13.3
Impact Factor
Theranostics 2023; 13(4):1302-1310. doi:10.7150/thno.82101 This issue Cite
Research Paper
Imaging DNA damage response by γH2AX in vivo predicts treatment response to Lutetium-177 radioligand therapy and suggests senescence as a therapeutically desirable outcome
1. MRC Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, UK
2. Nuclear Medicine and Molecular Imaging, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands
Received 2022-12-23; Accepted 2023-1-19; Published 2023-2-21
Abstract
Rationale: An effective absorbed dose response relationship is yet to be established for Lutetium-177 based radionuclide therapies such as 177Lu-DOTATATE and 177Lu-PSMA. The inherent biological heterogeneity of neuroendocrine and prostate cancers may make the prospect of establishing cohort-based dose-response relationships unobtainable. Instead, an individual-based approach, monitoring the dose-response within each tumor could provide the necessary metric to monitor treatment efficacy.
Methods: We developed a dual isotope SPECT imaging strategy to monitor the change over time in the relationship between 177Lu-DOTATATE and 111In-anti-γH2AX-TAT, a modified radiolabelled antibody that allows imaging of DNA double strand breaks, in mice bearing rat pancreatic cancer xenografts. The dynamics of γH2AX foci, apoptosis and senescence following exposure to 177Lu-DOTATATE was further investigated in vitro and in ex vivo tumor sections.
Results: The change in slope of the 111In-anti-γH2AX-TAT to 177Lu signal between days 5 and 7 was found to be highly predictive of survival (r = 0.955, P < 0.0001). This pivotal timeframe was investigated further in vitro: clonogenic survival correlated with the number of γH2AX foci at day 6 (r = -0.995, P < 0.0005). While there was evidence of continuously low levels of apoptosis, delayed induction of senescence in vitro appeared to better account for the γH2AX response to 177Lu. The induction of senescence was further investigated by ex vivo analysis and corresponded with sustained retention of 177Lu within tumor regions.
Conclusions: Dual isotope SPECT imaging can provide individualized tumor dose-responses that can be used to predict lutetium-177 treatment efficacy. This bio-dosimeter metric appears to be dependent upon the extent of senescence induction and suggests an integral role that senescence plays in lutetium-177 treatment efficacy.
Keywords: DOTATATE, γH2AX, senescence, DSBs, radiosensitivity
Introduction
Despite impressive improvements in progression free survival in the NETTER-1 trial, peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE remains a non-curative therapy, with limited evidence of extending overall survival [1]. 177Lu-DOTATATE remains highly effective at improving patients' quality of life [2], yet opportunities remain to improve therapeutic outcome by increasing the response rate. One aspect, increasing the administered dose of PRRT, is currently limited, given the high as safely administrable limit (AHASA) is the calculated absorbed dose of 23 Gy in the kidneys. This limit is based upon those established for external beam radiotherapy and concerns of renal toxicity from 90Y-DOTATATE [3]. While routine dosimetry is not (yet) widely adopted in the clinic, because quantitation of 177Lu uptake in tissues has its own challenges, there is promising evidence of improved response rates if patients are given administered doses up to this AHASA limit [4]. Although it may be tempting to advocate for ever increasing injected doses of 177Lu-DOTATATE, there are also valid uncertainties over long-term haematological toxicity including myeloid dysplasia and acute myeloid leukaemia [5], for which there are no known bone marrow dose-response relationships [6]. Such risks need to be taken into consideration when making an informed treatment decision, which is made more difficult with the current uncertainty over the necessary therapeutic dose. This minimum therapeutic dose needed to treat the tumor has not been established for neuroendocrine tumors, as it has been for radioiodine treatment of thyroid cancer [7]. To establish a dose threshold that is as low as reasonably achievable to reliably treat each patient, a relationship between administered and radiation absorbed dose and tumor response is required. It is likely that a general cohort-based dose-response may not be possible for neuroendocrine tumors due to its inherent biological heterogeneity [8]. A dose-response relationship based upon SPECT-image dosimetry was found in a cohort of pancreatic neuroendocrine patients [9]. However, when this same technique was applied to the more biologically diverse midgut neuroendocrine tumors, no significant radiation absorbed dose-effect correlation was observed [10], and there was a poorer dose-response correlation in pancreatic tumors with diameters less than 4 mm. Recent studies account for these differences between pancreatic and small intestine tumors by differences in tumor uptake of 177Lu across rounds of therapy [11, 12]. These results indicate not only inherent differences in tumor uptake across patients affected by tumor morphology and diffusion, but also differences in the biological response to each round of treatment [13]. Therefore, a direct metric of the biological response to 177Lu-DOTATATE therapy is warranted to account for these individual radiobiological differences, and establish a dose-response relationship taking into account the physical radiation dose from 177Lu and the (radio)biological response to that radiation dose.
One of the more cytotoxic types of DNA damage is the DNA double strand break (DSB). As part of DNA damage repair, one histone isoform, H2A.X, is phosphorylated around sites of DSBs, by kinases such as ATM, ATR, and DNA-PKcs. The S159-phosphorylated form of H2A.X is designated as γH2AX. We have previously shown that a radiolabelled modified anti-γH2AX antibody, 111In-anti-γH2AX-TAT, is capable of imaging the DNA double strand break damage [14-17], also following 177Lu-DOTATATE administration in vivo [18]. Lethal levels of DNA damage induced by the beta-particles emitted by lutetium-177 are thought to be a significant contributor to the therapeutic effect of radionuclide therapies such as 177Lu-DOTATATE. Therefore, a means to monitor the DNA damage response to damage inflicted by lutetium-177 would be invaluable to assessing the repair capability of each tumor, and to assess its response. This strategy aims to measure the dose-response within each tumor, within each patient. Here, we developed a metric that was predictive of survival of mice, bearing SSTR2-expressing neuroendocrine tumor xenografts, treated with 177Lu-DOTATATE, through quantifying 177Lu uptake, and concurrent imaging DNA damage with 111In-anti-γH2AX-TAT. While this approach has potential if applied within the clinic to establish optimized therapeutic doses and adaptive therapy, it also provided insight into the importance of DNA damage and senescence in response to 177Lu-DOTATATE treatment.
Imaging Concept
Due to the extended retention of lutetium-177 within the tumor, and prolonged low-dose rate irradiation of cancer cells, the presence of γH2AX foci may indicate either freshly formed or long-lived unresolved double stranded DNA breaks (DSBs). It is these unresolved residual γH2AX foci, existing for longer than 24 h that have been found to be predictive of clonogenic survival after external beam irradiation (EBRT), rather than the number of short-lived γH2AX foci (which are resolved within hours, representing repairable DNA damage) [19]. For example, de-Colle et al. measured the speed with which yH2AX foci were resolved, and observed a high level of intrinsic radiation sensitivity in prostate cancer samples, after ex vivo irradiation with EBRT [20]. The relative ability of tumors to repair DNA damage, and deal with unresolved DNA damage, would provide an essential insight into the functional radioresistance of tumors to lutetium-177 based therapies, particularly with heterogenous neuroendocrine and prostate tumors [8]. A functional approach to assessing radioresistance towards EBRT has recently been demonstrated by employing an ex vivo RAD51 foci formation assay [21]. However, unlike the single, brief EBRT exposure used in the RAD51 assay, it is challenging to distinguish non-invasively between the quickly resolved dsDNA breaks continually inflicted through lutetium-177 irradiation and residual foci at a single timepoint [22]. Furthermore, γH2AX foci have been shown recently to correlate with sstr2 immunofluorescence expression ex vivo as a proxy for 177Lu-DOTATATE dose [23].
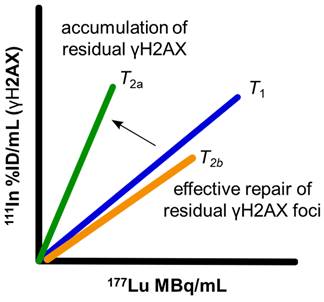
To address this need, the accumulation over time of γH2AX foci, relative to lutetium-177 uptake within the tumor can be visualized and quantified using SPECT imaging with 111In-anti-γH2AX-TAT, and 177Lu. By monitoring the change of this dual-isotope relationship, the accumulation of residual foci above and beyond the steady state repair of short-lived γH2AX foci can be quantified: If the proportion of γH2AX relative to lutetium-177 decreases or remains the same over time within a tumor then this would indicate the tumor is capable of continuing to successfully resolve DSBs until lutetium-177 is eventually cleared from the tumor (Figure 1). However, if the proportion of γH2AX relative to lutetium-177 increases over time, then this would indicate accumulation of unresolved γH2AX foci above the steady-state clearance of short-lived γH2AX foci. Therefore, any increase in the slope of this dose-response could be used to measure long-lived γH2AX foci which may be indicative of therapeutic success.
Relative accumulation of residual γH2AX foci can be measured through increases in the 177Lu to 111In (γH2AX) slope from an initial timepoint (T1), indicating an accumulation of residual foci (T2a) or maintenance of a slope (T2b) similar to slope at T1 indicating a steady-state of DNA damage repair.
Previously, this γH2AX dose-response slope has been generated by varying radiation absorbed dose irradiation in vitro or ex vivo to calculate relative radiosensitivity [20]. Therefore, to measure dose-response to lutetium-177 therapy in situ, a dose range of 177Lu uptake (as a proxy for radiation absorbed dose) would be required to establish relative radiosensitivity. This spectrum of lutetium-177 uptake can be achieved by utilising the heterogenous uptake of 177Lu-DOTATATE across a tumor, due to differences in diffusion [24] and receptor expression [23, 25].
Materials and Methods
Full materials and methods are presented in the supplementary material accompanying this manuscript.
Radiolabeling
177Lu-DOTATATE was prepared using previously described methods [26]. Carrier-free 177Lu was obtained from ITG (Germany), DOTATATE precursor was obtained from Cambridge Biosciences. 177Lu-DOTATATE was prepared to a molar activity of 50 MBq/nmol for in vitro use, and 86 MBq/nmol (60 MBq/μg) for in vivo experiments, unless otherwise stated. Radiolabeling yield was routinely >99.5%, as determined by ITLC. Immunoconjugate preparation and radiosynthesis of 111In-anti-γH2AX-TAT was performed using mouse monoclonal anti-γH2AX antibodies (Merck, clone JBW-301) as previously described [18]. JBW-301 binds human, murine and rat γH2AX.
In vivo imaging
All animal procedures were performed in accordance with the UK Animals (Scientific Procedures) Act 1986 and with local ethical committee approval. Female athymic nude mice (Envigo) were housed in IVC cages in groups of up to six per cage in an artificial day-night cycle facility with ad libitum access to food and water. Tumor xenografts were generated by subcutaneous injection of CA20948 cell suspensions (106 cells in 100 μL PBS) in the right hind flank in two staggered batches 4 days apart (2 x 15 mice) (Scheme S1). After 14 days post-inoculation mice were randomized into treatment groups. The 1st batch of inoculated mice received 177Lu-DOTATATE (5-30 MBq) in 100 µL PBS by intravenous injection and after 4 days received 111In-anti-γH2AX-TAT (5 MBq, 5 μg, in 100 µL PBS) by intravenous injection. The second batch of mice received both injections of 111In-anti-γH2AX-TAT and 177Lu-DOTATATE on the same day and SPECT/CT images were undertaken 24 h later and subsequently every 2nd day, representing days 1, 3, 5, 7, 9 and 11 post start of 177Lu-DOTATATE treatment. SPECT/CT images were acquired in list-mode for approximately 15 minutes using a VECTor4CT scanner (MILabs, Utrecht, the Netherlands) equipped with a HE-UHR-RM collimator containing pinhole apertures of 1.8 mm diameter. Tumor growth was monitored by calipers and mice were culled when they reached predefined ethical endpoints including tumors >1200 mm3.
Dual isotope voxel analysis
Reconstruction of both 177Lu and 111In SPECT images were performed using a SROSEM algorithm using the same number of subsets and iterations over all imaging sessions, (111In: 149-189 and 223-272 keV, 4 subsets, 4 iterations, no filter; 177Lu: 196-216 keV, 2 subsets, 4 iterations, no filter), using 0.6 mm3 voxels. Reconstruction of 177Lu and 111In signals using this approach were shown previously to provide reliable measurements of either isotope, by phantom studies using solutions with mixed isotope standards [27]. Reconstructed images were co-registered to CT images for attenuation correction. Tumor volumes of interest were determined by using a decay-corrected 0.5%ID/mL threshold for 177Lu, using PMOD v.3.38 (PMOD Technologies, Zurich, Switzerland). Linear regression of 111In (%ID/mL) and 177Lu (MBq/mL) in co-registered voxels was performed in GraphPad Prism v9 (GraphPad Software, San Diego, CA, USA). For the purpose of linear regression, the signal of 111In was decay corrected, whereas 177Lu-DOTATATE was not.
Results
The rat pancreatic CA20948 tumor model employed here has previously been widely investigated in syngeneic rat models of PRRT therapy. Our previous investigation provided proof-of-concept of an increase in γH2AX expression and uptake of 111In-anti-γH2AX-TAT at day 3 post administration in xenograft tumors in mice treated with 20 MBq of 177Lu-DOTATATE, compared to untreated animals or isotype matched 111In-anti-IgG-TAT controls [27]. Here, to determine whether 111In-anti-γH2AX-TAT imaging could be used to predict survival, a large range of doses of 177Lu-DOTATATE was used (0, 5, 10, 20, 30 MBq) in 30 mice (n = 6 per treatment group, Supplemental Scheme S1).
177Lu-DOTATATE dose-response
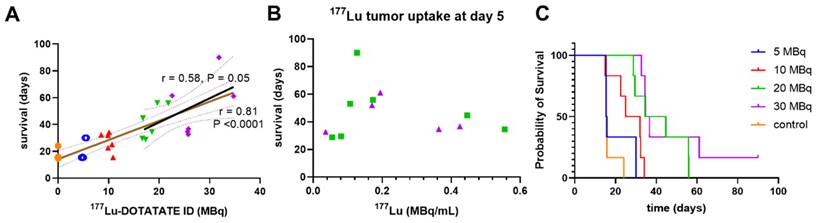
We observed a notable injected dose-response across all treatment groups with 177Lu-DOTATATE in this model that significantly correlated with survival (Figure 2A, Pearson r = 0.81, P < 0.0001). While this correlation was expected across a very wide dose range including very low injected doses, and using a monoclonal tumor cell-line derived xenograft, this correlation diminished markedly at therapeutically relevant higher injected doses in the 20-30 MBq range (Figure 2A, Pearson r = 0.58, P = 0.05). Similar to a clinical study in midgut neuroendocrine tumors [10], we observed no correlation between survival and mean tumor lutetium-177 uptake found for these higher-treated groups by SPECT (Figure 2B, Supplemental Figure S1). There was no statistical difference in survival between mice of the 5 MBq treatment cohort and controls (Figure 2C, Mantel-Cox, p = 0.31). Since the relatively fast growth rate of the 5 MBq treatment group tumors obscured comparison between imaging timepoints, and such a fast growth rate is not reflective of clinical experience they were excluded from further analysis.
Measuring dose-response to lutetium-177 using 111In-anti-γH2AX-TAT in vivo
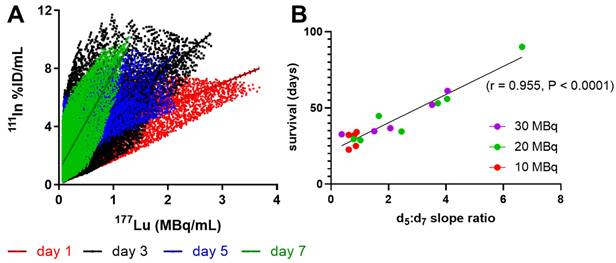
A large change between days 5 and 7 was observed in the shape of the voxel-per-voxel 111In vs. 177Lu scatter-plots in mice with longer survival times (Figure 3A). Comparing the ratio of the 111In:177Lu slopes at days 5 and 7 within mice of the 10, 20 and 30 MBq treatment groups, we observed excellent correlation with survival (Figure 3B, Pearson r = 0.95, P < 0.0001). This ratio appeared to be reflective of the DNA repair capacity of the tumor with ratios >>1 correlated with greater therapeutic success while tumors with a ratio ~1 were indicative of shorter survival and therefore within the repair capacity of the tumor.
Higher injected doses of 177Lu-DOTATATE (A) correlated with survival (r = 0.81, P < 0.0001), but (B) retention of 177Lu-DOTATATE at day 5 did not correlate with survival. (C) Higher injected doses of 177Lu-DOTATATE did increase the probability of survival.
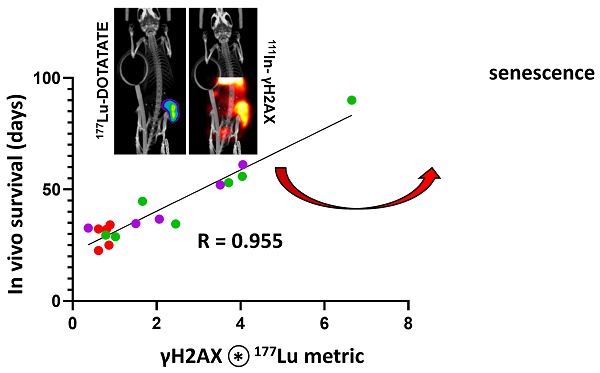
Shift in 111In-anti-γH2AX-TAT to 177Lu-DOTATATE dose response correlates with survival. Linear regression of all 111In to 177Lu values within each CA20948 tumor at each timepoint revealed a large shift in the slope of this trajectory between days 5 and 7. (A) representative mouse 111In to 177Lu trajectories imaged at days 1, 3, 5 and 7 injected with 25.8 MBq with a 37 day survival. (B) The ratio between slopes of the dose-response trajectories at day 5 and 7 significantly correlated with survival (Pearson r = 0.955, P < 0.0001).
(A) γH2AX foci (mean ± SD) correlated inversely with clonogenic survival at day 6 (Pearson r = -0.995, P < 0.0005). Clonogenic survival values (mean ± SD) for the doses of 177Lu-DOTATATE determined previously [1]. (B) Increased expression of γH2AX does not correspond to increases in apoptosis alone and sustained expression of γH2AX could reflect increased expression of senescence over time.
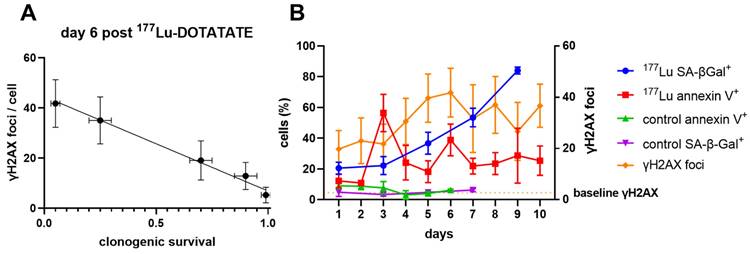
Investigating the time course of the in vitro γH2AX and 177Lu-DOTATATE dose response
To understand the dynamics of γH2AX across the spectrum of 177Lu-induced radiation damage, we enumerated γH2AX foci by immunofluorescence in CA20948 cells up to 11 days after a brief exposure to increasing doses of 177Lu-DOTATATE in vitro (Supplemental Figure S2). Only G1 cells were analysed to minimize counting of γH2AX foci induced by replicative stress. There was a consistent linear response of γH2AX foci relative to 177Lu-DOTATATE up to 0.32 MBq/mL up to day 5 (Supplemental Figure S3A). The consistency of this dose response slope in vitro supported our observations in vivo and may represent the steady state DNA repair capacity of the cells. Beyond the 0.32 MBq/mL dose threshold there was a very different dose response over time with effectively no dose-response up to day 3 (Figure S4). After day 4 the slope steadily increased up to day 6 and afterwards became highly variable. Day 6 also appears to be a critical timepoint in vitro, as γH2AX foci across all the doses was inversely correlated with clonogenic survival (Pearson R = -0.995, P < 0.0005, Figure 4A).
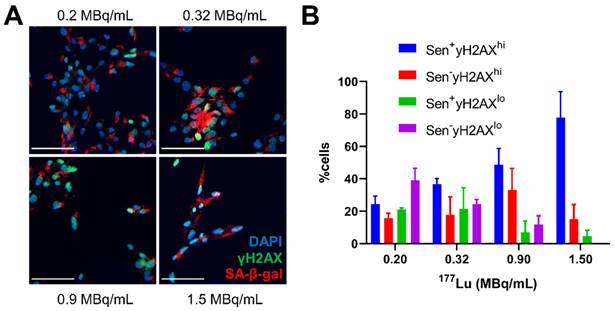
Since apoptosis and senescence could be contributing to the γH2AX foci expression over time, cells treated with 177Lu-DOTATATE (1.5 MBq/mL) were probed for apoptosis using Annexin V staining or for senescence by beta-galactosidase activity (SA-β-gal+) (Figure 4B). There appeared to be two distinct waves of apoptosis: at day 3 and then another at day 6. In addition, we observed a steady increase in the fraction of SA-β-gal+ positive cells after day 3 indicating increasing dominance of cellular senescence over time. This increased proportion of cells displaying a senescent phenotype was also dose-dependent with 177Lu-DOTATATE and was associated with increased expression of γH2AX (Figure 5).
Evidence of 177Lu-DOTATATE induced senescence ex vivo
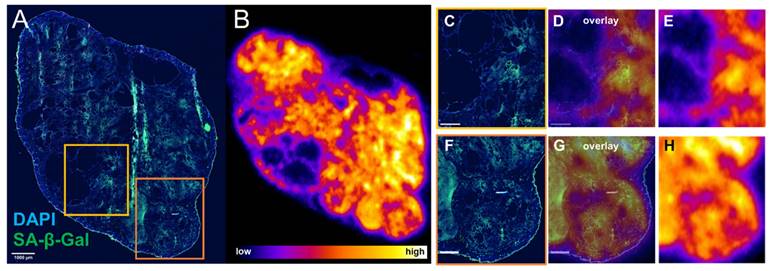
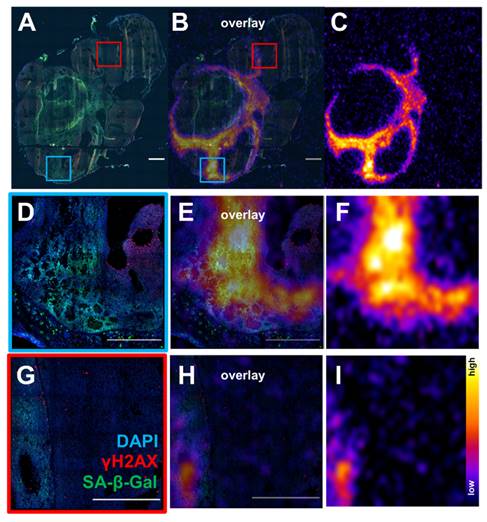
Since there appeared to be an association between sustained γH2AX expression and senescence in cells exposed to 177Lu-DOTATATE in delayed timepoints (day 5 onwards), the distribution of lutetium-177 by ex vivo autoradiography and SA-β-gal immunofluorescence of the tumor xenografts were investigated. Expression of SA-β-gal was associated with higher retention of 177Lu-DOTATATE within tumor tissue (Figure 6). These senescent tissue regions also appear capable of retaining 177Lu-DOTATATE for a long period, being observed at 24 days post-injection (Supplemental Figure S5), and even at 42 days post-injection (Figure 7). Even in mice therapeutically undertreated with as little as 5 MBq, we observed SA-β-gal association with lutetium-177 retaining remnant regions (Figure S6 and S7).
Discussion
This study determined a dose-response relationship with 177Lu-DOTATATE in a preclinical model based not on injected or accumulated lutetium-177 activity, but rather the accumulation of DNA damage, relative to 177Lu. Determining a dose-response with 177Lu-DOTATATE, and other lutetium-177 based therapies, has been a challenge in the clinic [11]. The heterogenous dose-response at therapeutically relevant doses was reflected in our study. Despite using a monoclonal cell culture derived xenograft grown in in-bred immune-compromised mice, considerable heterogeneity was seen in treatment response relative to similar injected 177Lu activity and 177Lu tumor uptake, when administering therapeutically relevant doses of 177Lu-DOTATATE (Figure 2). Differences in diffusion and blood vessel density between tumors and between patients is one of the challenges to establish an injected dose-response in patients treated with 177Lu-DOTATATE. Recent work showed diffusion weighted MRI to be an independent predictor of patient outcome [28]. In the present study, this inherent heterogenous distribution of 177Lu-DOTATATE was used by us to monitor dose-response changes for each tumor over time.
Senescence markers associated with γH2AX expression in vitro. (A) representative immunofluorescence images of CA20948 cells at day 5 post-treatment with varying concentrations 177Lu-DOTATATE (0.2-1.5 MBq/mL, 2 h), senescence associated β-galactosidase (red), DAPI (blue), γH2AX (green), scale bar is 100 µm. (B) Increasing percentage of cells with high expression of γH2AX foci with increasing 177Lu-DOTATATE dose concentration as well a higher proportion of these cells positive for SA-β-gal.
Senescence marker SA-β-gal is associated with retained expression of 177Lu-DOTATATE ex vivo. Mouse bearing CA20948 tumor was injected with 10.7 MBq 177Lu-DOTATATE and culled at day 11 post-injection. (A) Immunofluorescence of tumor tissue stained with DAPI (blue) and SA-β-gal (green) (scale bar 1 mm) was compared with (B) lutetium-177 autoradiography performed on an adjacent slide. (C,F) Regions with SA-β-gal expression were associated with higher levels of lutetium-177 (D,E and G,H scale bar 500 µm).
Ex vivo analysis of tumor from mouse treated with 16.8 MBq of 177Lu-DOTATATE sacrificed at day 42 post-treatment start (A) Immunofluorescence DAPI (blue) γH2AX (red) and SA-β-gal (green) (scale bar 1000 µm) (C) whole tumor 177Lu autoradiography from an adjacent slide and overlay (B). Regions with SA-β-gal expression were associated retention of lutetium-177 (D,E,F) and (G,H,I) (scale bar 500 µm). There were regions with high expression of γH2AX not associated with 177Lu expression and is likely due to replicative stress due to rapid tumor growth.
Previous ex vivo analysis of residual γH2AX foci after exposure to differing doses of external beam radiation provided functional measurement of tumor radiosensitivity. Due to inherent radiobiological differences between external radiation and radionuclide therapy, any assessment of relative radiosensitivity towards 177Lu-DOTATATE should not be assumed from external radiation. However, it is not possible to separately measure residual γH2AX foci from 177Lu-DOTATATE in vivo as γH2AX is constantly generated in response to ongoing lutetium-177 beta particle emissions. This study addressed this challenge by measuring the change in 111In-anti-γH2AX-TAT signal, as a proxy for γH2AX foci numbers, relative to varying 177Lu-DOTATATE doses over time, and within each tumor, to indirectly measure the accumulation of γH2AX induced by 177Lu-DOTATATE. While the slope of the dose-response curve was previously used as a metric for functional radiosensitivity in the ex vivo γH2AX assay [29]. Here we used the change in this slope as a measurement of γH2AX as a metric of residual γH2AX foci. The change in 111In:177Lu slope between days 5 and 7 post injection of 177Lu-DOTATATE proved to be highly predictive of survival (r = 0.955, P < 0.0001, Figure 3).
This aggregate measurement of the 177Lu dose-response curves provides some insight into potential thresholds of the injected dose required to elicit meaningful treatment of the tumor. Mice receiving 10 MBq 177Lu-DOTATATE were clustered just below a slope ratio of 1, suggesting minimal accumulation of γH2AX, and therefore well within the repair capacity of the tumor against 177Lu-DOTATATE. However, some mice that were given higher doses of 177Lu-DOTATATE in 20 and 30 MBq treatment groups could also be considered within the repair capacity of the tumor as they were also clustered around 1. The extent of this shift in dose-response slopes in the remaining mice demonstrated a linear relationship with tumor growth inhibition (Figure 2A). The sustained accumulation of γH2AX appeared critical to the success of 177Lu-DOTATATE treatment, particularly between days 5 and 7. This sustained expression of γH2AX despite the continued physical decay of 177Lu-DOTATATE has also been observed within CA20948 tumors and lung NCI-H69 tumors ex vivo [25]. Similarly, a γH2AX-luciferase reporter transfected into a lung cancer cell line showed a peak expression of γH2AX in vitro between day 5 and 7 in response to X-ray irradiation, and after day 5 following 56Fe ion exposure in vitro [30]. Importantly, this study found an elevated signal from luciferase in vivo after 5 days that was persistent until at least day 12 post X-ray irradiation. The late stage γH2AX expression in response to the environmental contaminant radionuclide cesium-137 has also demonstrated a pivotal change in γH2AX dose-response between days 5 and 7 [31], that was also replicated by variable dose-rate external 137Cs irradiator [32].
The implications of this sustained accumulation of γH2AX were interrogated further in vitro. The consistency of γH2AX foci generated in response to lower amounts of 177Lu-DOTATATE (≤ 0.32 MBq/mL) up to day 5 aligned well with the steady-state dose response relationship observed in vivo (Figure S3). Despite the decreasing dose-rate of 177Lu-DOTATATE over time, γH2AX foci instead increased over time, even after day 6 (Figure 4B). At day 6, the number of γH2AX foci per cell correlated well with decreased clonogenic survival (Figure 4A). This decrease in clonogenic survival appeared to be a result of contributions from both apoptosis and senescence. Since γH2AX expression is elevated in both apoptosis [33], and senescence [34], they may both be contributing to the increase of 111In-anti-γH2AX-TAT signal observed in vivo. However, due to the relatively slow kinetics of antibody-based imaging systems it is also likely that our imaging system is biased towards imaging of the kinetically slower target of senescent cells over the short-lived target of apoptotic cells. Furthermore, our dual-isotope imaging strategy relies upon the retention of 177Lu-DOTATATE, where apoptosis would result in the loss and clearance of 177Lu-DOTATATE.
We observed a dose-dependent increase in SA-β-Gal with 177Lu-DOTATATE observed in vitro. Within these SA-β-Gal-positive cells, an increased proportion of cells with high expression of γH2AX was found, with increasing dose of 177Lu-DOTATATE (Figure 5). The contribution of apoptosis in response to 177Lu-DOTATATE has been documented previously in vivo [25]. In addition, apoptosis could only partially account for the sustained induction of γH2AX in the in vitro γH2AX-luciferase reporter study [30]. The contribution of 177Lu-DOTATATE to tumor senescence by expression of SA-β-Gal towards lutetium-177 to our knowledge has not been documented and could account for this persistent accumulation of γH2AX, thought to be a potential trigger for senescence [35]. Tumor regions with retained lutetium-177 were associated with increased expression of SA-β-Gal by ex vivo analysis (Figure 6). Even though mice investigated by ex vivo analysis in this study did experience tumor regrowth, there were regions of tumors that appeared to be successfully treated, expressing SA-β-Gal and retaining 177Lu, even up to 42 days post-injection (Figure 7). While this study only investigated a single administration of 177Lu-DOTATATE, patients will receive additional doses of 177Lu-DOTATATE every 42-56 days and therefore the potential impact of re-treatment of these senescent regions warrants further investigation.
Conclusions
This study demonstrates that imaging DNA damage with 111In-anti-γH2AX-TAT is a suitable bio-dosimeter to predict the effectiveness of lutetium-177 based radionuclide therapy. The sustained and excessive γH2AX signal in tumors relative to retained lutetium-177 was associated with increased survival with 177Lu-DOTATATE therapy. If implemented in the clinic, this type of bio-dosimeter approach has the potential to guide radionuclide treatment plans according to each tumor's radiobiological response, rather than heterogenous cohort-based dose-response curves. The adoption of such (radio)biological bio-dosimeters in the clinic could provide the necessary regulatory justification to guide and optimise administration of radionuclide therapy beyond existing AHASA limits. In addition, this study highlighted the potential role of senescence following lutetium-177 treatment as a potential cause of the therapeutically successful sustained γH2AX response observed in vivo and therefore warrants further investigation as a potentially desirable outcome of radionuclide therapy.
Supplementary Material
Supplementary methods and figures.
Acknowledgements
This research was supported by MRC (MR/P018661/1).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar AE. et al. Final overall survival in the phase 3 NETTER-1 study of lutetium-177-DOTATATE in patients with midgut neuroendocrine tumors. J Clin Oncol. 2021;39:4112 -
2. Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M. et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. J Clin Oncol. 2018;36:2578-84
3. Geenen L, Nonnekens J, Konijnenberg M, Baatout S, De Jong M, Aerts A. Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [177Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours. Nucl Med Bio. 2021;102-103:1-11
4. Sundlöv A, Gleisner KS, Tennvall J, Ljungberg M, Warfvinge CF, Holgersson K. et al. Phase II trial demonstrates the efficacy and safety of individualized, dosimetry-based 177Lu-DOTATATE treatment of NET patients. Eur J Nucl Med Mol Imaging. 2022;49:3830-3840
5. Sonbol MB, Halfdanarson TR, Hilal T. Assessment of Therapy-Related Myeloid Neoplasms in Patients With Neuroendocrine Tumors After Peptide Receptor Radionuclide Therapy: A Systematic Review. JAMA Oncol. 2020;6:1086-92
6. Bergsma H, van Lom K, Raaijmakers MHGP, Konijnenberg M, Kam BLBLR, Teunissen JJM. et al. Persistent Hematologic Dysfunction after Peptide Receptor Radionuclide Therapy with <sup>177</sup>Lu-DOTATATE: Incidence, Course, and Predicting Factors in Patients with Gastroenteropancreatic Neuroendocrine Tumors. J Nucl Med. 2018;59:452-8
7. Maxon HR, Thomas SR, Hertzberg VS, Kereiakes JG, Chen IW, Sperling MI. et al. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N Engl J Med. 1983;309:937-41
8. O'Neill E, Cornelissen B. Know thy tumour: Biomarkers to improve treatment of molecular radionuclide therapy. Nucl Med Bio. 2022;108-109:44-53
9. Ilan E, Sandström M, Wassberg C, Sundin A, Garske-Román U, Eriksson B. et al. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J Nucl Med. 2015;56:177-82
10. Jahn U, Ilan E, Sandström M, Garske-Román U, Lubberink M, Sundin A. <sup>177</sup>Lu-DOTATATE Peptide Receptor Radionuclide Therapy: Dose Response in Small Intestinal Neuroendocrine Tumors. Neuroendocrinology. 2020;110:662-70
11. Jahn U, Ilan E, Sandström M, Lubberink M, Garske-Román U, Sundin A. Peptide Receptor Radionuclide Therapy (PRRT) with 177Lu-DOTATATE; Differences in Tumor Dosimetry, Vascularity and Lesion Metrics in Pancreatic and Small Intestinal Neuroendocrine Neoplasms. Cancers. 2021 13
12. Roth D, Gustafsson JR, Warfvinge CF, Sundlöv A, Åkesson A, Tennvall J. et al. Dosimetric quantities of neuroendocrine tumors over treatment cycles with <sup>177</sup>Lu-DOTA-TATE. J Nucl Med. 2022;63:399-405
13. Schiavo Lena M, Partelli S, Castelli P, Andreasi V, Smart CE, Pisa E. et al. Histopathological and Immunophenotypic Changes of Pancreatic Neuroendocrine Tumors after Neoadjuvant Peptide Receptor Radionuclide Therapy (PRRT). Endocr Pathol. 2020;31:119-31
14. Poty S, Mandleywala K, O'Neill E, Knight JC, Cornelissen B, Lewis JS. (89)Zr-PET imaging of DNA double-strand breaks for the early monitoring of response following alpha- and beta-particle radioimmunotherapy in a mouse model of pancreatic ductal adenocarcinoma. Theranostics. 2020;10:5802-14
15. Knight JC, Koustoulidou S, Cornelissen B. Imaging the DNA damage response with PET and SPECT. Eur J Nucl Med Mol Imaging. 2017;44:1065-78
16. Knight JC, Topping C, Mosley M, Kersemans V, Falzone N, Fernandez-Varea JM. et al. PET imaging of DNA damage using (89)Zr-labelled anti-gammaH2AX-TAT immunoconjugates. Eur J Nucl Med Mol Imaging. 2015;42:1707-17
17. Knight JC, Torres JB, Goldin R, Mosley M, Dias GM, Bravo LC. et al. Early Detection in a Mouse Model of Pancreatic Cancer by Imaging DNA Damage Response Signaling. J Nucl Med. 2020;61:1006-13
18. Cornelissen B, Kersemans V, Darbar S, Thompson J, Shah K, Sleeth K. et al. Imaging DNA damage in vivo using gammaH2AX-targeted immunoconjugates. Cancer Res. 2011;71:4539-49
19. Banáth JP, Klokov D, MacPhail SH, Banuelos CA, Olive PL. Residual γH2AX foci as an indication of lethal DNA lesions. BMC Cancer. 2010;10:4
20. De-Colle C, Yaromina A, Hennenlotter J, Thames H, Mueller AC, Neumann T. et al. Ex vivo γH2AX radiation sensitivity assay in prostate cancer: Inter-patient and intra-patient heterogeneity. Radiother Oncol. 2017;124:386-94
21. van Wijk LM, Vermeulen S, Meijers M, van Diest MF, Ter Haar NT, de Jonge MM. et al. The RECAP Test Rapidly and Reliably Identifies Homologous Recombination-Deficient Ovarian Carcinomas. Cancers (Basel). 2020;12:2805
22. van Oorschot B, Hovingh S, Dekker A, Stalpers LJ, Franken NAP. Predicting Radiosensitivity with Gamma-H2AX Foci Assay after Single High-Dose-Rate and Pulsed Dose-Rate Ionizing Irradiation. Radiat Res. 2016;185:190-8 9
23. Tamborino G, Nonnekens J, De Saint-Hubert M, Struelens L, Feijtel D, de Jong M. et al. Dosimetric evaluation of receptor-heterogeneity on the therapeutic efficacy of peptide receptor radionuclide therapy: correlation with DNA damage induction and in vivo survival. J Nucl Med. 2021
24. Bol K, Haeck JC, Groen HC, Niessen WJ, Bernsen MR, de Jong M. et al. Can DCE-MRI explain the heterogeneity in radiopeptide uptake imaged by SPECT in a pancreatic neuroendocrine tumor model? PloS one. 2013;8:e77076-e
25. Feijtel D, Doeswijk GN, Verkaik NS, Haeck JC, Chicco D, Angotti C. et al. Inter and intra-tumor somatostatin receptor 2 heterogeneity influences peptide receptor radionuclide therapy response. Theranostics. 2021;11:491-505
26. Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL. et al. [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med. 2001;28:1319-25
27. O'Neill E, Kersemans V, Allen PD, Terry SYA, Torres JB, Mosley M. et al. Imaging DNA Damage Repair In Vivo After <sup>177</sup>Lu-DOTATATE Therapy. J Nucl Med. 2020;61:743-50
28. Vandecaveye V, Dresen RC, Pauwels E, Van Binnebeek S, Vanslembrouck R, Baete K. et al. Early Whole-Body Diffusion-weighted MRI Helps Predict Long-term Outcome Following Peptide Receptor Radionuclide Therapy for Metastatic Neuroendocrine Tumors. Radiol Imaging Cancer. 2022;4:e210095
29. Olive PL, Banath JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;58:331-5
30. Li W, Li F, Huang Q, Shen J, Wolf F, He Y. et al. Quantitative, noninvasive imaging of radiation-induced DNA double-strand breaks in vivo. Cancer Res. 2011;71:4130-7
31. Turner HC, Lee Y, Weber W, Melo D, Kowell A, Ghandhi SA. et al. Effect of dose and dose rate on temporal γ-H2AX kinetics in mouse blood and spleen mononuclear cells in vivo following Cesium-137 administration. BMC Mol Cell Biol. 2019;20:13
32. Wang Q, Pujol-Canadell M, Taveras M, Garty G, Perrier J, Bueno-Beti C. et al. DNA damage response in peripheral mouse blood leukocytes in vivo after variable, low-dose rate exposure. Radiat Environ Biophys. 2020;59:89-98
33. Solier S, Pommier Y. The nuclear γ-H2AX apoptotic ring: implications for cancers and autoimmune diseases. Cell Mol Life Sci. 2014;71:2289-97
34. Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY). 2016;8:3-11
35. Siddiqui MS, François M, Fenech MF, Leifert WR. Persistent γH2AX: A promising molecular marker of DNA damage and aging. Mutat Res. 2015;766:1-19
Author contact
![]() Corresponding author: Professor Bart Cornelissen. University of Oxford, Oxford Institute for Radiation Oncology, Department of Oncology, Old Road Campus Research Building, Off Roosevelt Drive, Oxford OX3 7LJ, UK. bart.cornelissenox.ac.uk
Corresponding author: Professor Bart Cornelissen. University of Oxford, Oxford Institute for Radiation Oncology, Department of Oncology, Old Road Campus Research Building, Off Roosevelt Drive, Oxford OX3 7LJ, UK. bart.cornelissenox.ac.uk
 Global reach, higher impact
Global reach, higher impact