strike), and the combination of EB virus (two strikes) and environmental factors (three strikes) eventually leads to the carcinogenesis of this disease. These abnormal cells require the sequential acquisition of mutations from the prospective of Darwinian evolution “survival of the fittest” (26), with altered metabolic, mechanical, and cell communication contexts that act as ecological stress factors. Under these circumstances, cells need to compete with each other for survival, and thus the fittest cells can survive and form genetically distinct cell populations, the tumor. To summarize, I conclude that progressively transformed of normal cells to carcinogenesis could be viewed as an ecol-evolutionary process.
In the 1970s, Peter Nowell firstly described a landmark perspective on dynamic tumor evolution and progression (27). This theory implies that increasingly aggressive subclones in cancer cell populations are selected to evolve according to the Darwinian principles, which results in genetically heterogeneous tumors (intertumor and intratumor heterogeneity) and is potentially responsible for cancer formation, drug resistance and metastasis (28). Over the past decades, based on the advancing technologies such as microarray technology, genome-wide analysis and whole-exome sequencing, tumor heterogeneity, intraclonal genetic diversity and high genomic instability in NPC have been widely excavated and disclosed (29-31). These empirical findings should be the basis of portrait of genetic architectures and inferred clonal evolutionary trees. In recent few years, the application of single-cell transcriptomics has contributed to a more comprehensively and attentive understanding of the stromal landscape and immune dynamics and their interplay with cancer cells in the whole NPC (32-35). Temporal changes of the microenvironment's heterogeneity including enrichment of M2-polarized macrophages and LAMP3+ dendritic cells between the primary and recurrent NPC (rNPC) has been revealed (36). On the basis of the Darwinian principles (37,38), we should realize that, the heterogeneity of NPC could be the result of competition between various clones of cancer cells that act as competing species for resources in the tumor microenvironment. In turn, the subpulolations of NPC cells could acquire, through mutational and epigenetic changes, a variety of phenotypic traits that compound to allow territorial expansion by proliferative self-renewal, diaspora and therapeutic resistance.
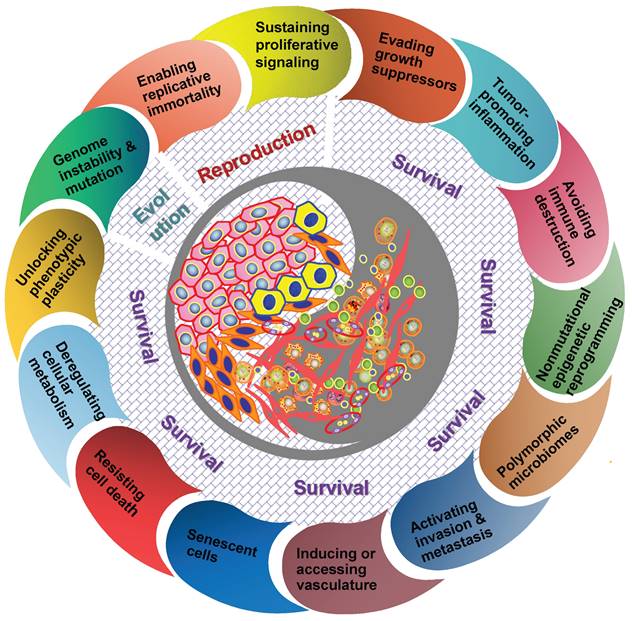
It should be understood that diverse clones of cancer cells need not to be genetically encoded. Tumor microenvironment has been proved to be a major determinant of tumor heterogeneity (39). The ecological interaction between cancer cells and their habitats is dynamic reciprocal. A tumor can be regarded as being an integrated ecosystem in which the co-evolution of neoplastic cells together with tumor microenvironment such as extracellular matrix, tumor vasculature and immune cells confers a highly fitness to exceptionally variable phenotypes of cancer cells (40,41). The selective advantage may be achieved by various fitness phenotypes (or ecological factors) so-called “hallmarks of cancer” , which includes the acquired capabilities for sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing/accessing vasculature, activating invasion and metastasis, reprogramming cellular metabolism, avoiding immune destruction, unlocking phenotypic plasticity, nonmutational epigenetic reprogramming, polymorphic microbiomes, and senescent cells (42). So the point I'm emphasizing here is that the great majority of these hallmarks ultimately affect cancer cell fitness through the ability of survival and/or reproduction (Figure 1). Most of hallmark phenotypic features governed by the crosstalk between cancer cells and tumor microenvironment in NPC have also been explored in more recent years (8,16,32-36,43,44). Here I'd like to emphasize that, “nothing in evolution makes sense except in the light of tumor microenvironment”.
The hallmarks of cancer act as ecological factors of population fitness. Genome instability and mutation lead to the diversity and evolutionary adaptation of population species, other cancer hallmarks affect ecological adaptation of cancer cells through the dynamic change of their survival ability and reproductive capacity in particular.
Tumor therapeutic resistance, relapse and distant metastasis is essentially an evolutionary process (45,46). Many cancers, if not all, a small subpopulation of cells in tumors termed as cancer stem cells (CSCs) should be the representative for elucidating this evolutionary process because of the capacity of maintaining tumor heterogeneity, unlimited proliferation and evading therapeutic predation (47,48). On the other side, CSCs can proactively remodel the surrounding tissue microhabitats to maintain a perivascular niche and phenotypic variation in fitness (that is, ecological engineering and niche construction) (49). During the past decades, diverse identifying approaches and experiments have been applied for the study of CSCs in NPC (50). The presence of CSCs in NPC was initially identified by label-retaining cell (LRC) approach by Yao KT et al. in 2007 (51). Subsequently, Zeng YX et al. reported that side population (SP) cells of NPC had stem cell-like properties with an enhanced ability to form tumors in vivo (52). Our previous study also showed that aldehyde dehydrogenase 1 (ALDH1) could be a novel CSCs marker in NPC, which was endowed with extensive proliferation, capable of self-renewal and generating tumors (53). Moreover, CSCs themselves are found to be overrepresented at the invasive front (tumor-host interface) of NPC tissues. For example, EBV-encoded LMP2A, FoxM1 could enhance the ability of tumorigenicity of NPC cell lines in mice xenograft, and the expressions of these proteins were mainly localized at the tumor invasive front (54,55). As such, CSCs at the invasive front acting as an driver might contribute greatly to metastatic features and worse prognosis of NPC patients.
The same species may generate different morphological structures and physiological characteristics under different conditions in which they live for a long time, and this type of biological adaptation is called “ecotype” (56). The morphological and/or physiological attributes are generally caused by the selection of habitat environment (57-60). The cancer cells in NPC specimens show light microscopic evidence of squamous differentiation, and their microenvironment comprises the very complex stromal components. As mentioned above, either the differentiated or undifferentiated subtype generally contains a proportion of neoplastic spindle cells, and these cells with fibroblast-like phenotype are observed predominently in the invasive tumor front. (14,61). Given such communities of species and their dynamic interactions with each other and their environments day and night, it is reasonable to assume that spindle cells are reflection of morphological adaptation to the external environment from squamous subtypes. In view of these cells possessing the epithelial-mesenchymal transition (EMT) and CSCs features, it might be considered as the morphological indicators of “mobile/migratory CSCs” and “invasive/metastatic NPC” (61). Additionally, vasculogenic mimicry, a new tumor microvascular paradigm of non-endothelial cells, is formed to adopt multiple cellular phenotypes including tube-like structure or endothelial-like properties, and contributes significantly to the progression and metastasis of NPC (62-65). On the other side, different populations also could display the same or similar adaptive characteristics through variation and selection under the long-term living in the same habitat (56). It is noteworthy that in some cases, NPC cells are quite similar to the morphology of benign cellular changes such as clusters of germinal centre cells and reactive lymphoid hyperplasia (11,12). In clinical practice, the computational application and deep learning model have been recently used by us and other groups for the successful identification (66-68), and largely assist pathologists on these difficult diagnoses (68).
What any ecologist sees is the result of evolution. Any script of evolution is staged on the stage of ecology. Now Dobzhansky's famous dictum has been taken on a whole new meaning “nothing in evolution or ecology makes sense except in the light of the other” by Pelletier F in 2009 (69). The same is true for human neoplasm. I advance here that, “nothing in cancer evolution or ecology makes sense except in the light of the other”, both of cancer evolution and ecology are interdependent to each other and mutually promoting. In a word, the essence of NPC as well as other kinds of human neoplasms is “unity of ecology and evolution”.
NPC as invasive species and its metastasis as an ecological dispersal
Population dispersal generally means that some individuals leave the original habitat and spread out (70-74); for example, terrestrial birds living in an archipelago seek a suitable habitat among different islands. The diaspora of NPC shares several common features with the ecological concept of “diaspora”. NPC metastasis can be thought as a form of ecological dispersal owing to invasive species destroying the local ecosystem and setting up a base in distant organs through their proliferation and spread after entering the circulation to undergo jump dispersal. One of the critical characteristics of a diaspora is that dispersed populations retain a sense of their uniqueness that maintains collective memory of their original habitats (75,76). This is mainly reflected by the establishment of a new habitat, and maintaining a identity as the same as their original homelands. NPC patients tend to have cervical lymph node metastases at early stage, and metastasize to bones, lungs, livers and other organs after therapy (4-6). In most of samples, the microscopically histopathological type of metastatic sites is consistent with primary cancer cells, whereby pathologists can easily identify metastatic NPC that is originated from the original tumors. On the other side, most of molecular characteristics of metastatic sites and those of primary tumors show no significant difference in many pathological cases.
Another critical aspect to understand the feature of biological diaspora is that native environments and homeostasis always are disrupted by invasive species (77). Whether in the primary sites or metastatic areas, the external environment is generally hostile to the invaders. These malignant cells confront with many physical barriers before they can invade, survive and favor the new environment for further colonization and dissemination. Consistent with the “seed and soil” theory of metastasis proposed by Paget in 1889 (78), the best predictors of the success of ecological dispersal are those variables that whether cancer cells have enough ecological adaptive and reproductive ability (79-81). In the primary sites, for example, NPC cells can secrete matrix metalloproteinases that physically alter their environments (82-84), contribute to angiogenesis via vascular endothelial growth factor (VEGF) production (85-87), manipulate the content and functions of extracellular vesicles (EVs) (88-92) or change tight vascular capillary endothelial walls by inducing endothelial-mesenchymal transition (EnMT) (93) of the organ ecosystem. This is well reflected in the detection of magnetic resonance imaging (MRI) with extensive local infiltration of adjacent soft tissues, erosion of skull base, etc. If observing through the microscope, we can find that the normal tissue architecture (e.g. lymph nodes, vascular architecture) is destroyed by clusters of cancer cells.
Jump dispersal refers to the fast movement of population species over a long distance, and then the establishment of individual organisms at the destination, for instance, the spreading of Argentine ants throughout the United States (94-96). Tumor cell metastasis through the circulation is a great example of jump dispersal (78,79). In the ecological system of Nature, multiple organisms encounter different kinds of abiotic or biotic barriers on their journey, such as the dispersal of shallow-water marine species is hampered by stretches of deep ocean constitute barriers in the tropical Pacific. Likewise, CSCs also encounter such dispersal barriers during their translocation to distant organs/tissues. Such according process can be grouped roughly under the following points: 1) The diameter of CTCs is three to four times that of the capillary pores in distant organs. So they would be trapped there before extravasating from blood and lymphatic vessels (97,98); 2) In the circulation system, these CTCs confront with a rather harsh habitat completely different from the original sites, where they are susceptible to anoikis and mechanical stress, and vulnerablely attacked by immune cells such as NK cells in the circulating (99,100). The latest study demonstrates that platelet-derived RGS18 protects CTCs from NK-mediated immune surveillance by hijacking immune checkpoint HLA-E:CD94-NKG2A (101); 3) When cancer cells reach the distant sites, they have to adapt to the new microenvironment such as ECM components and the immune system seem to don't welcome their arrivals (102). These disseminated tumor cells (DTCs) need to construct the local conditions as “ecological engineers” to set up their habitat to assist the survival (79-81,103), that is, creating pre-metastatic niche (niche construction) such as immunosuppression, tumor-promoting inflammation, inducing angiogenesis and ECM reprogramming, and aerobic glycolysis producing an acidic local environment (104-106). For instance, they attract angiogenesis vasculature to ensure a supply of oxygen and nutrients, and switch energy metabolism to glycolysis for hypoxia adaptation (107,108). Therefore, only the most adaptable phenotypes of CTCs could be successful to root in distant organs. The dynamic ecology of DTCs and the pre-metastatic microenvironment can be “pre-metastatic ecosystem”.
Let's briefly have a look at some advances of CTCs research in NPC. The first report about the capture of CTCs in peripheral venous blood was identified by CK-19 positive cells in NPC (109). Our previous study indicated that dissociated NPC cells in the circulation exhibiting EMT/CSCs features were endowed with their metastatic potentials (61). CTCs can be used for tumor stage determination and prognosis prediction of NPC at all stages, and especially epithelial/mesenchymal (E/M) hybrid CTCs predict adverse outcomes (110,111). To be more exhilarating, a study showed that CTCs can be a novel more sensitive biomarker for minimal residual disease (MRD) of NPC patients (112). As yet, we should realize that the area of “circulating ecology” and “metastatic ecology” in NPC remains largely undisclosed, for example, what is the different immune-tumor ecological interactions in primary, circulation and metastatic lesions, it is worth of further investigation.
Dormancy is an organism's ability to enter a dormant state of low metabolic activity when facing with adverse environmental conditions (113-115), as such increases fitness by delaying the time in the habitat. Likewise, a phenomenon called "tumor dormancy" is very common in clinical practice, that is, disseminated cancer cells can seed in the host tissues for years before the local recurrences or metastases can be detected (116,117). Tumor dormancy is considered as a conserved evolutionary process related to adaptation and resistance to selective stress that is imposed by dissemination and the hard microenvironment within the target organs (118-121). Up to now, molecular mechanisms of cancer dormancy have been widely investigated. For example, Weeraratna AT et al. reported that aged lung microenvironment can facilitate a permissive niche and promote the efficient reactivation of dormant melanoma cells in the lung through WNT5A, AXL and MER activators (122). For the dormancy of NPC, a study from Chinese Taiwan reported the high susceptibility of primary NPC cells following transferred IFN-γgene to the induction of tumor dormancy (123). Three-dimensional culture studies suggest that the LTBP-2 contribute to tumor cell dormancy in a growth factor favorable microenvironment (124). However, we have to admit that the microenvironment signals regulating the dormancy of NPC still remain poorly understood by now, which might hamper our fully understanding of the complexity of the disease.
It is generally accept that the metastasis of NPC is a complex, multifactorial and multi-step process. However, for the spatial knowledge about the recurrence and metastasis of NPC, we may often stay in the simple “one-way migration” mind, that is one-way trip “A to B”. Can this progression be thought at least as double trip “A to B then to A”? A great challenge in clinical practice is that distant/recurrent metastasis contributes to the major reason for therapeutic failure and most of these cancers are still incurable (75,97,125), that's really the main question before us. This harsh fact might indicate that conventionally describing metastasis as a simple one-way migration of cells from the primary sites to target organs is too far behind to elucidating the complex progression of cancer. One remarkable feature of population dispersal is that emigrants dispersing to habitat patches connect with the host each other through exchange of individuals and information. Indeed, cancer cells can seed distant sites as well as the primary tumor itself, and this phenomenon is so-called "self-seeding” (126,127). The “self-seeding” demonstrates that metastasis is a bidirectional process whereby cancer cells can seed the primary tumor itself by CTCs. This metastatic hypothesis has been validated in experimental models of breast cancer, colon cancer and melanoma (128). Exploiting clinical investigation within a self-seeding model may offer new possibilities for eradicating metastatic cancer. Unfortunately, a view of "self-seeding" process of NPC has not yet been proposed before. Currently, management of local recurrent NPC remains one of the most difficult challenges (129), and the related factors remain largely unknown. Emerging studies have showed that the local residual cancer cells (i.e. CSCs) might be responsible for this problem. Do self-seeding of CTCs or metastatic tumor cells at distant sites also work, or can their releasing soluble factors such as exosomes, cytokines, chemokines and growth factors self-feedback? On the other side, for patients with metastasis, can these metastatic population cells cause a second metastasis in other organs? From the perspective of the human body as the entire biosphere, this entirely makes sense. Two case reports as below can give us more or less such hints. One case is that this patient carried liver metastasis three months after clinical complete response, and after Transcatheter Hepatic Artery Chemoembolization (TACE) treatment, the liver metastases maintained stable for six months, but lung metastases were found to be established (130). Another case with occipital lymph node metastasis developed multiple distant metastases in the sternum, left ilium, bilateral internal mammary lymph nodes and spleen, while the nasopharynx and related regions remained well controlled without sign of recurrence (131). Considering the therapeutic complexity of NPC, the multidirectional process might reshape our thinking of cancer metastasis, and offer alternative opportunities for prevention and therapy of recurrent/metastatic NPC. Meanwhile, it should be noticed that we have a long way to go, more convincing data needs to be validated through the integration of mathematical, experimental and animal models.
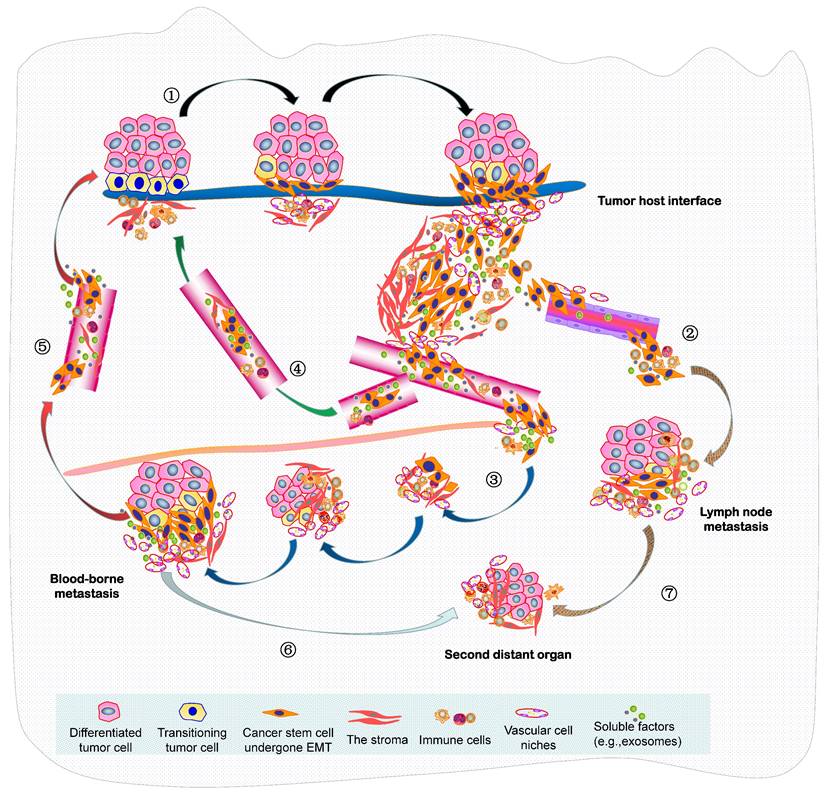
In summary, the seed and soil hypothesis indicates that the metastasis of NPC is analogous to the colonization of a new habitat, and this invasive and evolving progression should be better recognized as a form of ecological dispersal. Resulting from the increasingly limited space, high density, hypoxia and low nutrients within a tumor mass, this undoubtedly leads to fierce competition of cancer cells, there will then be selection for invasive species to facilitate the diaspora that can be seen as cell populations maximizing their average fitness. Such malignant progression is indeed “ecological invasion” (invasion ecology). In 2013, we constructed an integrated molecule-morphology model to elucidate the metastatic cascade of NPC (61). At the basis of it, a novel model about ecological dispersal of NPC progression is depicted here (Figure 2). The whole ecosystem of NPC can be classified into four interdependent parts: local primary ecosystem, circulating ecosystem, distant metastatic ecosystem and multidirectional ecosystem. I suggest that these classifications of ecosystems are not chaotic cancer cell masses, they could be dynamic cross-level organization in time-space manner. In order to promote survival rate and produce more offspring, various groups of tumor cells in the whole ecosystem interact (short- or medium- or long-range interactions) and communicate “intellectually” with each other and a complex microenvironment, they respond quickly to the stimulation of external factors and make the optimized decision through ecol-evolutionary pathways within cellular networks or intercellular networks. Their ecol-evolutionary adaptation courses are an exceptionally interesting area of future study.
Exploiting ecological principles to understand NPC progression
The population in ecology is defined as collection of individuals of the same species with unique characteristics coexisting at the specialized time and place (132). Population is an important unit of intertwined ecological and evolutionary process. Similarly, cancer cell population is considered as collection of subpopulations of cancer cells with different survival and reproductive abilities living together at regions of spatial heterogeneity within a tumor (37,133). The variation among individuals within a population is widespread. These differences are determined by a combination of specific genotypes and environmental influences. As described above, NPC is a complex ecological disease because these cancer cells are spatially and temporally heterogeneous, they continually interact and even evolve with each other and the microenvironment in order to increase the fitness of the cancer cells. In general, the malignant progression of NPC contains two parts of intraspecific relationship and interspecific relationship. The basic ecological principles to understand and manage this disease are exploited as below.
A novel ecological dispersal model of tumor multidirectional progression in NPC is proposed. During this process, ① NPC cells with CSCs characteristics undergo spindle-like phenotypes through EMT (mainly adapt to the selective pressure from the remodeling microenvironment) to dissociate from tumor-host interface (e.g. budding cells) and interplay with the various stroma components (local primary ecosystem); intravasate into the circulation (through either ② lymphangion or ③ blood vessels), survive the stresses of the circulating process, and extravasate to a metastatic site (lymph node or distant organ) (circulating ecosystem); enter slow-cycling states for dormancy, escape immune predation, engineer organ-specific niches to colonize micro/macro-metastases and later spread (distant metastatic ecosystem); Self-seeding of ④ CTCs or ⑤ metastatic tumor cells at distant sites, or their releasing soluble factors such as exosomes, cytokines and chemokines (self-feeding), or host cells include CAFs and immune cells (self-accomplice) return to primary tumor (multidirectional ecosystem) ; Additionally, metastatic cancer cells at ⑥ distant organ or ⑦ lymph node can produce the new populations in the second distant site.
Intraspecific relationship refers to the relationship between individuals within a population, which can be classified by the fitness effects of the individuals (neoplastic cells) on each other includes coexistence, competition, spatial behavior and communication behavior (45,134,135). As to specie coexistence, for example, the environment offers different types of foods or resources, and diet choice provides the most basic mechanism of coexistence. The ideal example of resource partitioning via diet choice hails from the Galapagos Islands. Different species of ground foraging Darwin's finches partition seeds according to the shape of the bird's beak and the size of the seed (132). Microscopically, the coexistence of two cell subtypes including DNKC and UDC is generally present in NPC tissues. In some cases, a certain content of spindle cells that might be the morphological characteristics of CSCs is also found. The cytokines secreted by cells within a tumor and abiotic environmental factors such as amino acids and trace nutrients provided through the blood consist of the diverse and heterogeneous pool of foods that could promote coexistence of different cell subtypes of this disease. Experimental observation under the microscope can find that the coexistence and proportion of these cancer cells are closely related to the surrounding environment such as the formation of tumor angiogenesis. The vascular niche is closely interacted with stem cell-like NPC at the invasive front (136), which has been proved to be crucial to tumor migration and metastasis by secretion of various angiocrine factors (104,137,138). All coins have two sides. As tumor cells divide, they can outstrip their blood supply, and create a hypoxia conditions, nutrient poor and acidic stagnant swamp rather than a healthy pond (139-141). The lysate of necrotic cells and the release of pro-inflammatory cytokines secreted by cancer cells combine to produce equal amounts of swamp gas, this model is so called toxic “cancer swamp” (141). The cancer swamp is exactly the environment in which cancer cells create a harsh habitat that will exert selective pressure and generate the adaptive clones with the abilities to migrate and metastasize. Interestingly, a novel imaging strategy was recently exploited for quantitative 3D evaluation of hypoxia heterogeneity in an orthotopic model of NPC (142).
Due to the diversity of natural environments, and the competition among individuals within the species, different populations can exhibit different spatial behaviors (143). In most situation, the populations are often distributed in groups. Commonly, collected distribution has greater resistance to adverse environment, meanwhile it also increases the competition among the individuals. Reproduction success rather than survival is the main criterion to measure biological adaptation of cancer populations. Competition for the limited space and resources deceases densities and population sizes of cancer cells. When it becomes harsh microenvironment like hypoxia and acidic conditions, better adapted populations will win the competition and grow in the invasive phenotypes (144,145). And that's clearly true here. Spatial behaviors of NPC microscopically are generally present with the format of “collective migration” including but not limited to the center of tumor mass displaying solid sheets, irregular islands or dyscohesive sheets. In contrast, collective movement of neoplastic cells at the tumor-host interface or in the stiffening stroma of NPC is often less ordered and has more mesenchyme-like phenotype.
Population communication is that an individual releases one or several stimulus signals, sends information, then another individual receives information and initiates specific behavior. Among them, chemical communication is a very common behavior for calling together with each other and acting together in groups. Intercellular communication through extracellular vesicles in cancer and evolutionary biology has been widely demonstrated (146-149). A recent report has found that EVs transferred from highly to poorly metastatic NPC cells could mediate cell-cell communication and enhance the metastatic potential of poorly metastatic cancer cells (150). In addition, an intriguing phenomenon is that cancer cells can move through an epithelial bridge, and seem to enable a new form of cell communication and migration (http://ki-galleries.mit.edu/2012/zani). Such communicating bridge was also observed among breast cancer clusters when I was working in Bissell MJ's lab at LBNL as visiting scholar. By on-top 3D cell culture, two separated cancer islands were found to be connected completely by the bridge (data not shown). It might be the novel communicating way for the direct transform and exchange of energy and message in cellular cancer ecosystem.
Interspecific relationship means that the interaction between the different species, which essentially includes interspecific competition, predation, parasitism, mutualism, etc. (10,132,151). These different ecological interactions can be found in various kinds of neoplasms including NPC, and that are key regulators of tumor ecosystem. Owing to technological advances, it has been clear that intercellular communication between cancer cells and stromal cells is established by extracellular vesicles including exosomes and ligand-receptor interactions, and other mechanisms involving cytokines and chemokines (152-154).
Predation is defined as one species attacks or kills another species and feeds on it. The former calls a predator, and the latter is a prey (135,151). Population predation might be best applicable to the interaction of neoplastic cells with the immune system. We must be aware that the research on immune cells of NPC has a long history. Since the 1970s, lots of efforts have been made to evaluate the expression levels of immune cells and their prognostic values (155-160) by immunohistological analysis, flow cytometry or other experimental methods in NPC, as well as the related molecular regulations (161-165). With the advancement of technologies such as single-cell transcriptomic analysis, the complex heterogeneity and dynamic landscape changes of tumor immune microenvironment (TIME) in NPC has been well described (32-36). For example, lasmacytoid dendritic cells (pDCs), CLEC9A+ DCs, natural killer (NK) cells, and plasma cells were significantly associated with improved survival outcomes in NPC, whereas enrichment of M2-polarized macrophages and LAMP3+ dendritic cells leads to immunotolerance, promoting tumor progression of NPC. The model of “hot” and “cold” NPC generally are classified on the basis of two functional subcategories of TIME cells: hot immune-infiltrated populations such as tumor-infiltrating lymphocytes (TILs), macrophages (M1-polarized), natural killer (NK) cells and mature dendritic cells (DCs), and cold immune-infiltrated cells includes myeloid-derived suppressor cells (MDSCs), mast cells, regulatory T cells (Tregs) and macrophages (M2-polarized). Therefore, predation by the systemic/local immune system is probably an important selective pressure for neoplastic cells in NPC, resulting in immune response. Tumor evolution also enables recruitment of inhibitory immune signals within the tumor stroma, which, in turn, leads to tumor immune escape. For instance, cancer cells are predated by M1 macrophages through phagocytosis; while M2-polarized phenotype (tumor-associated macrophages; TAMs) cooperates with NPC cells each other that encourages the interacted development of both populations (166). In general, the immune system can be converted from a predator into an accomplice (dual role) during cancer progression (167-169). Encouragingly, the exploration of NPC immune environment has advanced targeted immunotherapy of patients turning the TME from immunologically "cold" into "hot". Anti-PD-1/PD-L1 therapy is poised to advance standard for the treatment of recurrent or metastatic NPC (RM-NPC). Several randomized clinical trials in the first-line setting have displayed inspiring improvements in progression-free survival (PFS) with Anti-PD-1/PD-L1 therapy for the treatment of RM-NPC (170-173). In addition, various experimental and clinical studies have explored the use of cytotoxic T cells (CTLs), TILs, NK cells, and DCs in the treatment of NPC patients including refractory and locally advanced stages (174-178). Additionally, researchers have proposed reshaping the systemic tumor immune environment (STIE) to promote immunotherapy efficacy in human solid tumors including NPC (44). With the continuous research and the development of technique, the area of “NPC immunoecology” definitely provides the most promising strategy for the improving survival of patients in advanced stage.
Mutualism, it means that mutually beneficial interactions among individuals of different species (132,151). Sea anemones and hermit crabs is the mutually beneficial sample in nature. Sea anemones are fixed on the shells of hermit crabs to enable them to capture food more effectively; Conversely, sea anemones use toxic prickle cells to protect hermit crabs from natural enemies. The relationship between CAFs and neoplastic cancer cells within a tumor is the good case of mutualism, both of them can get a fitness advantage from the dynamic cooperation (37,179) and even co-evolve during the malignant progression (180-182). It has been well explained in many kinds of cancers, tumor cells strongly influence fibroblast population through the release of several growth factors such as fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), receptor tyrosine kinase (RTK) and reactive oxygen species (ROS), all of which contributing to fibroblast activation and proliferation (183,184). In turn, CAFs establish specific communications with tumor cells through secretion of various cytokines, chemokines, exosomes and other effector molecules to mediate the process of ECM remodeling such as increased tissue stiffness in order to promote cooperative mutualism as part of a collective strategy to benefit survival of both populations (185,186). In NPC, extracellular vesicle-packaged EBV-encoded LMP1 can activate normal fibroblasts into CAFs through the nuclear factor-kappa B (NF-kB) p65 pathway, and pre-metastatic niche is formed by activating CAFs in vivo (187). Another interesting report about the relation of mutualism in NPC is that exosomes released from EBV(+) NPC cells could activate YAP1/FAPα axis pathway to promote CAF-mediated characteristics includes fibrosis/remodeling of the ECM and tumor growth, meanwhile induce an immunosuppressive TME that benefits evading predation from immune system (188). On the other hand, the feed-forward loop between tumor cells and “cold ”immune cells is also important mutually beneficial relationship in NPC. One study showed that EBV+NPC cells can recruit monocytes by VEGF and generate a TAM-like phenotype by granulocyte-macrophage colony-stimulating factor (GM-CSF). In turn, TAM can induce EMT and promote NF-κB activation of tumor cells by CCL18 to enhance cancer metastasis (86). The dynamic crosstalk between cancer cells and tumor microenvironment including immune cells in NPC has been well summarized (8,16,189). To my view, such reciprocal interplay of NPC should be better essentially described as population “mutualism” in ecology. Mutualism is the remarkable feature of tumor interspecific relationship between cancer cells and stromal cells (here it means all noncancerous cells within the tumor). Through mutually beneficial exchanges with the extracellular environment and even their coevolution (an important connection between ecology and evolution) (180-182), all of these factors ultimately result in the greatest fitness, that is, maximize tumor survival and reproduction.
Reconstruction of the ECM environment extends to another interesting ecological issue (cancer/NPC stroma ecology). Morphological adaptation is one of the ecological features that populations have an exhibit to adapt to the living environment. For example, in order to accommodate the dry-heat desert environment, cactus plants can show obvious dryophytic characteristics such as the degradation of leaves to thorns. As a matter of fact, through a hybrid discrete-continuum (HDC) model, Anderson AR and the colleagues found that harsh microenvironment conditions (e.g. heterogenous ECM) could exert a dramatic selective force on the tumor, which ultimately grew as an invasive mass with finger-like margins (41). In the leading edge of NPC tissues surrounded by a abundance of stroma, spindle-like cells are prominently found, and often generate fingering edges (14,61,190). We suggest that it should be the more aggressive subtype in NPC, and could be a valuable morphological predictor for NPC concurrence and metastasis. Spindle cancer cells are a good example for the ecological adaptation of tumors. It could be that interactions between normal epithelial cells/tumor cells and the hard ECM environment could shape cancer cell morphological evolution for better-adapted phenotypes (191). A much better understanding of the special ecological mechanisms within different tissue context is required. The cell attractor model was initially constructed to explain the maintenance of normal cell phenotypes (192). The distinct cancer cell “types” (e.g. neoplastic spindle cells) observed in NPC tissues, or the CSCs-like (tumor-initiating) or metastatic states that can be thought as a cancer cell that has maximized its average fitness in NPC, would be such dynamical attractor states. Ernberg I et al. have demonstrated that the rare edge cells might represent cells transgressing the ridge between two basins (193). When the selective pressure of treatment is released after one treatment, the original population can be rebuilt by these temporarily resistant edge cells. Therefore, combination therapy specifically killing rare edge cells that are transiently drug-resistant may be necessary for human cancer including NPC.
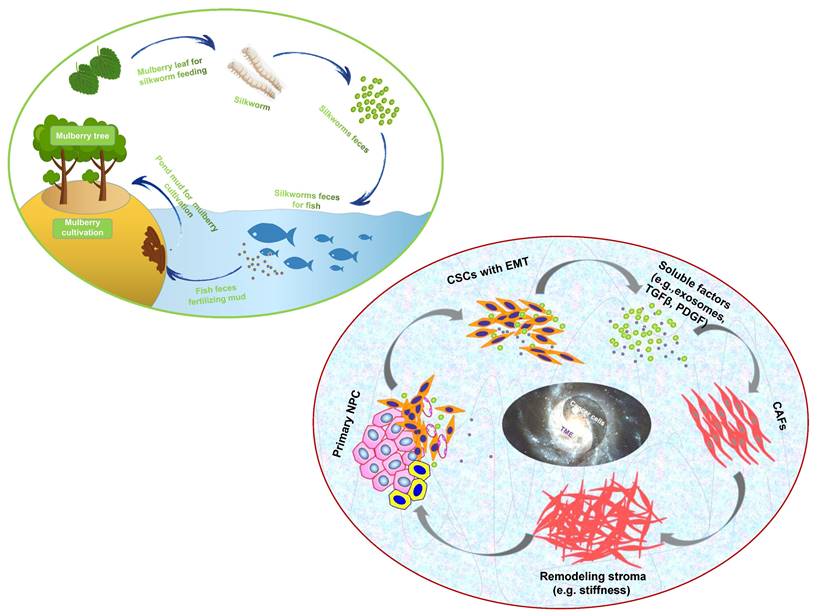
“Dynamic reciprocity” was firstly described in the early 1980's by Bissell MJ and her colleagues to explain the persistent biochemical and biophysical processes that occur between epithelial morphogenesis, breast cancer cells and the ECM (194). The mutualism relationship between these cancer cells and stromal components in NPC should also be recognized as “dynamic reciprocity”. A sustainable agriculture model that is very popular in southern China called “mulberry fish ponds” with a history of thousands of years (195). A special dike-pond mode means that the mulberry plants provide food and habitat for silkworm rearing; silkworms feces and any fallen and rotting mulberry can be used to fertilize fish ponds or as food for the fish; mud from the fish ponds is used for mulberry cultivation. The scientifically based man-made ecosystem - “mulberry-fish-ponds model” (Figure 3) could be a typical paradigm to elucidate the dramatic relationship of mutualism between cancer cells and the stromal cells such as immune cells, activated fibroblast, proliferating vascular cells, remodeling ECM and other abiotic factors (e.g. pH, metabolic conditions) in the whole cancer ecosystem. Once tissue-specific gene expression is altered through microenvironmental changes, affected cells may change their behaviors such as cell polarity and junctions, proliferation and invasion, no matter what they carry “cancer mutations” or not. In turn, cancer cells can produce an increasingly abnormal stressful microenvironment through the secretion of the abnormal ECM, and further lead to DNA mutations as a consequence of evolutionary adaptation. The solid evidence about dynamic reciprocity of cancer cell's morphological changes and stromal stiffness can be observed in the pathological specimens of NPC (61). What we can learn from the “mulberry fish ponds” model is that any disrupting in the dynamic interactions of cancer cells and its microenvironment would be an entry point for treatment approaches. Let's take an example. It has been proved that both of mechanical tissue stiffness and metabolic modulation affect directly each other to cause genome-wide changes in epigenetic regulatory states. ECM acts as a metabolic niche in tissue microenvironment, and its targeted therapeutic management might cause metabolic rewiring of tumor cells, and influence crosstalk between nonmalignant and malignant cell phenotypes in the metabolic ecosystem (196). As far as I know, it is the first time to introduce the mulberry-fish-ponds model to the area of cancer ecology.
The “mulberry-fish-ponds model” (top left) to elucidate the dynamic reciprocity of mutualism between (bottom right) cancer cells and TME (e.g. CAFs synthesizing ECM components such as collagen and FN contributes to stromal stiffness, which in turn promotes cancer progression) in cancer ecosystem. Cancer cells and TME work together to build “a community with a shared future” for tumor ecology (ecosystem).
Parasitism means that one species lives by parasitism in the body or on the surface of another species. Parasitic relationship is based on nutrition and spatial relationship. The adaptations of parasites to parasitic life are various, and at the same time, it will cause host immune response (132,151). The pathogenesis of NPC is closely associated with EBV infection of primary B cells, whereby parasitism is a remarkable characteristic among the interspecific relationships. As time goes by, the evolving cooperation of viral replication with primary B cells ultimately evolves into a mutually beneficial symbiotic relationship, and further promotes host genomic instability and derives somatic mutations and CNVs that are gradually accumulated in nasopharyngeal epithelial cells and often along with the enhancement of adaptative plasticity in response to the TME (6,197,198). Additionally, other pathogens with the parasite relationship including HPV, bacteria or mycetes have also been detected in NPC tissues (199,200). I would like to touch on briefly, the presence of tumor microbiota in NPC tissues was firstly reported in 2021 (201), and a retrospective cohort study has recently reported that intratumoral bacterial load is a value prognostic predictor for patients with NPC (202). Though the underlying impacts of bacteria on NPC cells has been carried out (203), more basic investigations and longitudinal clinical studies are expected to be performed in “NPC microecology” as well as other types of human neoplasms (204). We have reasons to believe that, following with the gradual understanding of the symbiosis relation of microbiota and NPC, as well as the mechanism of microorganisms in the occurrence and prognosis of this disease, and with the emerging development of small molecule microbiome modulator (SMMM), the research of NPC microecology will provide effective strategies for the prevention, diagnosis and treatment of NPC patients.
Population competition includes intraspecific competition and interspecific competition; the former refers to competition among individuals of the same species, and the latter means that competition among two or more species of organisms that are adversely affected by the use of common resources (46). For example, barnacles competing for space on rocks belongs to intraspecific competition, whereas the competition between Salvelinus malma and S. leucomaenis, Asterionella formosa and Synedra ulna fall inside interspecific competition (132). Cancer cell competition between tumor cells themself, or tumor cells and their microenvironment withing a tumor mass has been widely explored. Aggressive cancer cell populations achieve high fitness status and competitive abilities by displaying traits such as increased proliferation, de-differentiation and stemness governed by cell-competition genes P53, Myc and several signaling networks including the Warts/Hippo, PI3K/Akt/mTOR, JAK/STAT, and Wnt pathway (205,206). Spindle cells in NPC tissues having the EMT and stem cell-like features could be the more competitive cancer cell populations (14,61). In some cases of NPC biopsy, areas of coagulative necrosis are observed and can be extensive (12), which might be partly due to increased competition of cancer cells within a rapidly growing tumor encountering with the harsh conditions such as limited space, hypoxia and insufficient nutrient resources. Up to now, however, the competitive interactions and cellular/molecular competition pathways between tumor cell subpopulations or with the stromal tissues remain largely unexplored in NPC.
Heraclitus once said, "you cannot step into the same river twice”. From the above, we believe that the relationship between cancer cells and tumor microenvironment is not static, ecosystems themselves do not function in isolation. They grow and decline, transform, cooperate, select and co-evolve with each other in the whole pathological ecosystem spatially and temporally, just like the principle of “Yin-Yang theory”, which is created by the ancient Chinese people used by practitioner of Chinese medicine to explain the organizational structure, physiological function and pathological changes of the human body, and to guide the diagnosis and treatment of diseases.
Tumor-host interface as the ecological transition zone
Ecological transition zone (ecotone) is the transition zone along the edges of two adjacent ecological communities, the place where one ecological community meets another (132,151). Ecotone is a special area with the characteristics of adjacent communities and its own characteristics (207). It has several main characteristics, for example, a joint action and conversion area of various elements, with strong interaction and a greater biodiversity of species than either of the two separate ecosystems; the ecological environment is weak to resist interference and external forces; the ecological environment changes fast and has strong spatial dispersal ability and greater productivity. Accumulating studies have shown that tumor-stroma interface withing a tumor is the crucial part of the tumor (126). Its interface with the stromal components, also termed as the invasive tumor front (ITF), which is commonly defined as the three to six layers of caner cells at the invasive margin or the disseminated tumor groups between tumor and host tissue. The interaction of cancer interface with the surrounding stromal components attracts much attention in modern cancer biology (137, 208). In my standpoint, tumor-host interface could be considered as “ecological transition zone” where tumor cells meet so closely with the stromal components (Figure 4). This idea remains largely unexplored in the past decades.
Tumor-host interface shares the similar features with the ecological transition zone. Such is the case. Tumor-host interface has been proved to contain the most useful prognostic information for patients due to the acquisition of migratory and invasive features, secretion of proteolytic enzymes, reorganization of the extracellular matrix (209,210). Moreover, newly generated blood vessels, TAMs, CAFs, and other stromal cells are highly accumulated at the tumor-host interface (211,212). More importantly, such interface is a area inhabited by a particular species of highly resistant CSCs and the niches of TME (211). However, up to now, we don't know much about tumor-host interface in NPC. This concepts as well as "CSCs niche" and "migratory CSCs" were formally introduced in the research area of NPC (136,190). We found that stem cells-like features such as high expression of OCT4 and Nanog were more frequently located at the tumor-host interface, and correlated significantly with aggressive behaviors such as lymph node metastasis and distant metastasis of NPC patients (136). In addition, we indicates that cancer cells in the invasive front of NPC acquiring stem cell-like features should be maintained by vascular niches. Mathematics of tumor growth also shows that a tumor shaped like a solid sphere in which the stem-like cells are arranged at its surface only (126). Therefore, these findings imply that tumor cells localized within this region are more prone to promote adaptive responses to changes of the environment, thus evolve more capacity to promote drug resistance and tumor relapse. It has been found that tumor cells at tumor-host interface have transformed from an epithelial (low mobile/invasive potential) to a mesenchymal (high mobile/invasive potential) phenotype (EMT) (213). A single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head neck cancer showed that cells expressing the partial EMT (p-EMT) program spatially localized to the interface of primary tumors, and predict nodal metastasis (214). These p-EMT cells do not represent cloning regionalization of mutationally altered cells but represent epigenetically regulated plasticity of the fittest cells selected for invasive and malignant progression (42). As we have described, cancer cells are often present with spindle-like morphology at tumor-host interface of NPC. Moreover, EMT and hybrid E/M states such as membranous/cytoplasmic E-cadherin and nuclear vimentin and Snail, and CSCs marker ALDH1 are aberrantly expressed in the leading edge, all of these proteins are valuable predictors of poor survival in NPC (14,53,215-217). Similarly, one impressive report recently has also found that sarcomatoid carcinoma (SC) subtype (that is we call neoplastic spindle cells here) of NPC exhibits enriched EMT and invasion promoting genes, and significantly correlates with their morphological features (218). As may be inferred from this, these malignant cells in tumor-host interface exhibiting mesenchymal-like properties should be more likely to detach from the primary sites and diaspora at the early stage. Emerging evidence has showed that cancer cells with EMT properties induced by EBV-related factors such as LMP1, LMP2A and EBV-miR-BART7-3p can directly contribute to the metastatic progression of NPC in vitro and in vivo (54,65,219,220). In summary, tumor-host interface of NPC (cancer) representative of migratory/metastatic CSCs is the ideal battleground corresponds to a tumor microenvironment, and phenotypic plasticity change is one of the remarkable features of tumor-host interface area.
A close analogy of tumor-host interface and budding cells in NPC with the ecological nature. Land/sea transitional zone and isolated islands on earth (A,B). Tumor-host interface as the ecological transition zone, it often takes curved and finger-like shapes (C,D). Tumor buddings are similar to little islands separated from the continent (E, F). (A,B adapted from image.baidu; C-F adapted from our previous studies, Luo WR et al, (Ref.136,229) ).
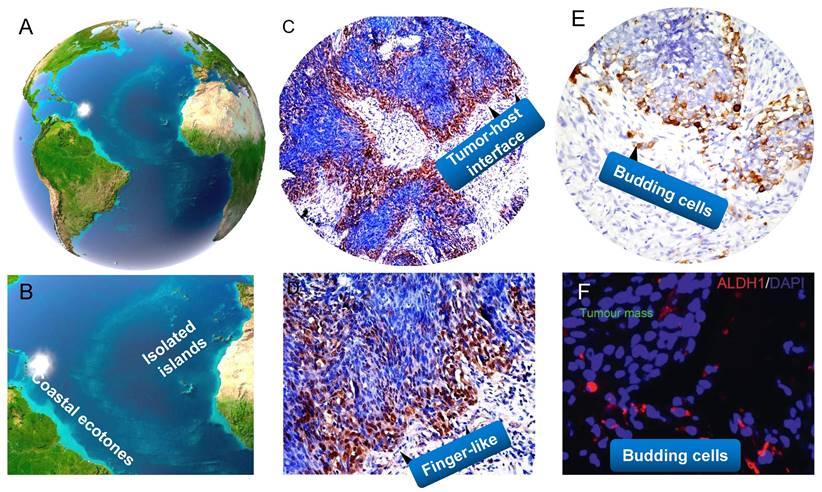
As described above, at tumor-host interface of tumor ecosystem, you can find that tumor‐promoting immune cells or vascular cells are particularly concentrated, and CSCs-like cancer cell populations with invasive features are specially adapted to the conditions of the transition zone. This interesting phenomenon is referred to the “edge effect” (151), which is an ecological concept that describes how there is a greater diversity and increased number of population species in the transition zone. The enhanced diversity and productivity deriving from the edge effect are commonly observed in the Nature (221,222). Wetland ecology and mangrove ecology (land/sea transitional zone) are typical representatives of the most highly productive natural systems. All of the resources such as water, humidity, air light and temperature, as well as the massive movement of nutrients and energy exchange and interact with each other in their interfaces. Indeed, tumor-host edge is such a battleground of fierce fighting in which there is repeated crosstalk between tumor cells and the stroma (e.g. stromal cells together with ECM and released factors from cancer cells such as exosomes, cytokines and growth factors), thus results in evolutionary plasticity of both cancer cells (e.g. changes in plasticity of cell junctions and EMT, transition from collective cell migration to individual cell migration) and the host environment (e.g. degradation, deposition and remodeling of ECM, alteration of ECM stiffness and porosity), and finally leads to invasive and metastatic progression of the tumor. It is therefore not difficult to see that a greater number of mutualism behaviors among the elements are present at the edges of cancer.
Nature does not like to be uniform. The meandering river, zigzag coastline, curved rivers and uneven surface are all its masterpieces. These diverse shape patterns of edges generate greater diversity of species and increase productivity (132,151). For example, the zigzag coastline of mainland increases the precipitation of the land, and diversity of nutrients and organisms; the meandering river flows through a wider area to irrigate more plants; the road of life is also as winding as a river. Similarly, the macrocosm pattern are reflected in structure of the human body (e.g. our blood circulation, intestines, or even inner mitochondrial membrane), for instance, curved blood capillary that blood can directly exchange nutrients and metabolites with tissues more effectively than if they are running in a straight line. As expected, when viewing at microscopic resolution, we can also see that the patterns of tumor-host interface in cancer tissues including NPC are not aligned, and they usually take winding, finger-like or other irregular shapes (14,136,215). I suppose that the adaptive potentials, such as sustaining proliferative signaling of tumor cells in the interface that serves as an ecological factor to the surroundings, are maximized by the curved patterns of shape, just like the finger-like "Pillars of Creation" in which a lot of newborn stars bred at its front. But then, another question is coming. Can we take the irregular phenotype of cancer cells back to normal? The answer is yes. The phenotypic reversion of cancer cells in 3D context actually has been well investigated by Bissell MJ's group (223,224). They found growth arrest and polarity restoration in disorganized breast cancer T4-2 cells in response to inhibition of β1-integrin. Another interesting study by Ewald AJ et al. in 2013 showed that, K14+ cells were enriched at the tumor-host interface leading collective invasion and lung metastasis in primary human breast tumors in 3D culture and in vivo. In contrast, knockdown of either K14 or p63 was sufficient to block the invasive protrusions of cancer cells subsequently with rounded cell borders (225). Deep investigating the molecular mechanisms of tumor-host interface is crucial for the development of “anti-ITF” ecological strategies in human cancers.
Tumor buddings as ecological islands separated from the continent
Tumor budding (TB) is an emerging prognostic biomarker in colorectal cancer (CRC) and other solid cancers (224-226). In particular, tumor budding has been listed as an additional prognostic factor in the 2017 UICC/AJCC TNM classification and is included in the 2019 WHO classification of tumors (227, 228). Our previous study firstly showed the presence of tumor buddings, and these cells migrated collectively or individually and correlated with aggressive tumor behaviour in NPC (229). Tumor buddings should be considered as ecological separated islands, and both of them shared the similar features. They have similar geographical locations, tumor buddings are generally defined as isolated single cancer cells or clusters of up to four cancer cells detaching from the tumor-stroma interface, and is thought to be the first step of tumor dissociation from the main tumor mass (226), whereas an isolated island is a small land separated from the mainland surrounded by water. More importantly, they all have important and unique features. The biological characteristics of isolation has a profound impact on the population and community living on the island (132). Physical distance and connectivity between the mainland and isolated areas might contribute to population dispersal and extinction rates. Just like isolated islands of low connectivity with the mainland, tumor buddings have the spatiotemporal isolation of areas and their specialized diversity. A recent review has postulated that the whole tumors are evolutionary island-like ecosystems that drive cancer cells' migration (230). It is noteworthy that tumor budding has been proved to be strongly associated with EMT property in a variety of cancers such as colon cancer, lung caner, pancreatic cancer and oral cancer (231-234). Photomicrograph of histologic specimens in NPC show that spindle-like morphology is frequently exhibited in tumor buds, and these cells possessed the EMT and CSCs characteristics in cancer tissues (61,229), which indicating that budding cells can acquire more invasive and unlimited proliferated abilities to approach the vascular architectures for intravasation and to seed the new populations. We demonstrate that tumor buddings could be valuable morphological predictors for NPC early invasion.
Therefore, the interaction between tumor buddings as a tumor-related factor and vascular cell, fibroblasts and immune cells as a host-related factor forms islands-like systems. Tumor buds can be seen as the heavy cavalry of cancer, and its ideal battleground is tumor-host interface (Figure 4). However, I have to say that, it is hard to distinguish budding cells from fibroblasts or other mesenchymal cells by haematoxylin and eosin (HE) staining or even pan-CK staining in NPC tissues, because these budding cells migrating the stroma commonly have a similar fibroblast-like phenotype, and the context is always complex with a dense infiltrate of lymphocytes and plasma cells. 3D cell culture and organiods might be ideal tools for better elucidating morphogenesis and ecological interrelations of tumor buds in NPC. A better understanding of the molecular and pathogenetic mechanisms disclosing tumor budding might contribute to the development of “anti-budding therapies” (226).
Selection driving factors and ecological therapy in NPC
Darwin's natural selection is an ecological process leading to adaptive evolution, which is occurred by various biotic and abiotic factors together in the complex habitats. The organisms constantly absorb substances from the habitat environment to meet their needs for survival and development, but is also changing the environment. In turn, the environment changes feed back into the organisms, and make the latter adapt to the new environmental changes (143). Any environmental factor (evolutionary driver) is the cause of natural selection, and differential survival and reproduction is the result for natural selection. The integration of ecological and evolutionary aspects holds promise for understanding evolution and metastatic progression of NPC, and the primary classification of selection factors is briefly discussed here.
The recognition of the importance of tumor microenvironment, ECM modification, niche construction and ecological relationships among cancer cells, stromal biotic/abiotic components has resulted in a promising cancer treatment so-called “ecological therapy”, which was initially proposed to for the therapeutic development of prostate cancer metastases (235). If we consider the context of darwinian evolution, the most efficient way to kill a species is to destroy its niche by altering the surrounding environment. The ecological “habitat” is an area inhabited by a particular species of animal, plant, or other type of organism (236,237). For example, an analogy to riparian ecosystems is drawn in human cancers, and tumor vascular network is thought to be a main cause of intratumoral heterogeneity (236). Habitat destruction such as hyperthermia, antiangiogenic therapy, pH alteration, matrix metalloproteinase inhibitors could lead to causes of species extinction, and it might be analogous cancer treatments (135). For example, we can unitize the principle of ecologic traps to induce cancer cells to a designate habitat where they can be better exposure for antigen presentation of cells of the immune system (76). Here I want to talk a little bit about the selection driving factor “heat”, which has been recognized to be a crucial microenvironmental and epigenetic factor as to biological development (238,239). A combination of cytotoxic drugs and hyperthermia have additive effects through the increase in ROS and subsequent DNA damage and apoptosis. Hyperthermia is especially effective in hypoxic and nutrient deprived areas of the tumor, and leads to an increased expression of immune checkpoint molecules (ICMs) when combined with radiotherapy. A model indicates that hyperthermia during the first radiotherapy fractions seems to be more effective in an artificial immune-tumor ecosystem (240). In the last few decades, hyperthermia (41-43°C) has been a successful treatment adjunct strategy in many types of cancer such as ovarian cancer, colorectal cancer, bladder cancer and breast cancer (241-245). A new study also showed that whole-body hyperthermia (WBH) combined with chemotherapy and IMR treatment can improve the 5-year overall survival (OS) of patients with advanced NPC (246). Recently, our results have revealed that hyperthermia alone or combination treatment of oridonin dramatically can increase the killing effect on NPC cells and CSCs-like population (247). Moreover, hyperthermia remarkably improves the sensitivity of NPC radiation-resistant cells and CSC-like cells to radiotherapy.
The early attempt of an ecologically-inspired approach known as “adaptive therapy” looks indeed very promising, in which cancer is treated by alternating different drugs that can suppress growth of resistant populations and avoid/delay drug resistance (45). Beyond that, other ecological approaches include adaptive immunotherapy targeting predation, oncolytic virus therapy as to parasitism and continuous pressure by metronomic therapy also provide promising potentials for the treatment of NPC patients.
Conclusions and perspectives
Gorky once said that there is no limit to the bold activities of science, nor should there be any limit. Over the past decades, a series of theories and results as to cancer ecology presented by a number of theory-minded cancer scientists have been put forward to explain carcinogenesis. As long ago as the early of 1960s, Smithers DW regarded cancer as a disease of organisation, in which “an abnormal cell may produce a clone of cells reacting abnormally with their environment, and environmental stress may disorganise the behaviour of cells within a sphere” (7). In the 1970s, Nowell PC published the pioneer work “the clonal evolution of tumor cell populations” (27). In the 1980s, the concept of a tumor as “an ecosystem” was proposed by Heppner GH (248,249). In the 1990s, Pienta K J et al. suggested that cancer can be viewed as “a complex adaptive system” (250), and firstly proposed the strategy “ecological therapy” for cancer in 2008 (235). Maley CC et al. demonstrated cancer as “an evolutionary and ecological process” in 2006 (37), and they classified the evolutionary and ecological features of neoplasms by Evo- and Eco-indices in 2017 (140). In 2018, Greaves M pointed out that “nothing in cancer makes sense except in the light of evolution” (18). In 2021, Massagué J et al. discussed the nature of metastasis-initiating cells (MIC) and their ecosystems for innovative treatments of metastatic cancers (251). At present, I declare that human cancer includes NPC is a multidimensional spatiotemporal “ecological and evolutionary unity” pathological ecosystem, “cancer is not a genetic disease but a disease with ecological and evolutionary unity”.
Treating cancer as “unity of ecology and evolution”, and application of evolutionary and ecological principles to cancer prevention and treatment should be deeply rooted in our mind. A comprehensive understanding of tumor ecology warrants further study although it has been gained increasing attention. In particular, nine overarching eco-evolutionary questions have been recently putted forward by Dujon AM et al. (252). Additionally, we should be aware that current study of “cancer ecology” is mainly limited to “general tumoriecology”, and the area attempts to apply the general principles and diverse models of ecology to the research of cancer ecology. Although they share the common “hallmarks of cancer”, tumors deriving from different systems and different sites should have their specific ecological and evolutionary properties. For example, distinct cancer sites such as pancreatic cancer, lung adenocarcinoma, glioblastoma and skin melanoma have different levels of tissue architectural heterogeneity, stroma to tumor ratios and fibrotic reaction (169); colorectal cancer and gastric cancer belong to the gastrointestinal neoplasms but reside in different microbiota niches; even incellular subsets of hematologic neoplasms such as chronic lymphocytic leukemia (CLL) and myelodysplastic syndromes (MDA) compete for marrow niche occupancy. Therefore, it is time for us to go deep into the systematic research of “system/organ tumoriecology”.
Ecological pathology: The "father of modern medicine” William Osler once said, “as is our pathology, so is our practice”. With the development of new theories and techniques, the research of various branches of pathology is so all-encompassing at present. What I want to emphasize here is that, we need a dynamic ecological view to truly understand and integrate human pathology, and it is essential to apply the ecological (-evolutionary) principles and approaches to study the etiology, pathogenesis, pathological changes and outcomes of human diseases. I collectively call this research subject as “ecological pathology”. Some inspiring explorations have been made in the branch of this field (253-256). For example, we should apply quantitative, spatially explicit methods from landscape ecology to define the heterogenous biological processes of cancer cells in clinical pathological samples (256). On the other side, we should study ecology of diseases from all of aspects of pathology such as histopathology, cytopathology, molecular pathology, ultrastructural pathology or computational and artificial intelligence (AI) pathology. According to the area of ecological oncology, the histopathology and related pathological techniques such as tissue clearing and 3D construction are important supports for its systematization and visualization (257). It is interesting to start from a pathological perspective to think about tumor ecology. For example, the ecological dynamics of tumor-host interface or budding cells across space and time, or a special pathological phenomenon or (sub)structure such as cell-in-cell/tunneling nanotubes/liquid-liquid phase separation (LLPS) (258-260), all of those are worthy of further ecological investigation. Not only that, this area should be jointed with emerging technologies in other medical subjects. Interestingly, computed tomographic (CT) imaging can identify intratumoral Darwinian dynamics before and during the treatment of cancer patients (261). Additionally, MRI can rapidly estimate intratumoral evolution of the molecular properties of cancer cells, which might be the equivalent of satellite images in landscape ecology (262). Such quantitative analysis of radiologic images for explicit measure of temporal and spatial evolutionary dynamics of cancer cells within their image-defied habitats may be termed as “ecological radiology”. Hence, it is hard to image what kind of sparks will the combination of ecological perspective of radiology-pathology bring about.
Multidimensional tumoriecology: Single-cell genomic, transcriptomic and epigenomic sequencing methods have grown rapidly over the past decade. These works are mainly depicted an interactive network among cancer cells, stromal cells, and immune cells in tumors. However, the complexity of ecosystem is far more than that, as described above. More importantly, disassociated tumor tissues have lost information about the crucial spatial location of cells in primary tissues by single-cell seq technology. We should remember that cancer is an eco-evolutionary process driven by the spatial feedback between evolving tumor cells and microenvironmentally temporal selection. In recent years, oncologists and biologists tried to uncover the ecosystem in several types of human cancer through single-molecule fluorescent in situ hybridization (smFISH), the RNAscope assay, and even by spatial transcriptomics (253). This “spatiotemporal tumoriecology” may lead to a comprehensive decoding of cancer and TME ecosystem in situ tissue samples. However, one of the technical challenges is that these current spatial omics-based images are still remained in the 2D histological stage that fails to possess the spatial organization and microenvironment of cancer tissues. It is hard for us to imagine the significance of studying landscape ecology without considering the interaction between spatial heterogeneity and ecological processes. Tumor is like a solid sphere rather than a planar (145). Just as Bissell MJ declared in 2017, we should “goodbye flat biology"-time for the 3rd and the 4th dimensions” (263). This is a critical point in the history of cancer research-we are at a crossroads, that is, an important juncture in combination of the perspective of spatial distribution, temporal variation of the populations, as well as a multi-dimensional perspective to comprehensively exploring of tumor ecology or understanding of the true colors of cancer (multidimensional spatiotemporal oncology/tumoriecology). With the integration of emerging approaches (264-269) such as the 3rd or 4th dimensions of the spatial omics and pathological tissue and cellular reconstruction, which could be a powerful platform for our understanding of the evolutionary and ecological process of human cancers. I would like to point out in particular that multidimension is a broad term here, it also includes cross-level of organization (gene, genome/epigenome/proteome/metabolome, organelle, cell, tissue, organ, system, organism, biosphere, etc.). Tumor ecosystem has the characteristics of hierarchical system in space. Cancer research has also been proposed to be viewed through the theory dimension, the systems dimension, the time dimension and the micro-/environment dimension (270). (Again, I think this holds for the study of cancer ecology).
Integrated tumoriecology: Tumor ecology is indeed a new interdisciplinary field, multidisciplinary fields such as physics, chemistry, computational modeling, biomechanics and ecology needs to be integrated (252). It has been proved that microbiomes/immune cells are important to the pathogenesis and prognostic outcome of patients, whereas the ecological and evolutionary roles of the microbiomes/immune cells, and how their eco-evolutionary dynamics determine host fitness in various cancers are still poorly understood. Therefore, investigators are encouraged to organize and develop the appropriate multidisciplinary team, for example, pathologists, modelers, ecologists, bioinformaticians, oncologists, geneticists, evolutionary ecologists, physicists, mathematicians, microbiologists and virologists, with the goal of integrating knowledge across disciplines to drive novel and comprehensive insights into the theoretical and experimental development of tumor ecology. A broad cross-disciplinary collaborations network in tumor ecology is formed between people engaged in scientific research because of their communications and collaborations in scientific researches, academic researches and practice, which is termed here as “integrated tumoriecology”. Ultimately, the integration of oncol-ecology will have a bright future in the greater benefit of patients.
In the end of this thesis, I advocate that the insight into the nature of NPC and according conceptual ideas can be generalized to all (cancer) cell types in our body. The role of DNA is not for reprogramming cells. The description such as “Cancer is a genetic disease” or “Cancer is caused by mutations in oncogenes” has overlooked an important fact that any cell behaviors is caused by numerous coordinated regulatory events that are heavily reliant on functional integration not only within single cells but also within tissues and the organism as a whole (270). The initiation and progression of human cancer (e.g. the status from the normal to diseased, early to late, local to diffuse), for example that we are familiar with, chronic cervicitis-cervical intraepithelial neoplasia (CIN)-micro-/invasive cervical cancer-metastasic cancer, acute/chronic viral hepatitis-early/late cirrhosis-primary/metastatic liver cancer and normal-polyp(adenoma)-different stages of colon cancer, which can be considered as an ecological disease, that is a multidimensional spatiotemporal ecological/-evolutionary process as a whole. In such an eco-pathological system with disorderedcellular tissue architecture, parenchymal cells dynamically interplay with their specific micro-/environments such as microorganisms, immune cells, stromal cells and abiotic factors (along with the communication of energy, message and matter), adapt to each other and even co-evolve in a cross-level manner. Emerging evidence reveals that microbiomes have an ecol-evolutionary role of modulating the functions of host innate immune responses in the development of human diseases including cancer (271,272). We can estimate optimistically, if regarding cancer as a pathological ecosystem, some newly arisen subjects and technologies, for example, applying the knowledge system and research methods of synthetic biology may be of great help with the damaged ecosystem such as the reconstruction/healing of diseased cellular niches, and further provide more promising treatment strategies for patients in the future (I'll refer to this as synthetic cancer ecology/synthetic ecological therapy). As indicated below, the cancer ecology tree is comprehensively constructed to show the complete frame and research interests of cancer ecology (Figure 5). The somatic mutation paradigm is still the mainstream in current cancer research, however, just like the famous cancer biologist Bissell MJ et al. said, the time has come for us to “rethink cancer” (270).
Abbreviations
NPC: nasopharyngeal carcinoma
EBV: epstein-Barr virus
DNKC: differentiated nonkeratinizing carcinoma
UDC: undifferentiated nonkeratinizing carcinoma
CSCs: cancer stem cells
LRC: label-retaining cell
SP: side population
ALDH1: aldehyde dehydrogenase 1
VEGF: vascular endothelial growth factor
EV: extracellular vesicles
ECM: extracellular matrix
EMT: epithelial-mesenchymal transition
p-EMT: partial EMT
EnMT: endothelial-mesenchymal transition
MRI: magnetic resonance imaging
DTCs: disseminated tumor cells
MRD: minimal residual disease
TACE: transcatheter hepatic artery chemoembolization
TIME: tumor immune microenvironment
pDCs: lasmacytoid dendritic cells
NK: natural killer
TILs: tumor-infiltrating lymphocytes
MDSCs: myeloid-derived suppressor cells
Tregs: regulatory T cells
TAMs: tumor-associated macrophages
CAFs: cancer-associated fibroblasts
CTLs: cytotoxic T cells
RM-NPC: recurrent or metastatic NPC
FGF: fibroblast growth factor
PDGF: platelet-derived growth factor
TGF-β: transforming growth factor-beta
RTK: receptor tyrosine kinase
ROS: reactive oxygen species
GM-CSF: granulocyte-macrophage colony-stimulating factor
NF-kB: nuclear factor-kappa B
HDC: hybrid discrete-continuum
ITF: invasive tumor front
TB: tumor buddings
CRC: colorectal cancer
HE: haematoxylin and eosin
WBH: whole-body hyperthermia
CLL: chronic lymphocytic leukemia
MDA: myelodysplastic syndromes
CT: computed tomographic
SmFISH: single-molecule fluorescent in situ hybridization
MIC: metastasis-initiating cells
SMMM: small molecule microbiome modulator
Acknowledgements
I am indebted to my family especially my father Dr. Shaowen Luo, his lifelong pursuit of medical knowledge and the holistic view about treating patients based on differentiation of syndromes in Chinese Medicine have deeply influenced me. I would like to extend my sincere gratitude to my mentors Prof. Yao KT (Academician of CAS) and Prof. Bissell MJ (Academician of AAS), their tireless pursuit of the exceptional science benefits me all my life.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81872202); Natural Science Foundation of Guangdong Province of China (Grant No. 2018A030313778), Natural Science Foundation of Shenzhen (Grant No. JCYJ20190809154603583, JCYJ20210324131210030).
Competing Interests
The authors have declared that no competing interest exists.
Human cancer ecology tree. The cancer ecology tree is unprecedentedly constructed to elucidate the framework and research direction of cancer ecology. The nature of human cancer includes NPC is an “ecological and evolutionary unity” disease.
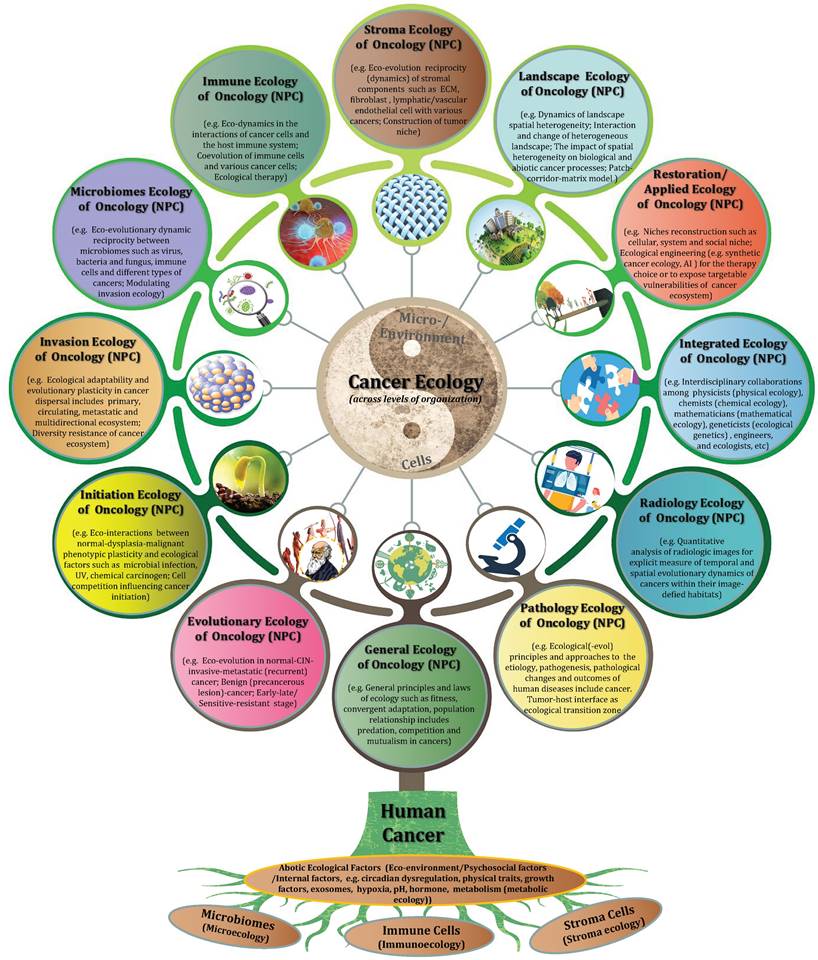
References
1. Trumper PA, Epstein MA, Giovanella VS. Epstein Barr virus and nasopharyngeal carcinoma. Lancet. 1976;1:686-7
2. Yao KT. Epidemiological characteristics and presumed carcinogenics of nasopharyngeal carcinoma in hunan province- based on 1973-1975 cancer mortality surgery. Bulletin of Hunan Medical College. 1982;7:10-7
3. Ou BX, Zeng Y. Etiology and Pathogenesis of Nasopharyngeal Carcinoma. People's Medical Publishing House. 1985
4. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-24
5. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64-80
6. Lo KW, Huang DP. Genetic and epigenetic changes in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:451-62
7. Smithers DW. Cancer, an attack on cytologism. Lancet. 1962;1:493-9
8. Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74
9. LIANG PC, CH'EN CC, CHU CC, HU YF, CHU HM, TSUNG YS. The histopathologic classification, biologic characteristics and histogenesis of nasopharyngeal carcinomas. Chin Med J. 1962;81:629-58
10. Su ZY, Siak PY, Leong CO, Cheah SC. Nasopharyngeal Carcinoma and Its Microenvironment: Past, Current, and Future Perspectives. Front Oncol. 2022;12:840467
11. Willis A. The ecosystem: an evolving concept viewed historically. Funct Ecol. 1997;11:268-71
12. Odum EP. Fundamentals of ecology. xii, 387 pp. W. B. Saunders Co, Philadelphia, Pennsylvania, and London, England. 1953
13. Zong YS, Wu QL, Liang XM, Liang YJ, He JH, Lin SX. Histological typing of primary nasopharyngeal carcinomas-a sum-up of experience from past 30 years. J Clin Exp Pathol. 2000;16:238-43
14. Luo WR, Chen XY, Li SY, Wu AB, Yao KT. Neoplastic spindle cells in nasopharyngeal carcinoma show features of epithelial-mesenchymal transition. Histopathology. 2012;61:113-22
15. Luo WR. Characterizing the Morphological and Immunohistochemical Features of Cancer Stem Cells and Their Clinical Implications in Nasopharyngeal Carcinoma. Guangzhou. Southern Medical University. 2012
16. Jiang J, Ying H. Revealing the crosstalk between nasopharyngeal carcinoma and immune cells in the tumor microenvironment. J Exp Clin Cancer Res. 2022;41:244
17. Dobzhansky T. Nothing in biology makes sense, except in the light of evolution. Amer Biol Teacher. 1973;35:125-9
18. Greaves M. Nothing in cancer makes sense except….BMC Biol. 2018;16:22
19. Wai Pak M, To KF, Lee JC, Liang EY, van Hasselt CA. In vivo real-time diagnosis of nasopharyngeal carcinoma in situ by contact rhinoscopy. Head Neck. 2005;27:1008-13
20. Shao X, He Z, Chen Z, Yao K. Expression of an Epstein-Barr-virus receptor and Epstein-Barr-virus-dependent transformation of human nasopharyngeal epithelial cells. Int J Cancer. 1997;71:750-5
21. Zhang H, Li Y, Wang HB, Zhang A, Chen ML, Fang ZX. et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat Microbiol. 2018;3:1-8
22. Brooks L, Yao QY, Rickinson AB, Young LS. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689-97
23. Xie L, Shi F, Li Y, Li W, Yu X, Zhao L. et al. Drp1-dependent remodeling of mitochondrial morphology triggered by EBV-LMP1 increases cisplatin resistance. Signal Transduct Target Ther. 2020;5:56
24. Fogg MH, Wirth LJ, Posner M, Wang F. Decreased EBNA-1-specific CD8+ T cells in patients with Epstein-Barr virus-associated nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 2009;106:3318-23
25. Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol. 2012;22:144-53
26. Balady GJ. Survival of the fittest-more evidence. N Engl J Med. 2002;346:852-54
27. Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23-8
28. Kyrochristos ID, Ziogas DE, Roukos DH. Drug resistance: origins, evolution and characterization of genomic clones and the tumor ecosystem to optimize precise individualized therapy. Drug Discov Today. 2019;24:1281-94
29. Fang W, Li X, Jiang Q, Liu Z, Yang H, Wang S. et al. Transcriptional patterns, biomarkers and pathways characterizing nasopharyngeal carcinoma of Southern China. J Transl Med. 2008;6:32
30. Bei JX, Li Y, Jia WH, Feng BJ, Zhou G, Chen LZ. et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42:599-603
31. Lin DC, Meng X, Hazawa M, Nagata Y, Varela AM, Xu L. et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet. 2014;46:866-71
32. Jin S, Li R, Chen MY, Yu C, Tang LQ, Liu YM. et al. Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res. 2020;30:950-65
33. Liu Y, He S, Wang XL, Peng W, Chen QY, Chi DM. et al. Tumour heterogeneity and intercellular networks of nasopharyngeal carcinoma at single cell resolution. Nat Commun. 2021;12:741
34. Gong L, Kwong DL, Dai W, Wu P, Li S, Yan Q. et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat Commun. 2021;12:1540
35. Chen YP, Yin JH, Li WF, Li HJ, Chen DP, Zhang CJ. et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity andimmune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 2020;30:1024-42
36. Peng WS, Zhou X, Yan WB, Li YJ, Du CR, Wang XS. et al. Dissecting the heterogeneity of the microenvironment in primary and recurrent nasopharyngeal carcinomas using single-cell RNA sequencing. Oncoimmunology. 2022;11:2026583
37. Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924-35
38. Axelrod R, Axelrod DE, Pienta KJ. Evolution of cooperation among tumor cells. Proc Natl Acad Sci U S A. 2006;103:13474-9
39. Tauriello DVF, Batlle E. Targeting the Microenvironment in Advanced Colorectal Cancer. Trends Cancer. 2016;2:495-504
40. Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346-54
41. Frankenstein Z, Basanta D, Franco OE, Gao Y, Javier RA, Strand DW. et al. Stromal reactivity differentially drives tumour cell evolution and prostate cancer progression. Nat Ecol Evol. 2020;4:870-84
42. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31-46
43. Ka-Yue Chow L, Lai-Shun Chung D, Tao L, Chan KF, Tung SY. et al. Epigenomic landscape study reveals molecular subtypes and EBV-associated regulatory epigenome reprogramming in nasopharyngeal carcinoma. EBioMedicine. 2022;86:104357
44. Xu L, Zou C, Zhang S, Chu TSM, Zhang Y, Chen W. et al. Reshaping the systemic tumor immune environment (STIE) and tumor immune microenvironment (TIME) to enhance immunotherapy efficacy in solid tumors. J Hematol Oncol. 2022;15:87
45. Enriquez-Navas PM, Wojtkowiak JW, Gatenby RA. Application of Evolutionary Principles to Cancer Therapy. Cancer Res. 2015;75:4675-80
46. Reynolds BA, Oli MW, Oli MK. Eco-oncology: Applying ecological principles to understand and manage cancer. Ecol Evol. 2020;10:8538-53
47. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983-8
48. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124-34
49. Prager BC, Xie Q, Bao S, Rich JN. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell. 2019;24:41-53
50. Lun SW, Cheung ST, Lo KW. Cancer stem-like cells in Epstein-Barr virus-associated nasopharyngeal carcinoma. Chin J Cancer. 2014;33:529-38
51. Zhang HB, Ren CP, Yang XY, Wang L, Li H, Zhao M. et al. Identification of label-retaining cells in nasopharyngeal epithelia and nasopharyngeal carcinoma tissues. Histochem Cell Biol. 2007;127:347-54
52. Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716-24
53. Wu A, Luo W, Zhang Q, Yang Z, Zhang G, Li S. et al. Aldehyde dehydrogenase 1, a functional marker for identifying cancer stem cells in human nasopharyngeal carcinoma. Cancer Lett. 2013;330:181-9
54. Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y. et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog. 2010;6:e1000940
55. Luo W, Gao F, Li S, Liu L. FoxM1 Promotes Cell Proliferation, Invasion, and Stem Cell Properties in Nasopharyngeal Carcinoma. Front Oncol. 2018;8:483
56. GREGOR JW. The Ecotype. Biol Rev. 1944;19:20-30
57. Gaither MR, Gkafas GA, de Jong M, Sarigol F, Neat F, Regnier T. et al. Genomics of habitat choice and adaptive evolution in a deep-sea fish. Nat Ecol Evol. 2018;2:680-7
58. Gavrilets S, Vose A, Barluenga M, Salzburger W, Meyer A. Case studies and mathematical models of ecological speciation. 1. Cichlids in a crater lake. Mol Ecol. 2007;16:2893-909
59. Gavrilets S, Vose A. Case studies and mathematical models of ecological speciation. 2. Palms on an oceanic island. Mol Ecol. 2007;16:2910-21
60. Duenez-Guzman EA, Mavárez J, Vose MD, Gavrilets S. Case studies and mathematical models of ecological speciation. 4. Hybrid speciation in butterflies in a jungle. Evolution. 2009;63:2611-26
61. Luo W, Yao K. Molecular characterization and clinical implications of spindle cells in nasopharyngeal carcinoma: a novel molecule-morphology model of tumor progression proposed. PLoS One. 2013;8:e83135
62. Wang HB, Lin YC, Zeng D, Lin W, Hong CQ, Lin WZ. et al. [Inhibitory effect of ginsenoside Rg3 on the tube-like structure formation in human nasopharyngeal carcinoma HNE-1 cell line in vitro]. Zhonghua Zhong Liu Za Zhi. 2010;32:739-42
63. Xiang T, Lin YX, Ma W, Zhang HJ, Chen KM, He GP. et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat Commun. 2018;9:5009
64. Jiang X, Deng X, Wang J, Mo Y, Shi L, Wei F. et al. BPIFB1 inhibits vasculogenic mimicry via downregulation of GLUT1-mediated H3K27 acetylation in nasopharyngeal carcinoma. Oncogene. 2022;41:233-45
65. Zhu N, Xu X, Wang Y, Zeng MS, Yuan Y. EBV latent membrane proteins promote hybrid epithelialmesenchymal and extreme mesenchymal states of nasopharyngeal carcinoma cells for tumorigenicity. PLoS Pathog. 2021;17:e1009873
66. Chuang WY, Chang SH, Yu WH, Yang CK, Yeh CJ, Ueng SH. et al. Successful Identification of Nasopharyngeal Carcinoma in Nasopharyngeal Biopsies Using Deep Learning. Cancers (Basel). 2020;12:507
67. Diao S, Luo W, Hou J, Lambo R, AL-kuhali HA, Zhao H. et al. Deep Multi-Magnification Similarity Learning for Histopathological Image Classification. IEEE J Biomed Health Inform. 2023 DOI: 10.1109/JBHI.2023.3237137
68. Diao S, Hou J, Yu H, Zhao X, Sun Y, Lambo RL. et al. Computer-Aided Pathologic Diagnosis of Nasopharyngeal Carcinoma Based on Deep Learning. Am J Pathol. 2020;190:1691-700
69. Pelletier F, Garant D, Hendry AP. Eco-evolutionary dynamics. Philos Trans R Soc Lond B Biol Sci. 2009;364:1483-9
70. Butler KD. Defining diaspora, refining a discourse. Diaspora: A Journal of Transnational Studies. 2001;10:189-219
71. Alzate A, Onstein RE. Understanding the relationship between dispersal and range size. Ecol Lett. 2022;25:2303-23
72. Lester SE, Ruttenberg BI, Gaines SD, Kinlan BP. The relationship between dispersal ability and geographic range size. Ecol Lett. 2007;10:745-58
73. Cohen R. The diaspora of a diaspora: the case of the caribbean. Social Sci Informat. 1992;31:159-69
74. Shuval JT. Diaspora migration: Definitional ambiguities and a theoretical paradigm. Int Migr. 2000;38:42-57
75. Weiss F, Lauffenburger D, Friedl P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat Rev Cancer. 2022;22:157-73
76. Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453-8
77. Sakai AK. et al. The population biology of invasive species. Annu Rev Ecol Syst. 2001;32:305-32
78. Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571-3
79. Pienta KJ, Robertson BA, Coffey DS, Taichman RS. The cancer diaspora: Metastasis beyond the seed and soil hypothesis. Clin Cancer Res. 2013;19:5849-55
80. Yang KR, Mooney SM, Zarif JC, Coffey DS, Taichman RS, Pienta KJ. Niche inheritance: a cooperative pathway to enhance cancer cell fitness through ecosystem engineering. J Cell Biochem. 2014;115:1478-85
81. Noorbakhsh J, Zhao ZM, Russell JC, Chuang JH. Treating Cancer as an Invasive Species. Mol Cancer Res. 2020;18:20-6
82. Yoshizaki T, Sato H, Furukawa M, Pagano JS. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci U S A. 1998;95:3621-6
83. Lan YY, Hsiao JR, Chang KC, Chang JS, Chen CW, Lai HC. et al. Epstein-Barr virus latent membrane protein 2A promotes invasion of nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction of matrix metalloproteinase 9. J Virol. 2012;86:6656-67
84. Li S, Luo W. Matrix metalloproteinase 2 contributes to aggressive phenotype, epithelial-mesenchymal transition and poor outcome in nasopharyngeal carcinoma. Onco Targets Ther. 2019;12:5701-11
85. Murono S, Inoue H, Tanabe T, Joab I, Yoshizaki T, Furukawa M. et al. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc Natl Acad Sci U S A. 2001;98:6905-10
86. Huang D, Song SJ, Wu ZZ, Wu W, Cui XY, Chen JN. et al. Epstein-Barr Virus-Induced VEGF and GM-CSF Drive Nasopharyngeal Carcinoma Metastasis via Recruitment and Activation of Macrophages. Cancer Res. 2017;77:3591-604
87. Li DK, Chen XR, Wang LN, Wang JH, Wen YT, Zhou ZY. et al. Epstein-Barr Virus Induces Lymphangiogenesis and Lympth Node Metastasis via Upregulation of VEGF-C in Nasopharyngeal Carcinoma. Mol Cancer Res. 2022;20:161-75
88. Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N. et al. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613-22
89. Mrizak D, Martin N, Barjon C, Jimenez-Pailhes AS, Mustapha R, Niki T. et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2014;107:363
90. Li F, Zhao X, Sun R, Ou J, Huang J, Yang N. et al. EGFR-rich extracellular vesicles derived from highly metastatic nasopharyngeal carcinoma cells accelerate tumour metastasis through PI3K/AKT pathway-suppressed ROS. J Extracell Vesicles. 2020;10:e12003
91. Nkosi D, Sun L, Duke LC, Meckes DG Jr. Epstein-Barr virus LMP1 manipulates the content and functions of extracellular vesicles to enhance metastatic potential of recipient cells. PLoS Pathog. 2020;16:e1009023
92. Lee PJ, Sui YH, Liu TT, Tsang NM, Huang CH, Lin TY. et al. Epstein-Barr viral product-containing exosomes promote fibrosis and nasopharyngeal carcinoma progression through activation of YAP1/FAPα signaling in fibroblasts. J Exp Clin Cancer Res. 2022;41:254
93. Li DK, Chen XR, Wang LN, Wang JH, Li JK, Zhou ZY. et al. Exosomal HMGA2 protein from EBV-positive NPC cells destroys vascular endothelial barriers and induces endothelial-to-mesenchymal transition to promote metastasis. Cancer Gene Ther. 2022;29:1439-51
94. Suarez AV, Holway DA, Case TJ. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc Natl Acad Sci U S A. 2001;98:1095-100
95. Kinlan BP, Gaines SD. Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology. 2003;84:2007-20
96. Cowen RK, Sponaugle S. Larval dispersal and marine population connectivity. Ann Rev Mar Sci. 2009;1:443-66
97. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559-64
98. Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science. 2013;341:1186-8
99. Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110-22
100. Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW. et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178-85
101. Liu X, Song J, Zhang H, Liu X, Zuo F, Zhao Y. et al. Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. 2023;41:272-287.e9
102. Strilic B, Offermanns S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell. 2017;32:282-93
103. Zhong H, Lambers H, Wong WS, Dixon KW, Stevens JC, Cross AT. Initiating pedogenesis of magnetite tailings using Lupinus angustifolius (narrow-leaf lupin) as an ecological engineer to promote native plant establishment. Sci Total Environ. 2021;788:147622
104. Kaplan RN, Rafii S, Lyden D. Preparing the "soil": the premetastatic niche. Cancer Res. 2006;66:11089-93
105. Sceneay J, Smyth MJ, Möller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449-64
106. Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell. 2016;30:668-81
107. Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer. 2020;1:672-80
108. Klotz R, Thomas A, Teng T, Han SM, Iriondo O, Li L. et al. Circulating Tumor Cells Exhibit Metastatic Tropism and Reveal Brain Metastasis Drivers. Cancer Discov. 2020;10:86-103
109. Lin JC, Tsai CS, Wang WY, Jan JS. Detection of circulating tumor cells in venous blood of nasopharyngeal carcinoma patients by nested reverse transcriptase-polymerase chain reaction. Kaohsiung J Med Sci. 2000;16:1-8
110. Wei JZ, Deng WM, Weng JJ, Li M, Lan GP, Li X. et al. Epithelial-mesenchymal transition classification of circulating tumor cells predicts clinical outcomes in progressive nasopharyngeal carcinoma. Front Oncol. 2022;12:988458
111. You R, Liu YP, Lin M, Huang PY, Tang LQ, Zhang YN. et al. Relationship of circulating tumor cells and Epstein-Barr virus DNA to progression-free survival and overall survival in metastatic nasopharyngeal carcinoma patients. Int J Cancer. 2019;145:2873-83
112. Ko JM, Vardhanabhuti V, Ng WT, Lam KO, Ngan RK, Kwong DL. et al. Clinical utility of serial analysis of circulating tumour cells for detection of minimal residual disease of metastatic nasopharyngeal carcinoma. Br J Cancer. 2020;123:114-25
113. Lennon JT, den Hollander F, Wilke-Berenguer M, Blath J. Principles of seed banks and the emergence of complexity from dormancy. Nat Commun. 2021;12:4807
114. Lennon JT, Jones SE. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat Rev Microbiol. 2011;9:119-30
115. Rees M. Evolutionary Ecology of Seed Dormancy and Seed Size. Phil Trans R Soc Lond. 1996;351:1299-308
116. Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611-22
117. Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer. 2020;20:398-411
118. Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834-46
119. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306-13
120. Scott JG, Hjelmeland AB, Chinnaiyan P, Anderson AR, Basanta D. Microenvironmental variables must influence intrinsic phenotypic parameters of cancer stem cells to affect tumourigenicity. PLoS Comput Biol. 2014;10:e1003433
121. Bakhshandeh S, Werner C, Fratzl P, Cipitria A. Microenvironment-mediated cancer dormancy: Insights from metastability theory. Proc Natl Acad Sci U S A. 2022;119:e2111046118
122. Fane ME, Chhabra Y, Alicea GM, Maranto DA, Douglass SM, Webster MR. et al. Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature. 2022;606:396-405
123. Chou AS, Chen HC, Li CR, Hsieh CH, Ting LL, Liao SK. Tumor dormancy resulting from subcutaneous injection to SCID mice with cultured nasopharyngeal carcinoma cells is mediated via IFN-γ induction of a highly differentiated phenotype. Cancer Biother Radiopharm. 2011;26:417-26
124. Chen H, Ko JM, Wong VC, Hyytiainen M, Keski-Oja J, Chua D. et al. LTBP-2 confers pleiotropic suppression and promotes dormancy in a growth factor permissive microenvironment in nasopharyngeal carcinoma. Cancer Lett. 2012;325:89-98
125. Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27:34-44
126. Comen E, Norton L, Massagué J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369-77
127. Leung CT, Brugge JS. Tumor self-seeding: bidirectional flow of tumor cells. Cell. 2009;139:1226-8
128. Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L. et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315-26
129. Lee AW, Fee WE Jr, Ng WT, Chan LK. Nasopharyngeal carcinoma: salvage of local recurrence. Oral Oncol. 2012;48:768-74
130. Liu Q, Wang J, Sun C, Xu J. The diagnosis and management of rare cystic liver metastases from nasopharyngeal carcinoma: A case report. Medicine (Baltimore). 2018;97:e11257
131. Yang J, Xia WX, Xiang YQ, Lv X, Ke LR, Yu YH. et al. Occipital lymph node metastasis from nasopharyngeal carcinoma: a special case report and literature review. Chin J Cancer. 2016;35:1
132. Begon M, Townsend CR, Harper JL. Ecology: From Individuals to Ecosystems (Fourth Edtion). Wiley Blackwell. 2006
133. Kenny PA, Nelson CM, Bissell MJ. The Ecology of Tumors: By perturbing the microenvironment, wounds and infection may be key to tumor development. Scientist. 2006;20:30
134. Basanta D, Anderson AR. Exploiting ecological principles to better understand cancer progression and treatment. Interface Focus. 2013;3:20130020
135. Walther V, Hiley CT, Shibata D, Swanton C, Turner PE, Maley CC. Can oncology recapitulate paleontology? Lessons from species extinctions. Nat Rev Clin Oncol. 2015;12:273-85
136. Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS One. 2013;8:e56324
137. Christofori G. New signals from the invasive front. Nature. 2006;441:444-50
138. Borovski T, De Sousa E Melo F, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634-9
139. Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437-43
140. Maley CC, Aktipis A, Graham TA, Sottoriva A, Boddy AM, Janiszewska M. et al. Classifying the evolutionary and ecological features of neoplasms. Nat Rev Cancer. 2017;17:605-19
141. Amend SR, Pienta KJ. Ecology meets cancer biology: the cancer swamp promotes the lethal cancer phenotype. Oncotarget. 2015;6:9669-78
142. Huang W, Wang K, An Y, Meng H, Gao Y, Xiong Z. et al. In vivo three-dimensional evaluation of tumour hypoxia in nasopharyngeal carcinomas using FMT-CT and MSOT. Eur J Nucl Med Mol Imaging. 2020;47:1027-38
143. Ma SJ. The ecological adaptation of insect population. Acta Ecologica Sinica. 1982;9:225-7
144. Rohani N, Hao L, Alexis MS, Joughin BA, Krismer K, Moufarrej MN. et al. Acidification of Tumor at Stromal Boundaries Drives Transcriptome Alterations Associated with Aggressive Phenotypes. Cancer Res. 2019;79:1952-66
145. Anderson AR, Quaranta V. Integrative mathematical oncology. Nat Rev Cancer. 2008;8:227-34
146. Möller A, Lobb RJ. The evolving translational potential of small extracellular vesicles in cancer. Nat Rev Cancer. 2020;20:697-709
147. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617-38
148. Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E. et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046-57
149. Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226-32
150. Li F, Zhao X, Sun R, Ou J, Huang J, Yang N. et al. EGFR-rich extracellular vesicles derived from highly metastatic nasopharyngeal carcinoma cells accelerate tumour metastasis through PI3K/AKT pathway-suppressed ROS. J Extracell Vesicles. 2020;10:e12003
151. Lin YZ, Fu RS. Ecology (2nd ). Science Press. 2011
152. Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126:1139-43
153. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887-99
154. Stuelten CH, Parent CA, Montell DJ. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat Rev Cancer. 2018;18:296-312
155. Jondal M, Klein G. Classification of lymphocytes in nasopharyngeal carcinoma (NPC) biopsies. Biomedicine. 1975;23:163-5
156. Galili U, Klein E, Klein G, Singh Bal I. Activated T lymphocytes in infiltrates and draining lymph nodes of nasopharyngeal carcinoma. Int J Cancer. 1980;25:85-9
157. Huang PS, Shen YY. [Immunohistochemical and cytochemical analysis of lymphocytes and plasma cells in nasopharyngeal carcinoma]. Zhonghua Zhong Liu Za Zhi. 1986;8:187-9
158. Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT. et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23:1393-403
159. Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML. et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462-73
160. Lu J, Chen XM, Huang HR, Zhao FP, Wang F, Liu X. et al. Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck. 2018;40:1245-53
161. Yuling H, Ruijing X, Li L, Xiang J, Rui Z, Yujuan W. et al. EBV-induced human CD8+ NKT cells suppress tumorigenesis by EBV-associated malignancies. Cancer Res. 2009;69:7935-44
162. Mrizak D, Martin N, Barjon C, Jimenez-Pailhes AS, Mustapha R, Niki T. et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2014;107:363
163. Wu TS, Wang LC, Liu SC, Hsu TY, Lin CY, Feng GJ. et al. EBV oncogene N-LMP1 induces CD4 T cell-mediated angiogenic blockade in the murine tumor model. J Immunol. 2015;194:4577-87
164. Guo X, Zheng H, Luo W, Zhang Q, Liu J, Yao K. 5T4-specific chimeric antigen receptor modification promotes the immune efficacy of cytokine-induced killer cells against nasopharyngeal carcinoma stem cell-like cells. Sci Rep. 2017;7:4859
165. Huo S, Luo Y, Deng R, Liu X, Wang J, Wang L. et al. EBV-EBNA1 constructs an immunosuppressive microenvironment for nasopharyngeal carcinoma by promoting the chemoattraction of Treg cells. J Immunother Cancer. 2020;8:e001588
166. Wang Y, Sun Q, Ye Y, Sun X, Xie S, Zhan Y. et al. FGF-2 signaling in nasopharyngeal carcinoma modulates pericyte-macrophage crosstalk and metastasis. JCI Insight. 2022;7:e157874
167. Alfonso JCL, Grass GD, Welsh E, Ahmed KA, Teer JK, Pilon-Thomas S. et al. Tumor-immune ecosystem dynamics define an individual Radiation Immune Score to predict pan-cancer radiocurability. Neoplasia. 2021;23:1110-22
168. Kalaora S, Nagler A, Wargo JA, Samuels Y. Mechanisms of immune activation and regulation: lessons from melanoma. Nat Rev Cancer. 2022;22:195-207
169. Salmon H, Remark R, Gnjatic S, Merad M. Host tissue determinants of tumour immunity. Nat Rev Cancer. 2019;19:215-27
170. Adkins DR, Haddad RI. Clinical trial data of Anti-PD-1/PD-L1 therapy for recurrent or metastatic nasopharyngeal Carcinoma: A review. Cancer Treat Rev. 2022;109:102428
171. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J. et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27:1536-43
172. Fang W, Yang Y, Ma Y, Hong S, Lin L, He X. et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19:1338-50
173. Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC. et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02). J Clin Oncol. 2021;39:704-12
174. Lee SP. Nasopharyngeal carcinoma and the EBV-specific T cell response: prospects for immunotherapy. Semin Cancer Biol. 2002;12:463-71
175. Comoli P, Pedrazzoli P, Maccario R, Basso S, Carminati O, Labirio M. et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942-9
176. Secondino S, Zecca M, Licitra L, Gurrado A, Schiavetto I, Bossi P. et al. T-cell therapy for EBV-associated nasopharyngeal carcinoma: preparative lymphodepleting chemotherapy does not improve clinical results. Ann Oncol. 2012;23:435-41
177. Smith C, Tsang J, Beagley L, Chua D, Lee V, Li V. et al. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 2012;72:1116-25
178. Chia WK, Wang WW, Teo M, Tai WM, Lim WT, Tan EH. et al. A phase II study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol. 2012;23:997-1005
179. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-48
180. Littlepage LE, Egeblad M, Werb Z. Coevolution of cancer and stromal cellular responses. Cancer Cell. 2005;7:499-500
181. Ishiguro K, Yoshida T, Yagishita H, Numata Y, Okayasu T. Epithelial and stromal genetic instability contributes to genesis of colorectal adenomas. Gut. 2006;55:695-702
182. Wu L, Wu W, Zhang J, Zhao Z, Li L, Zhu M. et al. Natural coevolution of tumor and immunoenvironment in glioblastoma. Cancer Discov. 2022 CD-22-0196
183. Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839-49
184. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792-804
185. Helms EJ, Berry MW, Chaw RC, DuFort CC, Sun D, Onate MK. et al. Mesenchymal Lineage Heterogeneity Underlies Nonredundant Functions of Pancreatic Cancer-Associated Fibroblasts. Cancer Discov. 2022;12:484-501
186. Hupfer A, Brichkina A, Koeniger A, Keber C, Denkert C, Pfefferle P. et al. Matrix stiffness drives stromal autophagy and promotes formation of a protumorigenic niche. Proc Natl Acad Sci U S A. 2021;118:e2105367118
187. Wu X, Zhou Z, Xu S, Liao C, Chen X, Li B. et al. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020;478:93-106
188. Lee PJ, Sui YH, Liu TT, Tsang NM, Huang CH, Lin TY. et al. Epstein-Barr viral product-containing exosomes promote fibrosis and nasopharyngeal carcinoma progression through activation of YAP1/FAPα signaling in fibroblasts. J Exp Clin Cancer Res. 2022;41:254
189. Huang SCM, Tsao SW, Tsang CM. Interplay of Viral Infection, Host Cell Factors and Tumor Microenvironment in the Pathogenesis of Nasopharyngeal Carcinoma. Cancers (Basel). 2018;10:106
190. Luo WR, Yao KT. Cancer stem cell characteristics, ALDH1 expression in the invasive front of nasopharyngeal carcinoma. Virchows Arch. 2014;464:35-43
191. Chaudhuri O, Koshy ST, Branco da Cunha C, Shin JW, Verbeke CS, Allison KH. et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970-8
192. Kauffman S. Origins of Order: Self-Organization and Selection in Evolution. Oxford University Press. 1993
193. Li Q, Wennborg A, Aurell E, Dekel E, Zou JZ, Xu Y. et al. Dynamics inside the cancer cell attractor reveal cell heterogeneity, limits of stability, and escape. Proc Natl Acad Sci U S A. 2016;113:2672-7
194. Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31-68
195. Zhong G. The Mulberry Dike-Fish Pond Complex: A Chinese Ecosystem of Land-Water Interaction on the Pearl River Delta. Hum Ecol. 1982;10:191-202
196. Sebestyén A, Dankó T, Sztankovics D, Moldvai D, Raffay R, Cervi C. et al. The role of metabolic ecosystem in cancer progression - metabolic plasticity and mTOR hyperactivity in tumor tissues. Cancer Metastasis Rev. 2021;40:989-1033
197. Nikitin PA, Yan CM, Forte E, Bocedi A, Tourigny JP, White RE. et al. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe. 2010;8:510-22
198. Saha A, Robertson ES. Mechanisms of B-Cell Oncogenesis Induced by Epstein-Barr Virus. J Virol. 2019;93:e00238-19
199. Dallai S, Gulisano M, Polli G, Pacini P. [Scanning electronic microscopy study of nasopharyngeal carcinoma]. Boll Soc Ital Biol Sper. 1990;66:303-8
200. Huang ES, Gutsch D, Tzung KW, Lin CT. Detection of low level of human papilloma virus type 16 DNA sequences in cancer cell lines derived from two well-differentiated nasopharyngeal cancers. J Med Virol. 1993;40:244-50
201. Huang T, Debelius JW, Ploner A, Xiao X, Zhang T, Hu K. et al. Radiation Therapy-Induced Changes of the Nasopharyngeal Commensal Microbiome in Nasopharyngeal Carcinoma Patients. Int J Radiat Oncol Biol Phys. 2021;109:145-50
202. Qiao H, Tan XR, Li H, Li JY, Chen XZ, Li YQ. et al. Association of Intratumoral Microbiota With Prognosis in Patients With Nasopharyngeal Carcinoma From 2 Hospitals in China. JAMA Oncol. 2022;8:1301-9
203. Zhou X, Matskova L, Zheng S, Wang X, Wang Y, Xiao X. et al. Mechanisms of Anergic Inflammatory Response in Nasopharyngeal Carcinoma Cells Despite Ubiquitous Constitutive NF-κB Activation. Front Cell Dev Biol. 2022;10:861916
204. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759-71
205. Parker TM, Henriques V, Beltran A, Nakshatri H, Gogna R. Cell competition and tumor heterogeneity. Semin Cancer Biol. 2020;63:1-10
206. van Neerven SM, Vermeulen L. Cell competition in development, homeostasis and cancer. Nat Rev Mol Cell Biol. 2023;24:221-236
207. Cavanaugh KC, Dangremond EM, Doughty CL, Williams AP, Parker JD, Hayes MA. et al. Climate-driven regime shifts in a mangrove-salt marsh ecotone over the past 250 years. Proc Natl Acad Sci U S A. 2019;16:21602-8
208. Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol. 2009;25:567-95
209. Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Laé M. et al. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844-53
210. Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26:319-31
211. Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225-238
212. Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744-9
213. Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A. et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J Pathol. 2014;234:410-22
214. Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S. et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell. 2017;171:1611-24.e24
215. Luo W, Fang W, Li S, Yao K. Aberrant expression of nuclear vimentin and related epithelial-mesenchymal transition markers in nasopharyngeal carcinoma. Int J Cancer. 2012;131:1863-73
216. Luo WR, Wu AB, Fang WY, Li SY, Yao KT. Nuclear expression of N-cadherin correlates with poor prognosis of nasopharyngeal carcinoma. Histopathology. 2012;61:237-46
217. Luo WR, Li SY, Cai LM, Yao KT. High expression of nuclear Snail, but not cytoplasmic staining, predicts poor survival in nasopharyngeal carcinoma. Ann Surg Oncol. 2012;19:2971-9
218. Ding RB, Chen P, Rajendran BK, Lyu X, Wang H, Bao J. et al. Molecular landscape and subtype-specific therapeutic response of nasopharyngeal carcinoma revealed by integrative pharmacogenomics. Nat Commun. 2021;12:3046
219. Horikawa T, Yang J, Kondo S, Yoshizaki T, Joab I, Furukawa M. et al. Twist and epithelial-mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are associated with metastatic nasopharyngeal carcinoma. Cancer Res. 2007;67:1970-8
220. Cai LM, Lyu XM, Luo WR, Cui XF, Ye YF, Yuan CC. et al. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene. 2015;34:2156-66
221. Ries L, Fletcher RJ, Battin J, Sisk TD. Ecological responses to habitat edge: mechanisms, models and variability explained. Ann Rev Ecol Evol Syst. 2004;35:491-522
222. Cadenasso ML, Pickett STA, Weathers KC, Jones CG. A framework for a theory of ecological boundaries. Bioscience. 2003;53:750-8
223. Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320-9
224. Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688-95
225. Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639-51
226. Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101-15
227. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (8th edn). Wiley Blackwell. 2017
228. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P. et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-8
229. Luo WR, Gao F, Li SY, Yao KT. Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology. 2012;61:1072-81
230. Chroni A, Kumar S. Tumors Are Evolutionary Island-Like Ecosystems. Genome Biol Evol. 2021;13:evab276
231. Pavlič A, Boštjančič E, Kavalar R, Ilijevec B, Bonin S, Zanconati F. et al. Tumour budding and poorly differentiated clusters in colon cancer - different manifestations of partial epithelial-mesenchymal transition. J Pathol. 2022;258:278-88
232. Thakur N, Ailia MJ, Chong Y, Shin OR, Yim K. Tumor Budding as a Marker for Poor Prognosis and Epithelial-Mesenchymal Transition in Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022;12:828999
233. Lawlor RT, Veronese N, Nottegar A, Malleo G, Smith L, Demurtas J. et al. Prognostic Role of High-Grade Tumor Budding in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis with a Focus on Epithelial to Mesenchymal Transition. Cancers (Basel). 2019;11:113
234. Jensen DH, Dabelsteen E, Specht L, Fiehn AM, Therkildsen MH, Jønson L. et al. Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236:505-16
235. Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1:158-64
236. Alfarouk KO, Ibrahim ME, Gatenby RA, Brown JS. Riparian ecosystems in human cancers. Evol Appl. 2013;6:46-53
237. Daoust SP, Fahrig L, Martin AE, Thomas F. From forest and agro-ecosystems to the microecosystems of the human body: what can landscape ecology tell us about tumor growth, metastasis, and treatment options? Evol Appl. 2013;6:82-91
238. Coffey DS, Getzenberg RH, DeWeese TL. Hyperthermic biology and cancer therapies: a hypothesis for the "Lance Armstrong effect". JAMA. 2006;296:445-8
239. Mantso T, Goussetis G, Franco R, Botaitis S, Pappa A, Panayiotidis M. Effects of hyperthermia as a mitigation strategy in DNA damage-based cancer therapies. Semin Cancer Biol. 2016;37-38:96-105
240. Scheidegger S, Mingo Barba S, Gaipl US. Theoretical Evaluation of the Impact of Hyperthermia in Combination with Radiation Therapy in an Artificial Immune-Tumor-Ecosystem. Cancers (Basel). 2021;13:5764
241. Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H. et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487-97
242. Westermann AM, Grosen EA, Katschinski DM, Jäger D, Rietbroek R, Schink JC. et al. A pilot study of whole body hyperthermia and carboplatin in platinum-resistant ovarian cancer. Eur J Cancer. 2001;37:1111-7
243. Hegewisch-Becker S, Gruber Y, Corovic A, Pichlmeier U, Atanackovic D, Nierhaus A. et al. Whole-body hyperthermia (41.8 degrees C) combined with bimonthly oxaliplatin, high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer: a phase II study. Ann Oncol. 2022;13:1197-204
244. Merten R, Ott O, Haderlein M, Bertz S, Hartmann A, Wullich B. et al. Long-Term Experience of Chemoradiotherapy Combined with Deep Regional Hyperthermia for Organ Preservation in High-Risk Bladder Cancer (Ta, Tis, T1, T2). Oncologist. 2019;24:e1341-50
245. Mu C, Wu X, Zhou X, Wolfram J, Shen J, Zhang D. et al. Chemotherapy Sensitizes Therapy-Resistant Cells to Mild Hyperthermia by Suppressing Heat Shock Protein 27 Expression in Triple-Negative Breast Cancer. Clin Cancer Res. 2018;24:4900-12
246. Zheng N, Xu A, Lin X, Mo Z, Xie X, Huang Z. et al. Whole-body hyperthermia combined with chemotherapy and intensity-modulated radiotherapy for treatment of advanced nasopharyngeal carcinoma: a retrospective study with propensity score matching. Int J Hyperthermia. 2021;38:1304-12
247. Lin TY, Jia JS, Luo WR, Lin XL, Xiao SJ, Yang J. et al. ThermomiR-377-3p-induced suppression of Cirbp expression is required for effective elimination of cancer cells and cancer stem-like cells by hyperthermia. J Exp Clin Cancer Res. 2022 (in proof)
248. Heppner GH. Tumor subpopulation interactions. In: A. Owen (ed.). Tumor Heterogeneity. pp. 225-236. New York. Academic Press. 1982
249. Miller BE, Miller FR, Heppner GH. Therapeutic perturbation of the tumor ecosystem in reconstructed heterogeneous mouse mammary tumors. Cancer Res. 1989;49:3747-53
250. Schwab ED, Pienta KJ. Cancer as a complex adaptive system. Med Hypotheses. 1996;47:235-41
251. Massagué J, Ganesh K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021;11:971-94
252. Dujon AM, Aktipis A, Alix-Panabières C, Amend SR, Boddy AM, Brown JS. et al. Identifying key questions in the ecology and evolution of cancer. Evol Appl. 2021;14:877-92
253. Rabbie R, Lau D, White RM, Adams DJ. Unraveling the cartography of the cancer ecosystem. Genome Biol. 2021;22:87
254. Nawaz S, Yuan Y. Computational pathology: Exploring the spatial dimension of tumor ecology. Cancer Lett. 2016;380:296-303
255. Gatenbee CD, Minor ES, Slebos RJC, Chung CH, Anderson ARA. Histoecology: Applying Ecological Principles and Approaches to Describe and Predict Tumor Ecosystem Dynamics Across Space and Time. Cancer Control. 2020;27:1073274820946804
256. Lloyd MC, Rejniak KA, Brown JS, Gatenby RA, Minor ES, Bui MM. Pathology to enhance precision medicine in oncology: lessons from landscape ecology. Adv Anat Pathol. 2015;22:267-72
257. Richardson DS, Lichtman JW. Clarifying Tissue Clearing. Cell. 2015;162:246-57
258. Fais S, Overholtzer M. Cell-in-cell phenomena in cancer. Nat Rev Cancer. 2018;18:758-66
259. Pinto G, Brou C, Zurzolo C. Tunneling Nanotubes: The Fuel of Tumor Progression? Trends Cancer. 2020;6:874-88
260. Mehta S, Zhang J. Liquid-liquid phase separation drives cellular function and dysfunction in cancer. Nat Rev Cancer. 2020;2:239-52
261. Gatenby RA, Grove O, Gillies RJ. Quantitative imaging in cancer evolution and ecology. Radiology. 2013;269:8-15
262. Kim JY, Gatenby RA. Quantitative Clinical Imaging Methods for Monitoring Intratumoral Evolution. Methods Mol Biol. 2017;1513:61-81
263. Bissell MJ. Goodbye flat biology - time for the 3rd and the 4th dimensions. J Cell Sci. 2017;130:3-5
264. Wu Y, Cheng Y, Wang X, Fan J, Gao Q. Spatial omics: Navigating to the golden era of cancer research. Clin Transl Med. 2022;12:e696
265. Wang M, Hu Q, Lv T, Wang Y, Lan Q, Xiang R. et al. High-resolution 3D spatiotemporal transcriptomic maps of developing Drosophila embryos and larvae. Dev Cell. 2022;57:1271-83.e4
266. Wessels D, Lusche DF, Voss E, Soll DR. 3D and 4D Tumorigenesis Model for the Quantitative Analysis of Cancer Cell Behavior and Screening for Anticancer Drugs. Methods Mol Biol. 2022;2364:299-318
267. Sheikh A, Abourehab MAS, Kesharwani P. The clinical significance of 4D printing. Drug Discov Today. 2023;28:103391
268. Schöneberg J, Dambournet D, Liu TL, Forster R, Hockemeyer D, Betzig E. et al. 4D cell biology: big data image analytics and lattice light-sheet imaging reveal dynamics of clathrin-mediated endocytosis in stem cell-derived intestinal organoids. Mol Biol Cell. 2018;29:2959-68
269. Noronha PM. Diving into deep-tissue imaging to help with cancer research. Nature. 2022;609:434
270. Strauss B, Bertolaso M, Ernberg I, Bissell MJ. Rethinking Cancer: A New Paradigm for the Postgenomics Era. The MIT Press. 2021
271. Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on humanmicrobe mutualism and disease. Nature. 2007;449:811-8
272. Pepper JW, Rosenfeld S. The emerging medical ecology of the human gut microbiome. Trends Ecol Evol. 2012;27:381-4
Author contact
![]() Corresponding author: Cancer Research Institute, Department of Pathology, The Second Affiliated Hospital of Southern University of Science and Technology, Shenzhen Third People's Hospital, National Clinical Research Center for Infectious Diseases, Shenzhen, China. Tel: +86-13509656082, Email: luoweirencom
Corresponding author: Cancer Research Institute, Department of Pathology, The Second Affiliated Hospital of Southern University of Science and Technology, Shenzhen Third People's Hospital, National Clinical Research Center for Infectious Diseases, Shenzhen, China. Tel: +86-13509656082, Email: luoweirencom
 Global reach, higher impact
Global reach, higher impact