13.3
Impact Factor
Theranostics 2023; 13(4):1264-1285. doi:10.7150/thno.81860 This issue Cite
Review
Engineered mesenchymal stem cell-derived extracellular vesicles: A state-of-the-art multifunctional weapon against Alzheimer's disease
1. Department of Neurology, Second Affiliated Hospital of Naval Medical University (Shanghai Changzheng Hospital), Shanghai 200003, China.
2. Department of Clinical Pharmacy, Xinhua Hospital; Clinical pharmacy innovation institute, Shanghai Jiao Tong University of Medicine, Shanghai 200000, China.
3. Changhai Clinical Research Unit, Shanghai Changhai Hospital, Naval Medical University, Shanghai 200433, China.
*These authors contributed equally to this work.
Received 2022-12-14; Accepted 2023-1-21; Published 2023-2-5
Abstract
With the increase of population aging, the number of Alzheimer's disease (AD) patients is also increasing. According to current estimates, approximately 11% of people over 65 suffer from AD, and that percentage rises to 42% among people over 85. However, no effective treatment capable of decelerating or stopping AD progression is available. Furthermore, AD-targeted drugs composed of synthetic molecules pose concerns regarding biodegradation, clearance, immune response, and neurotoxicity. Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) are essential intercellular communication mediators holding great promise as AD therapeutics owing to their biocompatibility, versatility, effortless storage, superior safety, and the ability to transport messenger and noncoding RNAs, proteins, lipids, DNAs, and other bioactive compounds derived from cells. The functionalisation and engineering strategies of MSC-EVs are highlighted (e.g. preconditioning, drug loading, surface modification, and artificial EV fabrication), which could improve AD treatment by multiple therapeutic effects, including clearing abnormal protein accumulation and achieving neuroprotection and immunomodulatory effects. Herein, this review summarises state-of-the-art strategies to engineer MSC-EVs, discusses progress in their use as AD therapeutics, presents the perspectives and challenges associated with the related clinical applications, and concludes that engineered MSC-EVs show immense potential in AD therapy.
Keywords: Mesenchymal stem cells, Extracellular vesicles, Alzheimer's disease, Drug delivery, Nanoparticles
Introduction
Alzheimer's disease (AD) is a neurodegenerative illness responsible for up to 80% of dementia cases and is typically characterised by memory loss, cognitive impairment, behavioural abnormalities, and difficulty in performing daily activities. The World Health Organisation reports that the global number of AD patients is close to 35 million, with 10 million new cases added every year. Typical pathological changes of AD are the accumulation of β-amyloid plaques and hyperphosphorylation of Tau in neurofibrillary tangles [1, 2]. Such pathological protein deposition damages the cerebral cortex and hippocampal neurons, while the release of inflammatory mediators by microglia and astrocytes during the brain immune response may in turn exacerbate the chronic neuroinflammation of AD patients [3, 4].
Traditional AD therapy relies on three cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and an uncompetitive N-methyl-D-aspartic acid receptor 2 modulator (memantine) [5-7]. Recently, two new AD therapeutics have been developed, namely sodium oligomannate and Aduhelm. Sodium oligomannate, which improves the cognitive ability of mild-to-moderate AD patients, was approved in China in 2019 and is the first AD drug targeting the brain-gut axis [8]. Aduhelm was approved by the Food and Drug Administration (FDA) of the United States and is the first AD drug targeting amyloid-β (Aβ) aggregates, although the related therapeutic effect remains to be discussed [9]. However, the aforementioned medicines only aim to improve life quality and prolong the lifetime without preventing disease progression, and novel AD treatment strategies are therefore highly sought. One such strategy relies on mesenchymal stem cells (MSCs), which exhibit immunoregulatory and tissue regeneration abilities [10, 11]. Preclinical studies revealed that MSC transplantation exerts promising therapeutic effects, such as improving hippocampal neurogenesis and synaptic activity and suppressing neuroinflammation [12]. However, MSC-based therapy is limited by cell heterogeneity, immune responses, and risk of tumour formation, and primarily relies on extracellular vesicle (EV) secretion rather than on the replacement of parenchymal brain cells [13]. Notably, MSCs also secrete membrane-bound EVs containing biomolecules such as growth factors and cytokines [14, 15]. Exosomes are nanosized EVs with diameters of 40-160 nm that are released by a wide range of cell lines, including those found in the brain, contributing to cell-cell communication [16-19]. As MSC-EVs can express the therapeutic potential of parental cells and demonstrate the merits of high penetrability through the blood-brain barrier (BBB) and low immunogenicity, they hold great promise for AD treatment [20, 21]. As reported, MSC-EVs can penetrate the BBB via their active transport, probably through the traverse of microvascular endothelial cell monolayers of the brain with transiently formed interendothelial gaps [22] and target particular acceptor cells [23, 24]. Rather than studying the actual brain delivery, most studies rely on therapeutic outcomes of MSC-EV treatment in AD models instead, though some researchers also directly demonstrated EV distribution [25]. To date, MSC-EV therapy has achieved exceptional efficacy in many neurological disease models [10, 11, 26, 27] by reducing pathological protein accumulation, alleviating neuroinflammation and oxidative stress, and protecting the nerves [28-31]. Further functionalisation of MSC-EVs has also been studied for improved therapeutic performance. Various engineering techniques, including preconditioning, drug loading, surface modification, and artificial EV fabrication, have been utilised to enhance the effectiveness of MSC-EVs as AD therapeutics. Although several reviews have discussed the potential and significance of MSC-EVs [32-34], none have presented an overview of the state-of-the-art engineering strategies for MSC-EVs and the future perspectives of these functionalised MSC-EVs for AD treatment. Therefore, the present review comprehensively discusses the methodology of MSC-EV engineering and summarises the results of MSC-EV-based AD therapy. Initially, we review the biogenesis of MSC-EVs and their isolation and purification methods, subsequently summarising the physiological functions of MSC-EVs in AD, e.g. the facilitation of Aβ degeneration, neuronal function restoration, and Aβ and Tau degradation. Subsequently, we discuss the recent developments in MSC-EV-based therapeutics with an emphasis on engineered MSC-EVs and their cargo and surface engineering techniques. Furthermore, the applications of natural and bioengineered MSC-EVs in AD treatment are discussed. Finally, we address the advantages and future challenges of MSC-EVs as bioactive nanocarriers for AD therapy, demonstrating the enormous potential of this therapy and its multiple applications.
2. EV biogenesis and isolation
2.1. Biogenesis of EVs
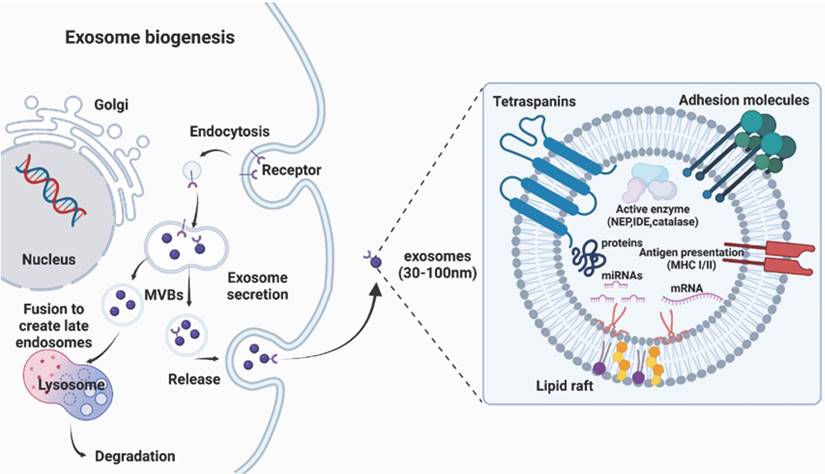
There are several routes for the generation of EVs, although the exact mechanism is still unclear. There are three broad categories of EVs: exosomes (30-150 nm in diameter), microvesicles (100-1000 nm), and apoptotic bodies (100-5000 nm). Because of the ambiguous boundary of EV subclasses implied in the guidelines presented by the International Society for EVs, this review primarily focuses on well-documented nanosized EVs called exosomes, and the genesis of exosomes, rather than all EVs categories, is presented in this article (Fig. 1). Exosome genesis begins with the formation of early inward-budding endosomes, which involve the trans-Golgi network and the endoplasmic reticulum and are triggered by cell membrane endocytosis. The invasion of late endosomes affords intraluminal vesicles (ILVs), which are then converted into multivesicular bodies (MVBs) that can fuse with the plasma membrane to release ILVs and produce EVs [35] or directly fuse with lysosomes/autophagosomes [19]. The fusion of MVBs with the lysosomes leads to the degradation of vesicular contents [36], while the fusion of MVBs with the plasma membrane of the cell releases vesicles via exocytosis, which are known as exosomes [37]. Since exosomes are formed by double invagination processes, their topologies are identical to those in cell plasma membranes [38].
2.2. Isolation of EVs
The first step of downstream analyses is to fabricate high-purity and high-quality EVs in high yield. However, as EVs contain multiple components and exhibit complex heterogeneity, efficient EV isolation and enrichment methods remain highly sought after, with a summary of available techniques presented in Table 1.
Ultracentrifugation, including differential and gradient ultracentrifugation, is widely used for EV separation and purification [46]. This method is useful in isolating EVs from large quantities of samples but is time-consuming [38]. Concerning gradient ultracentrifugation, EVs remain in the density equilibrium region and can therefore be separated from impurities [47]. However, repeated centrifugation and high centrifugal forces can irreversibly damage the isolated vesicles [48]. An innovative new technique for separating EVs from large samples is size-exclusion chromatography [49], relying on filtration through a column filled with a stationary phase containing porous beads of comparable size to the particles of interest [50]. This method offers the advantages of time savings, low cost, and repeatability but suffers from poor EV recovery and purity. Ultrafiltration, another size-based separation method, relies on EV interception by membranes with different pore sizes and offers the benefits of being efficient, economical, and easy and allows batch processing. However, ultrafiltration membranes possess narrow pores and are easily clogged when used to separate biological samples, while the excessive pressures used for ultrafiltration may induce EV deformation [51]. Additionally, EVs can be isolated using commercial kits that rely on polymer-based coprecipitation. Although this method eliminates the use of ultracentrifugation, which is costly and damages vesicles, it suffers from the potential coprecipitation of other macromolecules [38]. In general, the abovementioned methods exploit the physical properties of EVs and are not particularly specific.
The specificity of EV isolation can be improved using affinity-based capture strategies that utilise the high affinities of specific exosomal markers of their ligands [52]. Considering the similar morphologies, overlapping size ranges, and inherent heterogeneity of EVs, comprehensive characterisation and consistent purification processes that allow EV subtype isolation are lacking. There are advantages and disadvantages of each method, and several methods can be combined to maximise EV enrichment. Since of the main obstacles to the clinical use of EVs is the reproducibility of their extraction, optimized and standardized methods also need to be established.
Biogenesis and structure of exosomes, which originate from multivesicular bodies containing proteins (e.g. membrane transporters) and noncoding RNAs (e.g. microRNAs, long noncoding RNAs, and circular RNAs).
Overview of methods used for EV isolation.
| Methods | Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Differential ultracentrifugation | Differential Centrifugation based on density | High purity; Established protocols | Lengthy process; Large sample Volume | [39] |
| Gradient density ultracentrifugation | Density additional steps after centrifugation | High purity; Avoiding exosomal damage | Labour-intensive; Preliminary preparation; Cumbersome operation | [40] |
| Ultrafiltration | Based on particle size and molecular weight | High yields; High purity; Requires no special equipment; Easy to handle | Contamination of same-sized vesicles; Low specificity | [41] |
| Size-exclusion chromatography | Based on hydrodynamic Radius | Better repeatability; Time efficient; Less protein contamination | Low sample recovery | [42] |
| Immunoaffinity | Based on antibodies' interactions with specific membrane proteins in EVs | High specificity for EV subtypes isolation | High cost; marker dependent | [43] |
| Polymer-Based Precipitation | Precipitation with chemicals | Simple; fast | Lack specificity; much contamination; difficulty in scaling | [44] |
| Microfluidics-Based Isolation | Based on physical or mechanical properties | Fast; cheap; require less volume | Unsuitable for large samples; expensive equipment support | [45] |
The surface markers of MSC-EVs, storage, and routes of administration in disease treatment.
| EV source | Surface markers | Storage | Route of administration | References |
|---|---|---|---|---|
| hBMSCs | CD90, CD44, CD105, CD73, CD63, CD81, neuropilin 1 (+) CD34, CD45 (-) | storage at -80℃ in PBS with or without cryoprotective additives | lateral ventricle injection, IV, IN | [58-62] |
| hCMSCs | CD105 (+), CD90 (low), CD117 (-) | storage at -80℃ in PBS with or without cryoprotective additives | ND | [62, 63] |
| hUC-MSCs | CD90, CD73, CD105, CD44, CD9, CD63, CD81 (+) CD45, CD34, CD11b, CD19, HLA-DR (-) | storage at -80℃ in PBS with or without cryoprotective additives | IV | [62, 64, 65] |
hBMSCs, human bone marrow-derived mesenchymal stem cells; hCMSCs, human cardiac mesenchymal stromal cells; hUC-MSCs, human umbilical cord-derived MSCs; PBS, Phosphate‐buffered saline.
After isolation, EVs are mostly characterized by size measurement (nanoparticle tracking analysis [NTA], dynamic light scattering, etc.), morphology evaluation (electron microscopy or atomic force microscopy) or assessment of enriched protein expression (western blot, enzyme-linked immunosorbent assay, etc.) [53]. The most used surface markers to identify MSC-EVs are CD73, CD105 and CD90 [54]. However, the surface components of EVs derived from different MSCs are affected by their parent cells, so proteomics for MSC-EVs from different tissues needs further study (Table 2). The methodology for EV storage is rarely reported, although it affects both morphology and physicochemical parameters of EV simultaneously. As of now, 80°C seems to be the most promising storage temperature for EVs. However, the cost and transportation challenges of applying this mode may limit its application. It is therefore possible to enhance EV storage stability by using alternatives such as lyophilization and addition of additives [55]. The administration of MSC-EVs in AD treatment include localized administration (eg. dorsal hippocampus injection [56]) and systemic administration (eg. intravenous injection [57], intranasal administration [58]). Due to the BBB permeability of MSC-EVs, systemic administration has the advantage of minimally invasive over other administration routes.
3. Brain delivery of MSC-EVs
The BBB controls the entry of nutrients from the vascular system into the extracellular fluid of the central nervous system (CNS) and limits the passage of harmful exogenous molecules to maintain homeostasis, which severely limits the therapeutic efficacy of drugs against CNS diseases including AD [66]. Advantageously, MSC-EVs, which contain small-molecule nucleic acids, proteins, and other cell growth regulators, can penetrate the BBB to exert therapeutic effects in the brain and have therefore been adopted in a wide range of studies on different diseases, such as AD (Table 3) [67-71].
One of the most remarkable characteristics of MSC-EVs is their capacity to circulate in the bloodstream and cross the BBB. Although it is unclear how natural MSC-EVs cross the BBB, researchers have found that MSCs crossed the brain microvascular endothelial cell (BMEC) monolayers through transient intercellular gaps formed between BMECs [72]. Transmigration of MSCs across the endothelium in peripheral tissues is mediated by integrin-mediated adhesion or matrix metalloproteinase-dependent extracellular matrix degradation [73, 74]. Because the membrane of EVs derived from MSCs shares similar features with MSCs, these findings may offer clues for further investigation into the exact mechanism underlying BBB penetration by MSC-EVs. Moreover, as BMECs cannot represent the function of the complete BBB, a quantitative and qualitative method is needed for monitoring MSC-EV delivery in vivo to the brain [75].
Apart from the traditional intravenous (IV) delivery strategy, nasal delivery has also been studied as a non-invasive method to send MSC-EVs directly to the brain [58, 76], and the olfactory mucosa pathway in the olfactory region is the most essential route to the brain [77]. Intranasal administration of PKH-26 labelled MSC-EVs mainly reached neurons, compared with microglia and astrocytes [78], while another study observed a higher absorption by microglia and neurons in the hippocampus but not by astrocytes [58]. In addition, EVs labelled with C5 Maleimide-Alexa 594 were found in hippocampal neurons 24 hours after their IV injection [31]. Five hours after IV injection, MSC-EVs labelled with DiI were detected in the brain cortex, hippocampus, lungs, and spleen [57].
It is noteworthy that the lipid dyes used in MSC-EV studies have the limitations of non-specific marking and formation of aggregates from unbound dye [79], which can result in artefactual results and erroneous conclusions. Therefore, the derivation of MSC-EV delivery requires experimental methods such as advanced imaging techniques and rigorous controls [79].
4. Physiological functions of MSC-EVs in AD models
Initially, MSC therapies were demonstrated in bone and cartilage to induce tissue repair and regeneration [80]. Several MSC therapies became available soon after, including treatments for brain diseases [81]. MSC therapy studies conducted in mice and humans initially confirmed positive results, with clinical trials expected soon [82, 83]. Despite these positive results, negative effects soon occurred, including immunological rejection and cancerous growth due to the size of the cells and the risk of embolism complications [84]. These unexpected inconveniences lead to rapid reductions in MSC therapies, followed by the withdrawal of initial clinical trials. The discovery that MSC therapies had serious problems led to studies on their secreted vesicles [81, 85]. Several curative effects of MSCs have been recapitulated by MSC-EVs, which also leads to unexpected benefits, such as higher safety and tissue penetration [86]. Moreover, MSC-EVs are incapable of self-replicating, which prevents many risks of MSC therapy. In comparison with cell-based approaches, MSC-EV therapy demonstrated favourable effects like activating the immune system and prolonging the therapeutic effects [87].
Effects of native MSC-EVs on AD in animal models
| EV Source | Route of Administration | Therapeutic Effects | Mechanism of Action | References | |
|---|---|---|---|---|---|
| In vivo | |||||
| rBMSCs (Primary cell) | lateral ventricle injection | ↓Aβ deposition and soluble Aβ1-42 ↑Neuron viability ↓Neuron apoptosis rate ↓Pro-inflammatory factors (IL-1β, IL-6, and TNF-α) | ↑Expressions of NEP and IDE ↑Expression of miR-29c-3p ↓Expression of BACE1 ↑Activation of Wnt/β-catenin pathway | [97] | |
| mBMSCs (Primary cell) | IV | ↓Aβ deposition ↑Cognitive function | ↑Activation of SphK/S1P | [98] | |
| WJM-SCs (Primary cell) | IV | ↓Aβ deposition and astrocyte activation ↑Brain glucose metabolism and cognitive function | ↓Expression of HDAC4 ↑Expression of neuronal memory and synaptic plasticity-related genes | [32] | |
| hUCMSCs (Primary cell) | IV | ↓Immune memory in the brain ↓Deteriorated neuropathology caused by immune training | IL-10 dependent mechanism | [88] | |
| hUCMSCs (Primary cell) | IV | ↓Aβ deposition, neuronal loss and calcium transients ↑Cognitive function and hippocampal neuronal excitability Neuronal morphology alterations repair ↓mitochondrial changes | ↓Associated with Nrf2 defence system Nrf2, HO-1, iNOS, NQO1 ↑Keap1 | [31] | |
| mBMSCs (Primary cell) | lateral ventricle injection | ↑Cognitive function | ↑CA1 synaptic transmission ↓LTP and iNOS expression | [92] | |
| mBMSCs (Primary cell) | lateral ventricle injection/IV | ↑Cognitive function ↓Activation of microglia and astrocytes ↓IL-1β, IL-6, TNF-α, Aβ1-42, p-Tau | ↑Expression of BDNF | [99] | |
| hBMSCs (Primary cell) | Intracerebroventricular CSF exchange | ↑Cognitive function and neuronal counts ↓Astrocytic burden ↑Cell proliferation and neurogenesis | ND | [90] | |
| rBMSCs (Primary cell) | IV | Improves the degenerative effects on circumvallate taste buds and gustatory nerve fibres | ND | [91] | |
| in vitro | |||||
| hUCMSCs (Primary cell) | ↓Apoptosis rate and concentrations of inflammatory factors | ↑Expression of miR-223 Inhibition of PTEN-PI3K/Akt pathway | [89] | ||
| hADSCs (Primary cell) | ↓ Extracellular and intracellular Aβ levels | NEP associated mechanism | [100] |
BDNF, brain-derived neurotrophic factor; CSF, cerebrospinal fluid; hADSCs, human adipose tissue-derived mesenchymal stem cells; hUCMSCs, human umbilical cord tissue-derived mesenchymal stem cells; hBMSCs, human bone marrow-derived mesenchymal stem cells; IV, intravenous injection; LTP, long-term potentiation; mBMSCs, mouse bone marrow-derived mesenchymal stem cells; rBMSCs, rat bone marrow-derived mesenchymal stem cells; WJ-MSCs, Wharton's jelly-derived mesenchymal stem cells; ND, not detected.
Main pathological processes of AD and the key mechanisms by which MSC-EVs mitigate the related pathogenesis and induce nerve regeneration.
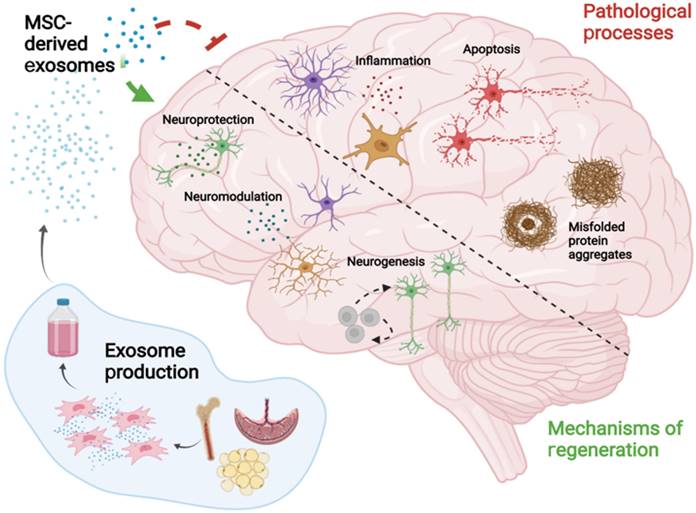
MSC-EVs were observed to improve pathological changes and cognition in AD mice by clearing abnormal protein accumulation [14, 29, 88], achieving neuroprotection [32, 58, 89], relieving inflammation [90, 91], and alleviating oxidative stress [31, 92, 93] (Fig. 2). MSC-EVs exhibit the homing ability and can target pathological regions in AD models, thus representing promising drug delivery platforms for AD treatment [94]. Nevertheless, the EVs that do not naturally express targeting ligands have the least penetrating capacity, and thus the functionalisation of MSC-EVs seems to become a solution, which has been discussed in 5.1.3.
Apart from MSC-EVs, AD has been studied using EVs derived from other cell lines. In the brain, astrocytes, which are the most abundant glial cells, are used to produce exosomes with ultrasound stimulation [95]. The result indicated that exosomes secreted by astrocytes can reduce in vitro toxicity and plaques caused by Aβ in AD mice. To manage the neuroinflammation linked to AD, yeast cell wall vesicles loaded with sitagliptin, a medication having an anti-inflammatory action, was created [96]. It was shown that the optimised vesicles were less toxic than the sitagliptin due to their spherical shape and negative surface charge.
In this review, we focus on MSC-EVs for the unique characteristics inherited from their parent cells and may perform superior functions to EVs derived from other cells, which has been discussed in section 8.1.
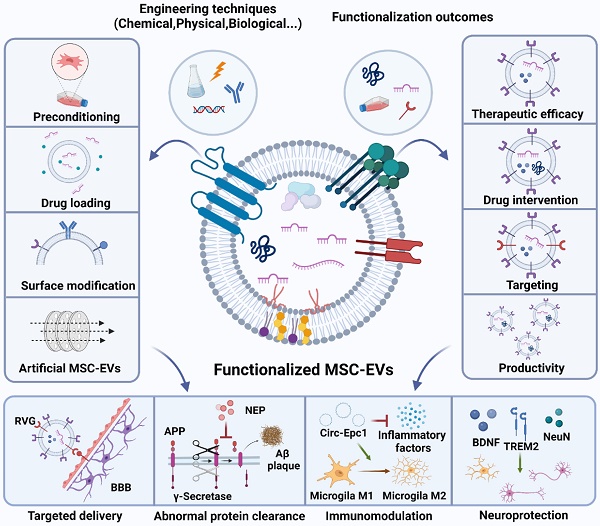
4.1. Clearance of abnormal protein accumulation by MSC-EVs
Pathological proteins, including Aβ plaques and hyperphosphorylated tau, which cause synaptic disruption and neuronal degeneration, are the key elements in the pathology of AD, and the removal of these abnormal proteins will significantly benefit AD treatment [101]. Notably, natural MSC-EVs have been discovered to have the potential for pathological protein clearance through modulating autophagy and regulating the levels of critical proteins in AD development with inherited proteins and microRNAs (miRs). miR is a set of non-coding RNA of 22-24 nucleotides long that can be found in EVs and attaches to the 3′ untranslated region of mRNA to silence the mRNA transcript [102]. Recent studies have investigated miRs associated with various pathways in AD patients [103, 104].
Recent research published in Nature Neuroscience has revealed that lysosome dysfunction that causes autophagy failure has a close correlation with AD pathology and Aβ deposition, that is, autolysosome acidification declines in neurons before extracellular amyloid deposition, associated with a significant decrease in v-ATPase activity and selective accumulation of Aβ/Aβ precursor protein (APP) within enlarged de-acidified autolysosomes [105]. In addition, autophagy activation was proven to be effective in Aβ plaque scavenging, indicating that autophagy activation represents a promising strategy against AD through clearance of abnormal protein accumulation [105]. It is noteworthy that MSCs and MSC-EVs show the intrinsic ability to activate autophagy to increase the reduction of abnormal protein, resulting in the remarkable amelioration of AD [106, 107].
In addition to the intrinsic ability to activate autophagy for the clearance of abnormal protein, MSC-EVs could also regulate the expression of a key protein that could clear abnormal protein. For example, researchers also found that MSC-EVs exert a therapeutic role in AD treatment through the regulation of sphingosine 1-phosphate (S1P) expression, which can stimulate autophagy, protect neurons, regulate the inflammatory response of glial cells, and antagonise apoptosis to inhibit the pathological changes induced by AD [108]. S1P receptors are expressed throughout the body and play key roles in angiogenesis, neurogenesis, immune cell transportation, endothelial barrier functioning, and vascular tone regulation [109]. It has been reported that MSC-EVs improve spatial cognition in AD mice and promote S1P and sphingosine kinase (SphK) 1 expression, which catalyses the phosphorylation of sphingosine to S1P [98]. Furthermore, MSC-EVs reduce amyloid levels and enhance the expression of the anti-neuronal core antigen of APP/PS1 mice, decreasing the concentrations of Aβ oligomers (AβOs), β-site APP-cleaving enzyme (BACE) and presenilin 1 and promoting the expression of neprilysin (NEP). In conclusion, the abovementioned studies found that MSC-EVs improve memory restoration in AD mice through the activation of the SphK/S1P signalling pathway and reduce Aβ deposition through various mechanisms, including autophagy modulation. In addition, NEP is a completely membrane-bound metallopeptidase that degrades neuropeptides and amyloid proteins and is therefore a potential target for AD treatment [110]. Importantly, NEP is known to exist on MSC-EVs, and its role in reducing Aβ deposition has been evaluated in vivo and in vitro [65, 100, 111].
MSC-EVs have also been discovered to have the potential for pathological protein clearance through their inherited miRs. APP proteolysis begins with cleavage by β-secretase, also denoted BACE, which makes BACE1 inhibitors promising AD therapeutics [112]. MiRs are noncoding RNAs closely related to AD pathogenesis and demonstrate enormous potential as therapeutic biomarkers [113]. Recent studies have demonstrated that BACE1 regulates AD progression via miRs [86, 114]. Sha et al. [97] discovered that miR-29c-3p delivered to neurons by bone marrow MSC-EVs (BMSC-EVs) inhibits BACE1 expression and activates the Wnt/β-catenin pathway to downregulate the level of BACE1[115] and thereby exert a therapeutic effect on AD. Jeong et al. demonstrated that compared with Aβ injection groups, human umbilical cord-derived mesenchymal stem cell (hUCMSC) groups exhibited a significant reduction in amyloid plaque and BACE1 and an increase in neurogenesis [65].
Overall, lysosome dysfunction, autophagy failure and accumulation of abnormal protein are the main characteristics of AD. MSC-EVs can effectively restore the autophagy function and accelerate the clearance of abnormal protein through multiple mechanisms, and finally provoke distinguished anti-AD effects, thus representing a promising strategy against AD.
4.2. Immunomodulatory effects of MSC-EVs
Neuroinflammation contributes significantly to AD pathogenesis, which is mainly characterised by overactive microglia, morphological and functional alterations in neurons, and upregulated inflammatory factors [116]. During the early stages of AD, activated microglia are primarily characterized by their neuroprotective and anti-inflammatory M2 phenotype. As AD progresses, hyperactivation of microglia becomes detrimental and pro-inflammatory, resulting in the M1 phenotype [117]. BMSC-EVs were reported to contain miR-146a, which contributes to microglial polarisation and is notably overexpressed in proinflammatory microglia (M1 type) [118]. In addition, a high level of miR-146a is detected in the brains of AD patients, and its expression is negatively regulated by nuclear factor-κB, therefore representing a promising therapeutic [81]. Nakano et al. reported that miR-146a from BMSC-EVs is taken up by astrocytes and inhibits inflammation in AD mice models [81]. The treatment of AD model mice with BMSCs was shown to improve synaptic density, alleviate inflammation within astrocytes, and reduce the M1 microglia ratio. The same function was found in EVs secreted by adipose tissue-derived mesenchymal stem cells [119].
IL-10 is a cytokine that suppresses immune responses in peripheral immunity. Studies aimed at understanding the antiphlogistic mechanisms of MSCs discovered that IL-10 plays an important role in macrophage phenotype switching [from the M1 (inflammatory) type to the M2 (anti-inflammatory) type] triggering MSC-induced repair [120]. Thus, IL-10 is essential to regulate aberrant immune activation and signalling associated with AD. Feng et al. discovered that MSC-EVs can mitigate trained immune responses in the CNS and demonstrated the important role of IL-10 in this process [88]. The development of taste disorders results from the activation of inflammatory pathways [121]. Exosomes derived from BMSCs can reduce the destructive changes caused by AD in circumvallate papilla taste buds [91].
MSC-EVs also exert a therapeutic effect on AD by reducing oxidative stress, which is vital for the pathogenesis of cognitive deficits and the development of neurological injuries [122]. Overexpressed or dysregulated proinflammatory cytokines (iNOS) contribute to AD pathology [123]. Wang et al. observed that exogenous Aβ-induced iNOS mRNA and protein expression was reduced by MSC-EVs, which exerted beneficial effects on APP/PS1 mice [92]. Currently, an increasing amount of evidence indicates that neurotrophic factor (NF)-E2-related factor like-2 (Nrf2) participate in the redox state and anti-inflammatory effects in neurodegeneration [124]. Notably, the Nrf2 signalling pathway increases antioxidant gene expression, inhibits microglia-mediated inflammation, and improves mitochondrial functioning in diseases of the nervous system, which indicates that Nrf2 activation is an innovative therapeutic approach to target AD development [125]. In APP/PS1 mice, the Nrf2 defence system participates in reducing neuronal deficits caused by MSC-EVs [31].
AD pathophysiology is affected by glial changes in morphology, function, and gene expression caused by AβO that lead to synaptotoxicity and neuronal death [126, 127]. Godoy et al. found that MSC-EVs naturally contain and carry endogenous catalase and provide protection against oxidative stress by delivering this enzyme to neurons and preventing synaptic loss in neurons exposed to AβOs [93].
In general, the above studies show that MSC-EVs alleviate inflammation through various mechanisms (e.g. microglial phenotype switching, inflammatory cytokine regulation, and oxidative stress reduction) and thus hold great promise for AD therapy.
4.3. Neuroprotective effects of MSC-EVs
MSC-EVs can improve AD symptoms by exerting neuroprotective effects and promoting neurogenesis. For example, miR-223 delivered by MSC-EVs protects neurons from apoptosis by activating the phosphatase and tensin homolog-phosphatidylinositol-3-kinase (PTEN-PI3K/Akt) pathway [28]. As astrocytes are the key cells that form synapses, recovery of astrocyte function may lead to synaptic occurrence and cognitive impairment. Nakano et al. discovered that the intracerebroventricular injection of BMSC-EVs containing miR-146a ameliorates cognitive impairment in AD mice by reducing astrocyte inflammation and improving synaptic development [81]. Similarly, Chen et al. found that in SH-SY5Y human neuroblastoma cells, MSC-EVs increase gene expression associated with synaptic plasticity and memory, including Homer1, GluR1, GluR2, NR2A, NR2B, Syp, and brain-derived NF (BDNF exon IV) [32]. Treatment with MSC-EVs can also be used to regulate the neuronal and astrocyte phases of AD mice [32]. Wang et al. reported that MSC-EV therapy restores the alterations of dendritic spines, calcium oscillations, abnormal action potential, and mitochondrial changes in AD models, highlighting the therapeutic efficacy of MSC-EVs toward AD [31]. Additionally, MSC-EV therapy through intranasal delivery [76] and cerebrospinal fluid (CSF) exchange [90] increases the neuronal density in the brain, which is typically related to reduced hippocampal shrinkage. Overall, these studies indicate that MSC-EVs can protect neurons and astrocytes and accelerate nerve regeneration in AD mice by recovering functionality and neural cells, thus holding great promise for AD treatment.
5. Engineered MSC-EVs for AD therapy
5.1. EV engineering techniques
Although MSC-EVs can be of therapeutic value for AD, their application is still hindered by several drawbacks, including low targeting efficiency, nonuniform treatment results, and low production yield [128]. Therefore, advanced engineering techniques are needed to functionalise MSC-EVs and thus increase their efficiency and potency as AD therapeutics. These techniques include parental cell preconditioning to enhance the inherent treatment effect, therapeutic cargo loading into MSC-EVs, MSC-EV surface modification to enhance targeting, and artificial MSC-EVs to improve productivity (Fig. 3).
5.1.1. Preconditioning
Preconditioning includes parent cell manipulations using specific culturing conditions, such as hypoxia, 3D culturing, and serum deprivation. Biochemical stimuli, including lipopolysaccharides, nitric oxide, and proinflammatory cytokines and exogenous genes such as plasmid DNA and miRNAs, can also be introduced into the culturing environment [129-131].
Preconditioning-based strategies are often used to enhance MSC functional characteristics before transplantation through culturing environment modification. EV generation by cellular metabolic processes is partly responsible for the genetic and phenotypic characteristics of the cells used for MSC-EV production, which can be altered by extrinsic stimulation [132]. Nevertheless, considering various physiological conditions of MSCs, the optimisation of preconditioning approaches needs further research. As preconditioning can stress parental cells, it is necessary to investigate the generated contents in EVs that might negatively impact therapeutic results.
5.1.2. Drug loading
As part of the cargo delivery system, EVs can be used to treat recipient cells by releasing both naturally formed contents and loaded therapeutics such as chemicals, proteins, peptides, and nucleic acids. Drug loading strategies can be divided into two categories: endogenous (drug-loaded EVs are collected after parental cell pretreatment using various approaches) and exogenous (exogenous drugs are loaded into isolated EVs) [133].
Strategies for the engineering of EVs: (A) preconditioning of parental cells to enhance the inherent treatment effect; (B) loading of therapeutic cargoes; (C) surface modification; and (D) fabrication of artificial EVs through the top-down disruption of parent cell membranes.
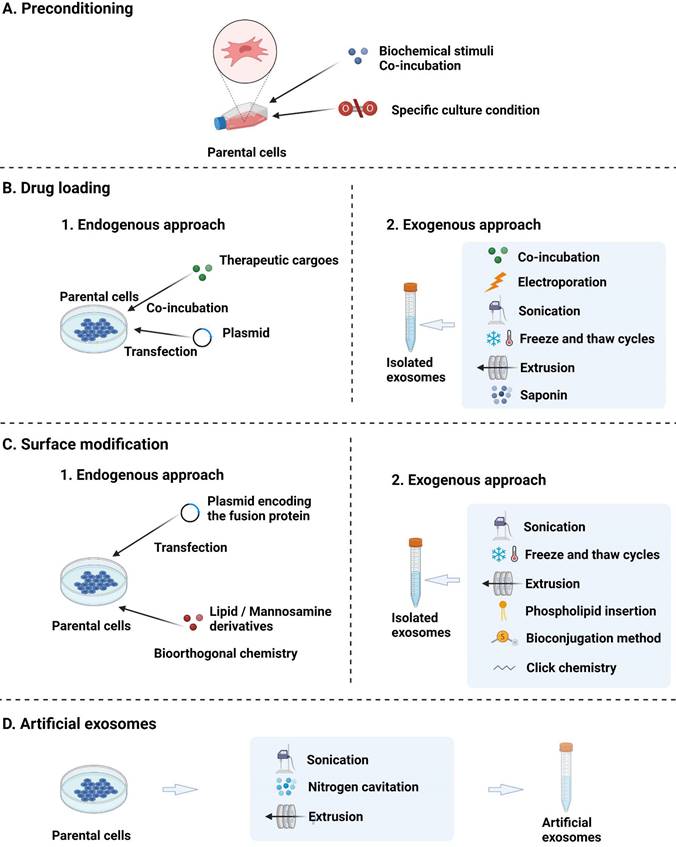
1) In the endogenous approach, therapeutic cargoes are loaded into EVs using the intrinsic cellular cargo transfer system and are packed in the secreted EVs [134]. The endogenous approach may be preferred to the exogenous approach, allowing EVs to be loaded with cargoes at the cellular level and facilitating small RNA expression induced by vector transfection [135]. However, the dependence on parent cell physiology may not allow for customised modification and result in uneven cargo levels in EVs [135]. The endogenous approach can be subdivided into two methods. In the first method, drugs are cocultured with parent cells and are naturally present in the secreted EVs. However, the achieved transfection efficiency is low, and this method is therefore mainly used for chemical drugs with low cytotoxicity [136]. In the second strategy, parent cells are transfected with chemical methods such as liposome transfection to carry drugs, although the transfection efficiency is low [137]. This method is mainly used for gene drugs, which can be transfected into parental cells to overexpress the elements of interest that are subsequently transferred into EVs and function within the target cells [138].
2) Exogenous strategies allow EVs to be loaded with specific drugs with better control over cargo loading and can be categorised as follows: 1) Coculturing of drugs with extracted EVs (for chemical drugs). The incubation of cargoes with EVs is a simple method, but the loading efficiency requires improvement, as the scope of loaded drugs is often limited to hydrophobic compounds [139]. 2) Transfer of drugs into EVs using physical strategies. A common physical approach to drug loading is electroporation, wherein charges are loaded into EVs using weak current pulses, and the phospholipid bilayer is broken down to form a recoverable hole that facilitates drug loading [140]. Other physical strategies, such as hypotonic dialysis, saponin incubation, extrusion, sonication, pH neutralisation, and freeze-thaw cycling, have also been used to exogenously load therapeutic drugs into natural EVs [141]. 3) Direct transfer of drugs into EVs by chemical (e.g. liposome) transfection methods. In this case, transfection reagents or permeabilisers are used to facilitate cargo entry into EVs without damaging the lipid bilayer structure. For example, acoustofluidics has been demonstrated as an effective and fast way of loading drugs into EVs [142]. In general, during postloading, EV aggregation, membrane damage, or the elicitation of inflammation should be cautiously avoided.
All the above-mentioned methods have been used to load cargo into EVs. However, as the drug loading efficiency may depend on the method used [140], the related techniques need to be optimised for maximal drug loading efficiency.
5.1.3. Surface modification
The surface modification allows natural EVs to be imbued with unique functionality, as exemplified by the targeting of peptides or sites for chemical modification via genetic manipulation [143]. Similar to cargo loading, surface functionalisation can be categorised into endogenous and exogenous functionalisation. 1) Endogenous approaches allow the EV surface to be genetically engineered to bear specific peptides or ligands by transfecting cells with vectors encoding these molecules [144]. Moreover, EVs can be endogenously modified using bioorthogonal chemistry, which is characterised by high efficiency and reproducibility. Even though endogenous approaches maintain important EV functionality and integrity, they suffer from heterogeneity in the secreted EV population and the complexity of the purification process used to separate functionalised EVs from native EVs.
2) In contrast to endogenous approaches, exogenous surface modification is performed directly on the EV membrane by physical and chemical means. Physical methods include extrusion, sonication, and freeze-thaw cycling [145] and allow for reagent-free functionalisation but may result in internal cargo loss. Chemical approaches include a series of facile click chemistry processes to covalently conjugate EV lipid or protein constructs with different linker groups to achieve various functionalities, as exemplified by thiol-maleimide [146] and azide-alkyne cycloadditions [147]. Regardless of the method used for EV functionalisation, it is essential to investigate whether surface modification affects the functionality, integrity, and therapeutic potential of EVs.
5.1.4. Artificial EVs
MSC-EV usage as therapeutics demands processes that afford EVs in high yields and are suitable for use in clinical settings. Unlike natural EVs, artificial EVs are usually produced by disintegrating the cell membrane with top-down strategies, including extrusion, microfluidic technologies, nitrogen cavitation, and sonication, and are therefore obtained in much higher yields [148]. Several intracellular elements of parental cells can be preserved in artificial EVs using these processes [149]. Notably, artificial EVs can contain up to twice as much RNA and protein as natural EVs [128] and can be alternatively loaded with therapeutic molecules. For example, drugs can be added into artificial EVs during the shearing process or by shearing cells preloaded with cargoes of interest [150]. Similar to EVs, these artificial molecules can also be loaded via electroporation and vector infection [151, 152]. Considering their similarity to their endogenous counterparts and, more importantly, their ability to bypass the time-consuming and labour-intensive isolation procedures of natural EVs, artificial EVs represent a promising alternative to natural EVs [153]. Because artificial EVs are a new field, there is a lack of information from both preclinical and clinical studies for AD therapy, further research on these nanocarriers in AD treatment is needed. There are still several difficulties in realising the clinical benefits of artificial EVs, including 1) contamination with other organelles and nuclei during the purification process, 2) low coating efficiency due to the limited quality control, and 3) the ratio of cell membranes if fused membranes are used [154]. Thus, for artificial EVs, standard operating procedures, quantification of cell membranes on cargoes, and the composition of hybrid cell membranes are required to be clarified for their further application in AD treatment.
5.2. Applications of bioengineered MSC-EVs in AD therapy
Previously, we discussed recent technological developments in the engineering of therapeutic EVs. As the use of artificial MSC-EVs in AD treatment has not yet been reported, sections 5.2.1-5.2.3 present a review of the current applications of preconditioned, drug-loaded, and surface-modified MSC-EVs in AD therapy (Table 4).
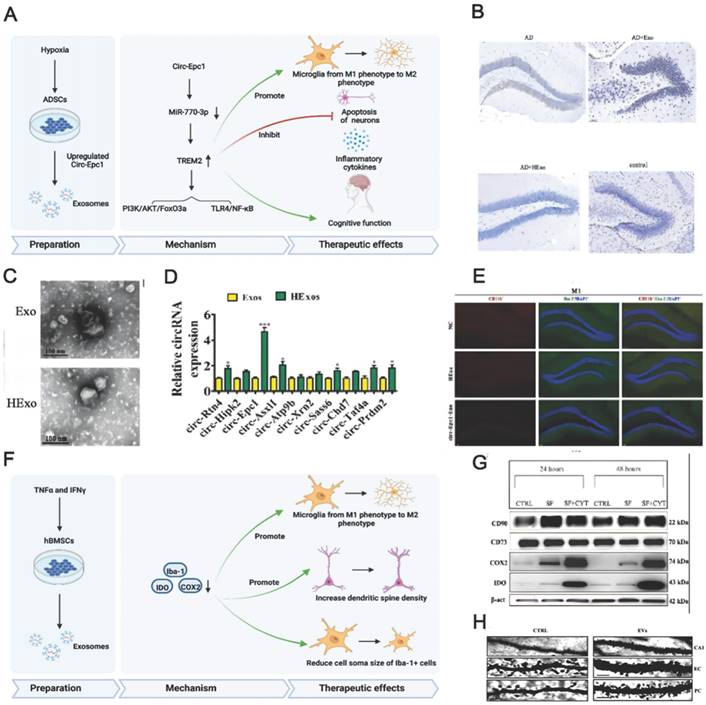
(A) Schematics of the use of hypoxia-preconditioned MSCs-derived exosomes in AD therapy. (B) Hippocampal neuron apoptosis detection by terminal deoxynucleotidyl transferase nick end labelling. (C) ADSC-exosome morphology under a transmission electron microscope. Scale bar: 100 nm. (D) Reverse transcription-quantitative polymerase chain reaction detection of circRNAs. (E) Detecting macrophage polarisation with immunofluorescence. Reproduced from ref. 156. Copyright © 2022 Haining Liu et al. (F) Schematics of the use of TNFα and IFNγ-preconditioned MSC-derived exosomes in AD therapy. (G) Typical stemness and immunoregulatory markers expressed by the MSCs (CTRL: control; SF: serum‐free; CYT: cytokines, TNFα and interferon gamma). (H) Photomicrographs of Golgi‐Cox-stained dendritic segments. Reproduced from ref. 58. Copyright © 2022 Morris Losurdo et al.
Applications of engineered MSC-EVs in AD treatment.
| EV Source | Modification | Route of Administration | Therapeutic Effects | Mechanism of Action | References |
|---|---|---|---|---|---|
| mBMSCs (Primary cells) | Surface Modification with RVG peptide | IV | ↑Learning and memory function | ↓Aβ deposition and activation of astrocytes ↓Pro-inflammatory factors ↑Anti-inflammatory factors | [30] |
| rBMSCs (ND) | Loaded miR-29 | dorsal hippocampus injection | ↑Cognitive and learning function | ↓Expression of BACE1 ↑Activation of PKA/CREB | [56] |
| mADSCs (ND) | Loaded miR-22 | IV/coculture | ↑Cognitive and learning function | ↓Pro-inflammatory factors ↓Neuroinflammation ↓Damage to nerve cells ↓Inflammation and pyroptosis ↓Expression of NLRP3, GSDMD and p30‐GSDMD | [155] |
| hUCMSCs (Primary cells) | Loaded NEP | IV | ↑Memory function | ↓Aβ deposition and inflammatory Response ↓Expression of BACE-1 ↑Expression of NEP ↑BDNF levels and neurogenesis | [65] |
| mBMSCs (Primary cells) | preconditioned in vitro in an AD environment | IV/IN | ↑Memory function and lifespan | ↓Aβ deposition and AβO concentration ↓Neuroinflammation ↑Neuronal density | [76] |
| mADSCs (Primary cells) | hypoxic preconditioned | ND | ↑Cognitive function | ↓Nerve apoptosis Circ-Epc1 associated mechanism ↓Expression of miR-770-3p and inflammatory factors ↑Expression of TREM2 Shifting microglial M1/M2 polarisation | [156] |
| mBMSCs (Primary cells) | hypoxic preconditioned | IV | ↑learning function ↑Memory function | ↓Aβ deposition and pro-inflammatory factors ↑Expression of synapsin 1 and PSD95 ↓Activation of astrocytes and microglia ↓Shifting microglial M1/M2 polarisation ↑Anti-inflammatory factors and level of miR-21 ↓Phosphorylation of STAT3 and NF-kB activation | [57] |
| hBMSCs (Primary cells) | TNFα and INFγ -preconditioned | IN | ND | ↓Shift microglial M1/M2 polarisation, microglia activation ↑Dendritic spine density | [58] |
| hUCB-MSC (Primary cells) | Graphene scaffold 3D culture | Coculture/hippocampus injection | ↑Learning and Memory Function | ↑Expression of NEP, IDE, HSP70 ↓Aβ deposition, TNF-α, IL1β | [157] |
GSDMD, gasdermin D; IN, intranasal administration; IV, intravenous injection; hBMSC, human bone marrow-derived mesenchymal stem cells; hUCBMSC, human umbilical cord blood-derived mesenchymal stem cells; hUCMSCs, human umbilical cord tissue-derived mesenchymal stem cells; mADSCs, mouse adipose-derived stem cells; mBMSCs, mouse bone marrow-derived mesenchymal stem cells; NEP, neprilysin; NLRP3, Nucleotide oligomerization domain-like receptor thermal protein domain associated protein 3; rBMSCs, rat bone marrow-derived mesenchymal stem cells; RVG, rabies viral glycoprotein; TNF, tumor necrosis factor; ND, not determined.
5.2.1. Preconditioned MSC-EVs for AD therapy
Preconditioned MSC-EVs can be used for AD treatment and are most prepared using hypoxic culturing and preconditioning with proinflammatory cytokines. Having MSCs accustomed to the hypoxic environment in advance may help them cope with the barren environment in vivo. High levels of hypoxia-inducible factor (HIF-1α), ROS (reactive oxygen species), and anti-inflammatory cytokines may enhance the neuroprotective ability of normoxic- and hypoxic-cultured MSCs [158]. Additionally, hypoxia participates in AD development, and intensive hypoxic training may prevent neuronal loss associated with AD and promote cognitive functions [159]. Using adipose tissue-derived stem cells (ADSCs) pretreated with hypoxia, Liu et al. revealed that ADSC-derived exosomes improved cognition by reducing neuronal damage in AD models [156]. Circular RNA-Epc1 (Circ-Epc1), which is upregulated in these exosomes, was responsible for improving cognitive functions, protecting neurons, and shifting microglial M1/M2 polarisation in exosome-treated APP/PS1 mice (Fig. 4A-E). Similarly, Cui et al. found that miR-21 overexpression in hypoxia-preconditioned MSC-EVs not only decreased Aβ deposition but also reduced proinflammatory factors, including TNF-α (tumor necrosis factor-α) and IL-1β (interleukin-1β) [57]. In addition, EVs derived from MSCs preconditioned with hypoxia improved cognitive function by preventing synaptic malfunction and alleviating inflammation in AD mice.
The immunoregulatory capacity of MSC-EVs can also be increased by preconditioning with proinflammatory cytokines. Losurdo et al. discovered that EVs derived from TNF-α and INFγ -preconditioned MSCs decreased microglial activation and increased dendritic spine density in AD to promote immunomodulatory and neuroprotective effects (Fig. 4F-H) [58]. Paracrine mechanisms are activated when infused MSCs are exposed to an injured environment by releasing bioactive factors in their secretome.
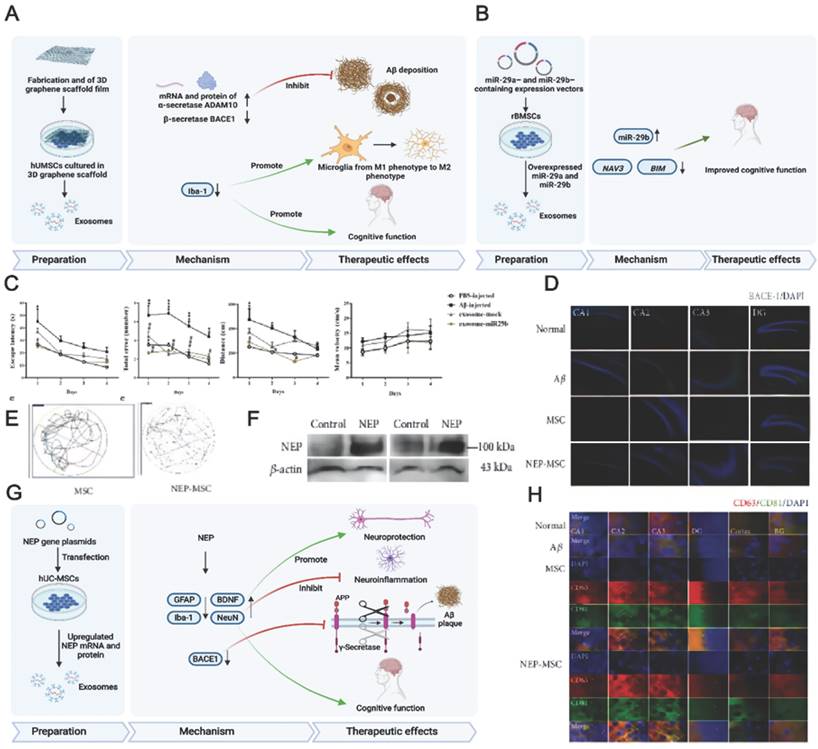
A previous study demonstrated that using a secretome collected from MSCs preconditioned in vitro in an AD environment led to sustained memory recovery, neuropathology repair, increased neuronal density, and reduced hippocampal shrinkage in APP/PS1 mice while increasing their lifespan [76]. Growing evidence has demonstrated that 3D cultured cells can produce a more realistic physiology. For example, treatment with 3D cultured exosomes was shown to upregulate the expression of β-secretase and thus reduce Aβ production in vivo and in vitro (Fig. 5A) [157].
5.2.2. Drug-loaded MSC-EVs for AD therapy
MSC-EVs can be loaded with diverse therapeutics, including nucleic acids, proteins, and drugs. AD patients showed reduced RNA-29 (microRNA-29) expression, which contributed to AD pathogenesis. Jahangard et al. revealed that treatment with miR-29b-loaded BMSC-EVs helps to restore the learning function and prevent memory loss in an Aβ-induced AD model (Fig. 5B-C) [56]. In this study, BMSCs were transfected with vectors containing miR-29b to subsequently derive miR-29b-containing exosomes. The underlying therapeutic effect of miR-29b against AD is based on the downregulation of NAV3 (neuron navigator), β-site amyloid precursor protein cleaving enzyme 1 (BACE1) and Bcl-2 interacting mediator of cell death (BIM) expression. Similarly, exosomes derived from miR-22-transfected ADMSCs exhibited therapeutic effects on both P12 cells and AD mice [155]. EV treatment also resulted in the acceleration of neuron regeneration, the alleviation of neuroinflammation, and the downregulation of inflammatory factors while improving the behavioural and memory abilities of AD mice.
Proteins as important regulator molecules, play a critical role in various disease therapies. A growing number of studies show that genetically engineered MSC-EVs containing therapeutic proteins were used in disease treatments [160-162]. For example, in the context of AD, Xu F et al. infected MSCs with lentivirus encoding the Src homology 2 domain-containing protein tyrosine phosphatase-2 (SHP2) gene to obtain MSC-EVs with high level of SHP2 [162]. As a result, mitophagy is significantly induced by MSC-EVs-SHP2, which ameliorates mitochondrial damage-mediated apoptosis and NLRP3 activation in neuronal cells.
In the brain, NEP plays an important role in Aβ clearance [163]. Jeong et al. overexpressed NEP in human umbilical cord tissue-derived MSCs (hUCMSCs) by transfection [65], revealing that NEP plasmid transfection into hUCMSCs increases NEP gene expression and protein levels. The transplantation of NEP-enhanced hUCMSCs into AD mice improved their memory and cognitive function. The underlying therapeutic mechanism of NEP-enhanced hUCMSCs includes the suppression of Aβ accumulation and BACE1 expression, the regeneration of brain-derived BDNF activity, neurogenesis promotion, and increased NEP expression. The therapeutic efficacy of hUCMSCs was attributed to MSC-EVs rather than to MSC penetration, which shows that MSC-EVs are promising for AD treatment (Fig. 5D-H).
5.2.3. Surface-modified MSC-EVs for AD therapy
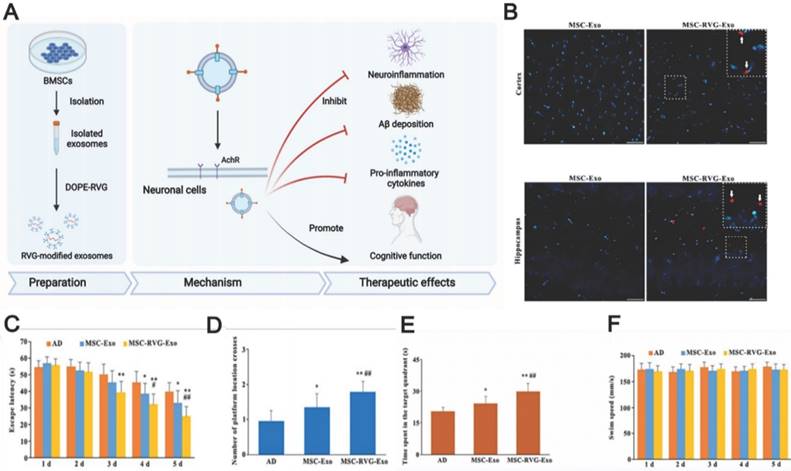
Even though MSC-EVs possess native homing properties, innovative technologies providing more specific targeting to drug-loaded EVs remain in high demand. The currently studied EV display technique achieved EV delivery to the brain by facilitating BBB penetration using cell-penetrating peptides such as the rabies virus glycoprotein (RVG), which specifically binds to the acetylcholine receptor or γ-aminobutyric acid (GABA) receptor to enter into neuronal cells through transcytosis. These receptors are widely distributed in microvascular endothelial cells and neurons [164, 165]. Cui et al. prepared RVG-decorated EVs by incubating BMSC-EVs with dioleoylphosphatidylethanolamine (DOPE)-RVG obtained by combining RVG and DOPE-N-hydroxysuccinimide [30]. The resulting MSC-EVs were effective in treating AD in mice, effectively reducing Aβ accumulation, activating microglial cells, and achieving a balanced inflammatory response (Fig. 6). Compared to those treated with non-RVG-tagged exosomes, mice treated with RVG-tagged exosomes exhibited better targeting to the cortex and hippocampus, decreased Aβ protein accumulation, and reduced microglial activation, which highlights the possibility of RVG-modified MSC-EVs in AD treatment. In addition, other peptides, such as those derived from candoxin and brain metastatic breast cancer membranes, have been used to facilitate the entry of red blood cells and polymeric nanoparticles into the brain [166, 167]. These peptides may also provide a promising way to enhance MSC-EV penetration. Thus, further experiments are necessary to investigate better methods of enhancing the targeting ability of MSC-EVs through surface modification.
6. MSC-EVs for AD therapy in clinical trials
Even though the number of related in vitro and animal studies is on the rise, clinical evaluations of MSC-EVs remain limited. EVs derived from allogenic adipose mesenchymal stem cells are among the few EV-based AD therapeutics that have progressed to phase I/II clinical trials (NCT04388982). This study was sponsored by the Ruijin Hospital and involved nine participants divided into three groups that were administered MSC-exos intranasally at different dosages (5, 10, and 15 μg) for 12 weeks. The primary outcome measures involved liver or kidney function and adverse events related to treatment evaluated by CTCAE 4.0 (common terminology criteria for adverse events), while the secondary outcome measures included cognitive function, quality of life, magnetic resonance neuroimaging results, and positron emission computed tomography neuroimaging results. Another outcome that was measured was the change in Aβ levels in serum and CSF. This study has yet to be concluded.
Another clinical study, Focused Ultrasound and Exosomes to Treat Depression, Anxiety, and Dementias (NCT04202770), was chaired by M.D. Sheldon Jordan of the Neurological Associates of West Los Angeles. This study is designed to facilitate the delivery of factors to localised targets using focused transcranial ultrasound before the IV infusion of exosomes derived from healthy full-term caesarean section amniotic fluid. The Quick Dementia Rating Scale is the primary outcome measure, and neuropsychological status and Montreal cognitive assessment results are secondary outcome measures. Starting in December 2019, this study is expected to continue for five years.
The rarity of such studies is ascribed to the need for more robust large-scale production; the ambiguous nature of MSC-EV components; the lack of standardised isolation methods, safety, and doses for clinical use; and methods for MSC-EV characterisation and quantitation.
(A) Schematics of the preparation, action mechanism, and therapeutic effects of 3D-cultured MSC-EVs in AD therapy. Ref. 157. (B) Schematics of the use of miR-29-loaded MSC-derived exosomes in AD therapy. (C) The escape latency, total errors, distance, and mean velocity of AD mice after transplantation of the engineered MSC-EVs containing miR-29b. Reproduced from ref. 56. Copyright © 2020 Yavar Jahangard et al. (D) BACE-1 expression in the hippocampus. Reproduced from ref. 65. Copyright © 2021 HyeJu Jeong et al. (E) The swimming path of the mice in Morris water maze test. (F) The change of NEP level in hUCMSC-EVs. (G) Schematics of the preparation, action mechanism, and therapeutic effects of NEP mRNA- and protein-loaded MSC-EVs in AD therapy. (H) The colocalisation of CD63 and CD81-positive hUCMSC-EVs in the hippocampus. Reproduced from ref. 65. Copyright © 2021 HyeJu Jeong et al.
(A) Schematics of the use of RVG-modified MSC-exo for AD therapy. (B) The injected exosomes in the brain. (C, D, E) Morris water maze test results of MSC-RVG-exo and MSC-exo injected AD mice. (F) The swimming speed of the groups. Reproduced from ref. 30. Copyright © 2019 Guo-Hong Cui et al.
7. Advantages and challenges of MSC-EV use for AD treatment
Along with the ability to penetrate the BBB, MSC-EVs possess properties similar to those of their parents, including immunomodulation, neuroprotection, and active migration [29], and are therefore ideal therapeutics for AD. The following section summarises the advantages and challenges of AD treatment with MSC-EVs.
7.1. Advantages
7.1.1. High biocompatibility and low immunogenicity
Compared with MSCs and traditional nanocarriers such as liposomes, MSC-EVs show higher biocompatibility and lower immunogenicity [168, 169]. In contrast to MSCs, EVs do not reproduce and undergo uncontrolled division, thus posing a lower risk of tumour formation [170]. Furthermore, unlike artificial nanoparticles, which can cause hypersensitivity owing to the inherent immune response toward foreign materials [171], EVs are less immunogenic, less toxic, and more stable in circulation considering their endogenous origin and special surface composition [172]. Compared with EVs derived from other cells, MSC-EVs would enhance the useful live and cargo bioavailability of EV-based nanocarriers, owing to the immunomodulatory properties inherited from the parent cells [173].
7.1.2. High efficiency of drug delivery
As highly biocompatible nanoscale materials, MSC-EVs can improve drug absorption and delivery. As natural EVs are capable of surviving hypoxia, they may be crucial to treating diseases characterised by hypoxic tissues, e.g. AD [168]. Once injected into the body, nanoparticles are subject to both physical and biological barriers (e.g. shearing force, phagocyte phagocytosis, and renal clearance) before they reach their intended targets. Natural EVs are free from these drawbacks. Moreover, EVs can penetrate the BBB and deliver the cargo into target cells while showing high stability in circulation, making them attractive nanocarriers for AD treatment [174]. In addition to intravenous injection, intranasal administration has become an attractive approach with the advantages of rapid onset of action, improved patient compliance, effective brain targeting, and reduced systemic side effects [175]. Unlike MSCs, EVs can be stored at -80 °C for up to a year without significant component breakdown [176].
7.1.3. Ease of modification
Bioactive molecules can be loaded into MSC-EVs before or after isolation, as described above. Cell transfection and parental cell incubation with drugs are two main ways of packaging drugs into MSC-EVs [177], allowing the efficient production of EVs loaded with the molecules of interest while preserving the integrity of EV membranes. Drug loading into MSC-EVs after isolation can be achieved in several ways, e.g. hydrophobic drugs can be combined with EVs, while hydrophilic drugs can be made to enter EVs through physical or chemical approaches [178]. Drug loading after EV isolation is more effective and significantly increases drug loading capacity [179]. Aside from the homing ability, the surface modification of MSC-EVs also increases their ability to penetrate the BBB [180]. Moreover, unlike liposomes, which exhibit a low packaging efficiency of hydrophilic cargoes, EVs have a high affinity for nucleic acids, which allows for the more efficient packaging and higher efficiency of these substances [181]. Apart from MSCs, immune cells and cancer cells are also popular cell sources for producing EVs. However, EVs derived from immune cells are currently being studied and applied in clinical settings for their antigen-presenting abilities, which are capable for designing vaccination avenues with intrinsically or extrinsically loaded antigens [182]. Cancer cell-derived EVs are mostly used in targeted cancer therapy through cancer-associated antigen presentation and have not been reported for AD therapy. Moreover, endogenous oncogenic factors could be carried in cancer cell-derived EVs, contributing to the risk of tumour formulation [183]. Hence, MSCs are well suited to mass-producing ideal drug-delivery EVs.
7.1.4. Ease of industrialisation
Although diverse cells can secrete EVs, human MSCs appear to be most promising for commercially sustainable EV production, as they are easily accessible, can be derived from nearly all ethically uncontroversial tissues in the human body and are highly proliferative [184]. Besides, The FDA has also approved their use for clinical purposes [84]. Thus, MSCs are well suited for producing ideal EVs for drug delivery.
7.2. Challenges
7.2.1. Poorly defined therapeutic mechanisms and quality control of MSC-EVs
Though MSC-EVs are rich in various bioactive and functional molecules, their composition is still complex and ambiguous. MSCs secrete detrimental cytokines via paracrine mechanisms, which demonstrate the need to identify EV contents and remove interferences from unknown secretions. It is also essential to determine the source of EVs and consider the differences in the content and therapeutic effect of EVs produced in different culturing environments, such as hypoxic and cytokine-containing environments [185]. The EV components are affected by the origin and physiology of parent cells [186]. Additionally, MSCs obtained from diverse sources vary in cell yield, survival, and differentiation abilities [187]. For example, synaptic structures are generated more efficiently by adipose tissue derived mesenchymal stem cells (AT-MSCs) than by stem cells derived from other sources [188]. Furthermore, although EVs exert therapeutic effects on AD, the mechanisms underlying these effects are not completely understood. MSC-EVs are characterised by complex characteristics, making their specification and characterisation difficult due to their limited quality attributes. As a result, apart from specifying and characterising the final product, quality control of raw materials and manufacturing processes is essential for MSC-EVs [189]. Therefore, quality and manufacturing controls should be implemented to guarantee EV quality, efficiency, and safety. Therefore, there is a need for further research to pinpoint the exact components of MSC-EVs that play a significant role in AD treatment. Currently, no research exists that compares the components of EVs from different MSCs, and differences in the therapeutic efficacy of such EVs are still unclear. To further elucidate the mechanism of MSC-EVs in AD therapy, one should identify the exact composition of EVs using multiomics methods.
Particle and protein concentrations differ according to the experimental methods, making comparisons more difficult [190]. Researchers found large inconsistencies in the doses used in 64 preclinical studies across several disease areas. They suggested adopting the parameters of therapeutic effects rather than quantification in EV dose determination [191].
While EV therapy was used for the first time in 2005 for cancer treatment [192], no standard methods have been established for quality control of EVs for clinical use. Strategies like ELISA, aptamer‐ and carbon nanotube‐based colourimetric assays, and bead‐based flow cytometry can be used to quantify the specific cargoes in EV products [193]. For reproducibility and quality assurance of MSC-EVs, reliable quality control of the contained particles must be established. For example, the minimal information for studies of EV (MISEV) recommends showing at least one transmembrane protein or extracellular lipid-bound protein on EVs, initially developed to identify EVs.
7.2.2. Complicated procedures
EV separation methods have inherent advantages and disadvantages, and there is no consensus regarding the best isolation approach. Moreover, the protein and RNA contents of EVs significantly depend on the isolation method [194]. Better production methods for MSC-EVs are required to facilitate their clinical use. Recent studies have demonstrated that size-exclusion chromatography is more effective at separating MSC-EVs than differential centrifugation. However, the limitation still involves labour-intensive processes and does not ensure the complete clearing of co-contamination with proteins and lipoproteins [195]. Considering their small yield and physicochemical heterogeneity, MSC-EVs are challenging to isolate in high yield or purity. Therefore, simpler, inexpensive, and mass-production-suitable methods should be developed to step forward in the clinical research and application of MSC-EVs. Moreover, no standard method currently exists for EV isolation. Thus, MSC-EV therapeutics need further regulatory oversight before they can be safely translated from the bench to the bedside [196].
8. Concluding remarks and outlook
Ninety-eight per cent of small molecules and almost 100% of large molecules are unable to penetrate through the BBB, despite a substantial number of neurotherapeutics being developed in different disease models. Due to their natural tendency to cross the BBB and their therapeutic potential, MSC-EVs have attracted attention as a new-generation drug delivery system for AD therapy. Despite such potential, challenges remain, such as low targeting efficiency, nonuniform treatment results, and low production yield. Therefore, advanced engineering techniques are needed to functionalise MSC-EVs and thus increase their efficiency and potency as AD therapeutics.
MSC-EVs exhibit many advantages, e.g. high biocompatibility, low immunogenicity, high drug delivery efficiency, ease of modification, and ease of industrialisation, and can alleviate AD symptoms by releasing loaded therapeutic agents to promote Aβ degradation, immunomodulation, and neuroprotection. Because AD pathology is unclear and considered to be multifactorial, MSC-EVs serve as a therapy that can target various pathogeneses of AD. In this review, we have summarised the advances in the use of MSC-EVs for AD treatment, focusing on naturally secreted MSC-EVs, bioengineered MSC-EVs, and functionalisation strategies while highlighting the encouraging preclinical results reported recently. The design principles for engineered MSC-EVs for future clinical application are summarised in Figure 7.
Developing EV-based therapies for clinical use is hampered by the limited availability of large-scale well manufactured EV production. Currently, the 2D culture method is the most used strategy for large-scale EV production, which is restricted by cell cloning, differentiation, and expansion [197]. In comparison to this method, approximately 40 times more EVs are produced by hollow fibre bioreactor cultures, which may serve as an alternative culture method to obtain EVs on a large scale. [198]. Despite this, 3D cell culture still has limitations. It is difficult to manually enlarge hanging objects using the 3D culture method and because exosome production efficiency depends on sphere size, production stability is inconsistent [199].
Design principles for engineered MSC-EVs as nanocarriers for future clinical application.
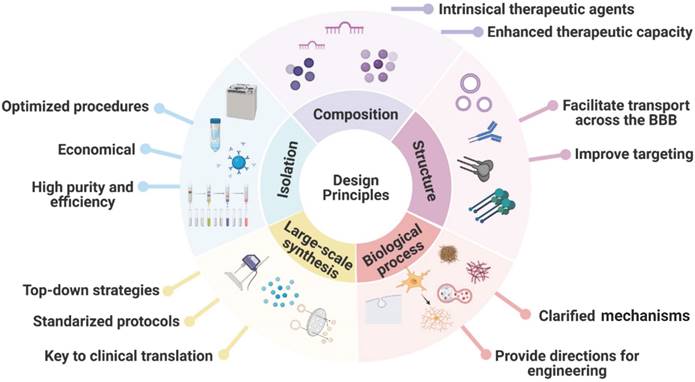
New drug delivery platforms based on MSC-EVs have recently been developed. The three main ways of adding the desired functionality to MSC-EVs are the enhancement of MSC-EV contents by the preconditioning of parent cells, EV surface modification for specific delivery of therapeutic cargoes, and EV loading with exogenous cargoes. Using artificial EVs allows one to conquer the disadvantages of natural EVs, including low productivity and tedious isolation methods, even though artificial EVs have not been used in AD therapy. The combination of MSC-derived membranes with nanomaterials featuring unique physical/chemical properties is also a promising solution for future AD therapy, catalysing the development of innovative biomaterials that possess multiple functions, high biocompatibility, and negligible immunogenicity for AD treatment. We expect this review to raise awareness concerning the potential of MSC-EVs as efficient nanocarriers for AD treatment, in addition to exhibiting an inherent therapeutic effect.
Even with the active progress in MSC-EV usage in AD therapy, many challenges, including those associated with isolation, yield, and standardisation processes, remain to be overcome before the related clinical translation. The large-scale production of MSC-EVs with acceptable batch-to-batch variation is challenging because of their heterogeneity, which is influenced by the culturing environment and the MSC source. To ensure a safe and effective MSC-EV therapy, it is important to select the right cell source. When using autologous cells, mismatched antigens are avoided, reducing the risk of host immune responses. Conversely, allogenic (donor) cells can provide readily available cell sources when needed. Nonetheless, in an analogous way to organ transplantation, antigen matching is essential for avoiding host immune responses. Consequently, MSC-EVs should be produced on a large scale with stringent quality control. Building donor cell banks that are easily accessible for the selection of proper cell sources may be an alternative approach for future clinical applications. Gene-editing techniques may also facilitate the deletion of unwanted immunogenic proteins by deleting specific genes. The use of CRISPR/Cas9 and zinc finger nuclease, for example, has demonstrated the feasibility of removing human leukocyte antigen genes and generating more immune-compatible stem cells [200, 201]. Long-term treatments could be achieved by MSC-EVs derived from these modified cells. Additionally, quality control standards and normative methods of isolation and characterisation should be established. Despite these challenges, MSC-EVs still represent a potential multifunctional weapon for AD therapy owing to their unique characteristics and biofunctions.
Abbreviations
Aβ oligomer (AβO); Aβ precursor protein (APP); adipose tissue stem cell (ADSC); Alzheimer's disease (AD); amyloid-β (Aβ); β-site amyloid precursor protein cleaving enzyme 1 (BACE1); Bcl-2 interacting mediator of cell death (BIM); β-site APP-cleaving enzyme (BACE); blood-brain barrier (BBB); brain-derived neurotrophic factor (BDNF); bone marrow mesenchymal stem cell-derived extracellular vesicle (BMSC-EV); brain microvascular endothelial cell (BMEC); brain microvascular endothelial cell (BMEC); central nervous system (CNS); cerebrospinal fluid (CSF); dioleoylphosphatidylethanolamine (DOPE); extracellular vesicle (EV); gasdermin D (GSDMD); human adipose tissue-derived mesenchymal stem cells (hADSCs); human bone marrow-derived mesenchymal stem cells (hBMSCs); human umbilical cord tissue-derived mesenchymal stem cells (hUCMSCs); human umbilical cord blood-derived mesenchymal stem cells (hUCBMSCs); hypoxia-inducible factor (HIF-1α); intraluminal vesicle (ILV); intranasal administration (IN); intravenous injection (IV); long-term potentiation (LTP); mouse adipose-derived stem cells (mADSCs); mouse bone marrow-derived mesenchymal stem cells (mBMSCs); mesenchymal stem cell (MSC); mesenchymal stem cell-derived extracellular vesicle (MSC-EV); multivesicular bodies (MVBs); NOD-like receptor thermal protein domain associated protein 3 (NLRP3); not detected (ND); neprilysin (NEP); neurotropic factor (NF); phosphatase and tensin homolog-phosphatidylinositol-3-kinase (PTEN-PI3K/Akt); Phosphate‐buffered saline (PBS); rabies viral glycoprotein (RVG); rat bone marrow-derived mesenchymal stem cells (rBMSCs); sphingosine kinase (SphK); sphingosine 1-phosphate (S1P); Src homology 2 domain-containing protein tyrosine phosphatase-2 (SHP2); Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs). γ-aminobutyric acid (GABA).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 82072051, 82173891), Shanghai Natural Science Foundation (No. 22ZR147750, China), Science and Technology Support Projects in Biomedicine Field of Shanghai Science and Technology Commission (No. 19441907500, China), Innovative Clinical Research Project of Changzheng Hospital (No. 2020YLCYJ-Y02, China), Characteristic Services of Veteran Cadres Memory Disorders Diagnosis and Treatment in Changzheng Hospital (No. 2020CZWJFW12, China), and Science and Technology Commission of Shanghai Municipality (Project No20ZR1435400).
Author contributions
Tong Yin: Writing-Original Draft, Resources. Yan Liu: Investigation, Writing-Original Draft. Wenbo Ji: Resources, Investigation. Jianhua Zhuang: Validation. Xiaohan Chen: Visualization. Baofeng Gong: Resources. Jianjian Chu: Investigation. Wendanqi Liang: Resources. Jie Gao: Writing-Review & Editing, Conceptualization, Supervision. You Yin: Writing-Review & Editing, Supervision, Funding Acquisition. All authors have approved the final article.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Joe E, Ringman JM. Cognitive symptoms of Alzheimer's disease: clinical management and prevention. BMJ. 2019;367:l6217
2. Ji W, Gong B, Jin H, Chen X, Li P, Cheng W. et al. Recent Progress Towards Vaccines and Antibody-based Therapies Against Alzheimer's Disease. Mini Rev Med Chem. 2021;21:3062-72
3. Neves AF, Camargo C, Premer C, Hare JM, Baumel BS, Pinto M. Intravenous administration of mesenchymal stem cells reduces Tau phosphorylation and inflammation in the 3xTg-AD mouse model of Alzheimer's disease. Exp Neurol. 2021;341:113706
4. Kosyreva AM, Sentyabreva AV, Tsvetkov IS, Makarova OV. Alzheimer's Disease and Inflammaging. Brain Sci. 2022 12
5. Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25:59-70
6. Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61-7
7. Li P, Xu J, Gu H, Peng H, Yin Y, Zhuang J. Memantine ameliorates cognitive deficit in AD mice via enhancement of entorhinal-CA1 projection. BMC Neurosci. 2021;22:41
8. Syed YY. Sodium Oligomannate: First Approval. Drugs. 2020;80:441-4
9. Han J, Du Z, Lim MH. Mechanistic Insight into the Design of Chemical Tools to Control Multiple Pathogenic Features in Alzheimer's Disease. Acc Chem Res. 2021;54:3930-40
10. Chen HX, Liang FC, Gu P, Xu BL, Xu HJ, Wang WT. et al. Exosomes derived from mesenchymal stem cells repair a Parkinson's disease model by inducing autophagy. Cell Death Dis. 2020;11:288
11. Zhang Y, Zhang Y, Chopp M, Zhang ZG, Mahmood A, Xiong Y. Mesenchymal Stem Cell-Derived Exosomes Improve Functional Recovery in Rats After Traumatic Brain Injury: A Dose-Response and Therapeutic Window Study. Neurorehabil Neural Repair. 2020;34:616-26
12. Hernández AE, García E. Mesenchymal Stem Cell Therapy for Alzheimer's Disease. Stem Cells Int. 2021;2021:7834421
13. Liew LC, Katsuda T, Gailhouste L, Nakagama H, Ochiya T. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer's disease. Int Immunol. 2017;29:11-9
14. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812-23
15. Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP. et al. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics. 2012;2012:971907
16. Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L. et al. Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136:41-56
17. Baker S, Polanco JC, Götz J. Extracellular Vesicles Containing P301L Mutant Tau Accelerate Pathological Tau Phosphorylation and Oligomer Formation but Do Not Seed Mature Neurofibrillary Tangles in ALZ17 Mice. J Alzheimers Dis. 2016;54:1207-17
18. Polanco JC, Scicluna BJ, Hill AF, Götz J. Extracellular Vesicles Isolated from the Brains of rTg4510 Mice Seed Tau Protein Aggregation in a Threshold-dependent Manner. J Biol Chem. 2016;291:12445-66
19. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 367
20. Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8
21. Ryan ST, Hosseini-Beheshti E, Afrose D, Ding X, Xia B, Grau GE. et al. Extracellular Vesicles from Mesenchymal Stromal Cells for the Treatment of Inflammation-Related Conditions. Int J Mol Sci. 2021 22
22. Chen YX, Wei CX, Lyu YQ, Chen HZ, Jiang G, Gao XL. Biomimetic drug-delivery systems for the management of brain diseases. Biomater Sci. 2020;8:1073-88
23. Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y. et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926-44
24. Lankford KL, Arroyo EJ, Nazimek K, Bryniarski K, Askenase PW, Kocsis JD. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018;13:e0190358
25. Vandendriessche C, Kapogiannis D, Vandenbroucke RE. Biomarker and therapeutic potential of peripheral extracellular vesicles in Alzheimer's disease. Adv Drug Deliv Rev. 2022;190:114486
26. Tsivion-Visbord H, Perets N, Sofer T, Bikovski L, Goldshmit Y, Ruban A. et al. Correction: Mesenchymal stem cells derived extracellular vesicles improve behavioral and biochemical deficits in a phencyclidine model of schizophrenia. Transl Psychiatry. 2020;10:341
27. Perets N, Hertz S, London M, Offen D. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol Autism. 2018;9:57
28. Wei H, Xu Y, Chen Q, Chen H, Zhu X, Li Y. Correction: Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020;11:431
29. Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN. et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11:8129-42
30. Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J. et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease. Immun Ageing. 2019;16:10
31. Wang H, Liu Y, Li J, Wang T, Hei Y, Li H. et al. Tail-vein injection of MSC-derived small extracellular vesicles facilitates the restoration of hippocampal neuronal morphology and function in APP / PS1 mice. Cell Death Discov. 2021;7:230
32. Chen YA, Lu CH, Ke CC, Chiu SJ, Jeng FS, Chang CW. et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer's Disease Pathology and Improve Cognitive Deficits. Biomedicines. 2021 9
33. Guo M, Yin Z, Chen F, Lei P. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer's disease. Alzheimers Res Ther. 2020;12:109
34. Harrell CR, Volarevic A, Djonov V, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes as New Remedy for the Treatment of Neurocognitive Disorders. Int J Mol Sci. 2021 22
35. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
36. Luzio JP, Gray SR, Bright NA. Endosome-lysosome fusion. Biochem Soc Trans. 2010;38:1413-6
37. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89
38. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem Rev. 2018;118:1917-50
39. Yu D, Li Y, Wang M, Gu J, Xu W, Cai H. et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21:56
40. Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R. et al. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol. 2021;9:811971
41. Boriachek K, Islam MN, Möller A, Salomon C, Nguyen NT, Hossain MSA. et al. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small. 2018 14
42. Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A. et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031
43. Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S. et al. Methodological Guidelines to Study Extracellular Vesicles. Circ Res. 2017;120:1632-48
44. Weng Y, Sui Z, Shan Y, Hu Y, Chen Y, Zhang L. et al. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst. 2016;141:4640-6
45. Massey AE, Malik S, Sikander M, Doxtater KA, Tripathi MK, Khan S. et al. Clinical Implications of Exosomes: Targeted Drug Delivery for Cancer Treatment. Int J Mol Sci. 2021 22
46. Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L. et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684-707
47. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-82
48. Xu K, Jin Y, Li Y, Huang Y, Zhao R. Recent Progress of Exosome Isolation and Peptide Recognition-Guided Strategies for Exosome Research. Front Chem. 2022;10:844124
49. Suthar J, Parsons ES, Hoogenboom BW, Williams GR, Guldin S. Acoustic Immunosensing of Exosomes Using a Quartz Crystal Microbalance with Dissipation Monitoring. Anal Chem. 2020;92:4082-93
50. Kaddour H, Tranquille M, Okeoma CM. The Past, the Present, and the Future of the Size Exclusion Chromatography in Extracellular Vesicles Separation. Viruses. 2021 13
51. Jalaludin I, Lubman DM, Kim J. A guide to mass spectrometric analysis of extracellular vesicle proteins for biomarker discovery. Mass Spectrom Rev. 2021: e21749.
52. Benjamin-Davalos S, Koroleva M, Allen CL, Ernstoff MS, Shu S. Co-Isolation of Cytokines and Exosomes: Implications for Immunomodulation Studies. Front Immunol. 2021;12:638111
53. Shekari F, Nazari A, Assar Kashani S, Hajizadeh-Saffar E, Lim R, Baharvand H. Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: a systematic review. Cytotherapy. 2021;23:277-84
54. van Balkom BWM, Gremmels H, Giebel B, Lim SK. Proteomic Signature of Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles. Proteomics. 2019;19:e1800163
55. Jeyaram A, Jay SM. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. Aaps j. 2017;20:1
56. Jahangard Y, Monfared H, Moradi A, Zare M, Mirnajafi-Zadeh J, Mowla SJ. Therapeutic Effects of Transplanted Exosomes Containing miR-29b to a Rat Model of Alzheimer's Disease. Front Neurosci. 2020;14:564
57. Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL. et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. Faseb j. 2018;32:654-68
58. Losurdo M, Pedrazzoli M, D'Agostino C, Elia CA, Massenzio F, Lonati E. et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl Med. 2020;9:1068-84
59. T LR, Sánchez-Abarca LI, Muntión S, Preciado S, Puig N, López-Ruano G. et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14:2
60. Nolan JP, Duggan E. Analysis of Individual Extracellular Vesicles by Flow Cytometry. Methods Mol Biol. 2018;1678:79-92
61. Munshi A, Mehic J, Creskey M, Gobin J, Gao J, Rigg E. et al. A comprehensive proteomics profiling identifies NRP1 as a novel identity marker of human bone marrow mesenchymal stromal cell-derived small extracellular vesicles. Stem Cell Res Ther. 2019;10:401
62. Görgens A, Corso G, Hagey DW, Jawad Wiklander R, Gustafsson MO, Felldin U. et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J Extracell Vesicles. 2022;11:e12238
63. Kang IS, Suh J, Lee MN, Lee C, Jin J, Lee C. et al. Characterization of human cardiac mesenchymal stromal cells and their extracellular vesicles comparing with human bone marrow derived mesenchymal stem cells. BMB Rep. 2020;53:118-23
64. Tan KL, Chia WC, How CW, Tor YS, Show PL, Looi QHD. et al. Benchtop Isolation and Characterisation of Small Extracellular Vesicles from Human Mesenchymal Stem Cells. Mol Biotechnol. 2021;63:780-91
65. Jeong H, Kim OJ, Oh SH, Lee S, Reum Lee HA, Lee KO. et al. Extracellular Vesicles Released from Neprilysin Gene-Modified Human Umbilical Cord-Derived Mesenchymal Stem Cell Enhance Therapeutic Effects in an Alzheimer's Disease Animal Model. Stem Cells Int. 2021;2021:5548630
66. Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discov. 2021;20:362-83
67. Cui Y, Guo Y, Kong L, Shi J, Liu P, Li R. et al. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact Mater. 2022;10:207-21
68. Li F, Wu J, Li D, Hao L, Li Y, Yi D. et al. Engineering stem cells to produce exosomes with enhanced bone regeneration effects: an alternative strategy for gene therapy. J Nanobiotechnology. 2022;20:135
69. Lin Z, Xiong Y, Meng W, Hu Y, Chen L, Chen L. et al. Exosomal PD-L1 induces osteogenic differentiation and promotes fracture healing by acting as an immunosuppressant. Bioact Mater. 2022;13:300-11
70. Thakur A, Parra DC, Motallebnejad P, Brocchi M, Chen HJ. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater. 2022;10:281-94
71. Xin L, Wei C, Tong X, Dai Y, Huang D, Chen J. et al. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: A therapy for intrauterine adhesions. Bioact Mater. 2022;12:107-19
72. Matsushita T, Kibayashi T, Katayama T, Yamashita Y, Suzuki S, Kawamata J. et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. 2011;502:41-5
73. De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M. et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440-9
74. Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072-84
75. Choi H, Choi K, Kim DH, Oh BK, Yim H, Jo S. et al. Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges. Pharmaceutics. 2022 14
76. Santamaria G, Brandi E, Vitola P, Grandi F, Ferrara G, Pischiutta F. et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer's mice. Cell Death Differ. 2021;28:203-18
77. Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44-52
78. Ma X, Huang M, Zheng M, Dai C, Song Q, Zhang Q. et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease. J Control Release. 2020;327:688-702
79. Verweij FJ, Balaj L, Boulanger CM, Carter DRF, Compeer EB, D'Angelo G. et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat Methods. 2021;18:1013-26
80. Kirker-Head CA. Recombinant bone morphogenetic proteins: novel substances for enhancing bone healing. Vet Surg. 1995;24:408-19
81. Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N. et al. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer's disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10:10772
82. Ballas CB, Zielske SP, Gerson SL. Adult bone marrow stem cells for cell and gene therapies: implications for greater use. J Cell Biochem Suppl. 2002;38:20-8
83. Devine SM. Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem Suppl. 2002;38:73-9
84. Zhou T, Yuan Z, Weng J, Pei D, Du X, He C. et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24
85. Huang YC, Lai LC. The potential roles of stem cell-derived extracellular vesicles as a therapeutic tool. Ann Transl Med. 2019;7:693
86. Meldolesi J. News about Therapies of Alzheimer's Disease: Extracellular Vesicles from Stem Cells Exhibit Advantages Compared to Other Treatments. Biomedicines. 2022 10
87. Tieu A, Lalu MM, Slobodian M, Gnyra C, Fergusson DA, Montroy J. et al. An Analysis of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Preclinical Use. ACS Nano. 2020;14:9728-43
88. Feng Y, Guo M, Zhao H, Han S, Dong Q, Cui M. Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Mitigate Trained Immunity in the Brain. Front Bioeng Biotechnol. 2020;8:599058
89. Wei H, Xu Y, Chen Q, Chen H, Zhu X, Li Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020;11:290
90. Benhamron S, Nitzan K, Valitsky M, Lax N, Karussis D, Kassis I. et al. Cerebrospinal Fluid (CSF) Exchange Therapy with Artificial CSF Enriched with Mesenchymal Stem Cell Secretions Ameliorates Cognitive Deficits and Brain Pathology in Alzheimer's Disease Mice. J Alzheimers Dis. 2020;76:369-85
91. Hassan R, Rabea AA, Ragae A, Sabry D. The prospective role of mesenchymal stem cells exosomes on circumvallate taste buds in induced Alzheimer's disease of ovariectomized albino rats: (Light and transmission electron microscopic study). Arch Oral Biol. 2020;110:104596
92. Wang SS, Jia J, Wang Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppresses iNOS Expression and Ameliorates Neural Impairment in Alzheimer's Disease Mice. J Alzheimers Dis. 2018;61:1005-13
93. de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB. et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J Biol Chem. 2018;293:1957-75
94. Perets N, Betzer O, Shapira R, Brenstein S, Angel A, Sadan T. et al. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019;19:3422-31
95. Deng Z, Wang J, Xiao Y, Li F, Niu L, Liu X. et al. Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-β-induced neurotoxicity. Theranostics. 2021;11:4351-62
96. Mozafari N, Dehshahri A, Ashrafi H, Mohammadi-Samani S, Shahbazi MA, Heidari R. et al. Vesicles of yeast cell wall-sitagliptin to alleviate neuroinflammation in Alzheimer's disease. Nanomedicine. 2022;44:102575
97. Sha S, Shen X, Cao Y, Qu L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer's disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging (Albany NY). 2021;13:15285-306
98. Wang X, Yang G. Bone marrow mesenchymal stem cells-derived exosomes reduce Aβ deposition and improve cognitive function recovery in mice with Alzheimer's disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell Biol Int. 2021;45:775-84
99. Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR. et al. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation. 2022;19:35
100. Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K. et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197
101. Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T. et al. A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett. 2015;589:84-8
102. O'Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018;9:402
103. Angelucci F, Cechova K, Valis M, Kuca K, Zhang B, Hort J. MicroRNAs in Alzheimer's Disease: Diagnostic Markers or Therapeutic Agents? Front Pharmacol. 2019;10:665
104. Wei W, Wang ZY, Ma LN, Zhang TT, Cao Y, Li H. MicroRNAs in Alzheimer's Disease: Function and Potential Applications as Diagnostic Biomarkers. Front Mol Neurosci. 2020;13:160
105. Lee JH, Yang DS, Goulbourne CN, Im E, Stavrides P, Pensalfini A. et al. Faulty autolysosome acidification in Alzheimer's disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat Neurosci. 2022;25:688-701
106. Ceccariglia S, Cargnoni A, Silini AR, Parolini O. Autophagy: a potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy. 2020;16:28-37
107. Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ. et al. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy. 2014;10:32-44
108. Crivelli SM, Giovagnoni C, Visseren L, Scheithauer AL, de Wit N, den Hoedt S. et al. Sphingolipids in Alzheimer's disease, how can we target them? Adv Drug Deliv Rev. 2020;159:214-31
109. McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet. 2021;398:1184-94
110. Nalivaeva NN, Zhuravin IA, Turner AJ. Neprilysin expression and functions in development, ageing and disease. Mech Ageing Dev. 2020;192:111363
111. Elia CA, Tamborini M, Rasile M, Desiato G, Marchetti S, Swuec P. et al. Intracerebral Injection of Extracellular Vesicles from Mesenchymal Stem Cells Exerts Reduced Aβ Plaque Burden in Early Stages of a Preclinical Model of Alzheimer's Disease. Cells. 2019 8
112. Ferreira JPS, Albuquerque HMT, Cardoso SM, Silva AMS, Silva VLM. Dual-target compounds for Alzheimer's disease: Natural and synthetic AChE and BACE-1 dual-inhibitors and their structure-activity relationship (SAR). Eur J Med Chem. 2021;221:113492
113. Iranifar E, Seresht BM, Momeni F, Fadaei E, Mehr MH, Ebrahimi Z. et al. Exosomes and microRNAs: New potential therapeutic candidates in Alzheimer disease therapy. J Cell Physiol. 2019;234:2296-305
114. Xiao G, Chen Q, Zhang X. MicroRNA-455-5p/CPEB1 pathway mediates Aβ-related learning and memory deficits in a mouse model of Alzheimer's disease. Brain Res Bull. 2021;177:282-94
115. Parr C, Mirzaei N, Christian M, Sastre M. Activation of the Wnt/β-catenin pathway represses the transcription of the β-amyloid precursor protein cleaving enzyme (BACE1) via binding of T-cell factor-4 to BACE1 promoter. Faseb j. 2015;29:623-35
116. Saito T, Saido TC. Neuroinflammation in mouse models of Alzheimer's disease. Clin Exp Neuroimmunol. 2018;9:211-8
117. Shen Z, Bao X, Wang R. Clinical PET Imaging of Microglial Activation: Implications for Microglial Therapeutics in Alzheimer's Disease. Front Aging Neurosci. 2018;10:314
118. Liang C, Zou T, Zhang M, Fan W, Zhang T, Jiang Y. et al. MicroRNA-146a switches microglial phenotypes to resist the pathological processes and cognitive degradation of Alzheimer's disease. Theranostics. 2021;11:4103-21
119. Sánchez-Castillo AI, Sepúlveda MR, Marín-Teva JL, Cuadros MA, Martín-Oliva D, González-Rey E. et al. Switching Roles: Beneficial Effects of Adipose Tissue-Derived Mesenchymal Stem Cells on Microglia and Their Implication in Neurodegenerative Diseases. Biomolecules. 2022 12
120. Lau SF, Fu AKY, Ip NY. Cytokine signaling convergence regulates the microglial state transition in Alzheimer's disease. Cell Mol Life Sci. 2021;78:4703-12
121. Wang H, Zhou M, Brand J, Huang L. Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci. 2009;1170:596-603
122. Tewari D, Sah AN, Bawari S, Nabavi SF, Dehpour AR, Shirooie S. et al. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr Neuropharmacol. 2021;19:114-26
123. Cinelli MA, Do HT, Miley GP, Silverman RB. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med Res Rev. 2020;40:158-89
124. Kobayashi E, Suzuki T, Yamamoto M. Roles nrf2 plays in myeloid cells and related disorders. Oxid Med Cell Longev. 2013;2013:529219
125. Saha S, Buttari B, Profumo E, Tucci P, Saso L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer's and Parkinson's Diseases. Front Cell Neurosci. 2021;15:787258
126. Fontana IC, Zimmer AR, Rocha AS, Gosmann G, Souza DO, Lourenco MV. et al. Amyloid-β oligomers in cellular models of Alzheimer's disease. J Neurochem. 2020;155:348-69
127. Gong B, Ji W, Chen X, Li P, Cheng W, Zhao Y. et al. Recent Advancements in Strategies for Abnormal Protein Clearance in Alzheimer's Disease. Mini Rev Med Chem. 2022
128. Kim HY, Kwon S, Um W, Shin S, Kim CH, Park JH. et al. Functional Extracellular Vesicles for Regenerative Medicine. Small. 2022: e2106569.
129. Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X. et al. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol. 2020;235:8010-22
130. Gala D, Mohak S, Fábián Z. Extracellular Vehicles of Oxygen-Depleted Mesenchymal Stromal Cells: Route to Off-Shelf Cellular Therapeutics? Cells. 2021 10
131. Domenis R, Cifù A, Quaglia S, Pistis C, Moretti M, Vicario A. et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018;8:13325
132. Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y. et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018;16:81
133. Claridge B, Lozano J, Poh QH, Greening DW. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front Cell Dev Biol. 2021;9:734720
134. Xu M, Yang Q, Sun X, Wang Y. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front Bioeng Biotechnol. 2020;8:586130
135. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148-56
136. Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC. et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769-79
137. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-5
138. Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N. et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185-91
139. Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894-906
140. Chen L, Wang L, Zhu L, Xu Z, Liu Y, Li Z. et al. Exosomes as Drug Carriers in Anti-Cancer Therapy. Front Cell Dev Biol. 2022;10:728616
141. Ma Y, Dong S, Li X, Kim BYS, Yang Z, Jiang W. Extracellular Vesicles: An Emerging Nanoplatform for Cancer Therapy. Front Oncol. 2020;10:606906
142. Wang Z, Rich J, Hao N, Gu Y, Chen C, Yang S. et al. Acoustofluidics for simultaneous nanoparticle-based drug loading and exosome encapsulation. Microsyst Nanoeng. 2022;8:45
143. Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C. et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137-49
144. Gaurav I, Thakur A, Iyaswamy A, Wang X, Chen X, Yang Z. Factors Affecting Extracellular Vesicles Based Drug Delivery Systems. Molecules. 2021 26
145. Rayamajhi S, Aryal S. Surface functionalization strategies of extracellular vesicles. J Mater Chem B. 2020;8:4552-69
146. Ramasubramanian L, Kumar P, Wang A. Engineering Extracellular Vesicles as Nanotherapeutics for Regenerative Medicine. Biomolecules. 2019 10
147. Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW. et al. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. 2014;25:1777-84
148. Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S. et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. 2021;19:242
149. Jo W, Kim J, Yoon J, Jeong D, Cho S, Jeong H. et al. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6:12056-64
150. Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J. et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698-710
151. Tao SC, Rui BY, Wang QY, Zhou D, Zhang Y, Guo SC. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25:241-55
152. Yang Z, Xie J, Zhu J, Kang C, Chiang C, Wang X. et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release. 2016;243:160-71
153. Goh WJ, Zou S, Ong WY, Torta F, Alexandra AF, Schiffelers RM. et al. Bioinspired Cell-Derived Nanovesicles versus Exosomes as Drug Delivery Systems: a Cost-Effective Alternative. Sci Rep. 2017;7:14322
154. Le QV, Lee J, Lee H, Shim G, Oh YK. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm Sin B. 2021;11:2096-113
155. Zhai L, Shen H, Sheng Y, Guan Q. ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer's disease. J Cell Mol Med. 2021;25:7513-23
156. Liu H, Jin M, Ji M, Zhang W, Liu A, Wang T. Hypoxic pretreatment of adipose-derived stem cell exosomes improved cognition by delivery of circ-Epc1 and shifting microglial M1/M2 polarization in an Alzheimer's disease mice model. Aging (Albany NY). 2022;14:3070-83
157. Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G. The Regulatory Functionality of Exosomes Derived from hUMSCs in 3D Culture for Alzheimer's Disease Therapy. Small. 2020;16:e1906273
158. Guo Y, Peng Y, Zeng H, Chen G. Progress in Mesenchymal Stem Cell Therapy for Ischemic Stroke. Stem Cells Int. 2021;2021:9923566
159. Meng SX, Wang B, Li WT. Intermittent hypoxia improves cognition and reduces anxiety-related behavior in APP/PS1 mice. Brain Behav. 2020;10:e01513
160. Xu F, Fei Z, Dai H, Xu J, Fan Q, Shen S. et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles with High PD-L1 Expression for Autoimmune Diseases Treatment. Adv Mater. 2022;34:e2106265
161. Tsai HI, Wu Y, Liu X, Xu Z, Liu L, Wang C. et al. Engineered Small Extracellular Vesicles as a FGL1/PD-L1 Dual-Targeting Delivery System for Alleviating Immune Rejection. Adv Sci (Weinh). 2022;9:e2102634
162. Xu F, Wu Y, Yang Q, Cheng Y, Xu J, Zhang Y. et al. Engineered Extracellular Vesicles with SHP2 High Expression Promote Mitophagy for Alzheimer's Disease Treatment. Adv Mater. 2022;34:e2207107
163. Feygina EE, Katrukha AG, Semenov AG. Neutral Endopeptidase (Neprilysin) in Therapy and Diagnostics: Yin and Yang. Biochemistry (Mosc). 2019;84:1346-58
164. Lentz TL, Burrage TG, Smith AL, Crick J, Tignor GH. Is the acetylcholine receptor a rabies virus receptor? Science. 1982;215:182-4
165. Wang Q, Cheng S, Qin F, Fu A, Fu C. Application progress of RVG peptides to facilitate the delivery of therapeutic agents into the central nervous system. RSC Adv. 2021;11:8505-15
166. Chai Z, Hu X, Wei X, Zhan C, Lu L, Jiang K. et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J Control Release. 2017;264:102-11
167. Kumar P, Treuren TV, Ranjan AP, Chaudhary P, Vishwanatha JK. In vivo imaging and biodistribution of near infrared dye loaded brain-metastatic-breast-cancer-cell-membrane coated polymeric nanoparticles. Nanotechnology. 2019;30:265101
168. Askenase PW. Ancient Evolutionary Origin and Properties of Universally Produced Natural Exosomes Contribute to Their Therapeutic Superiority Compared to Artificial Nanoparticles. Int J Mol Sci. 2021 22
169. Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13:24
170. Guy R, Offen D. Promising Opportunities for Treating Neurodegenerative Diseases with Mesenchymal Stem Cell-Derived Exosomes. Biomolecules. 2020 10
171. Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int J Mol Sci. 2019 20
172. Ma TJ, Gao J, Liu Y, Zhuang JH, Yin C, Li P. et al. Nanomedicine Strategies for Sustained, Controlled and Targeted Treatment of Alzheimer's Disease. Mini Rev Med Chem. 2018;18:1035-46
173. Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S. et al. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015;4:1131-43
174. Busatto S, Morad G, Guo P, Moses MA. The role of extracellular vesicles in the physiological and pathological regulation of the blood-brain barrier. FASEB Bioadv. 2021;3:665-75
175. Kashyap K, Shukla R. Drug Delivery and Targeting to the Brain Through Nasal Route: Mechanisms, Applications and Challenges. Curr Drug Deliv. 2019;16:887-901
176. Purushothaman A. Exosomes from Cell Culture-Conditioned Medium: Isolation by Ultracentrifugation and Characterization. Methods Mol Biol. 2019;1952:233-44
177. Zhou L, Kodidela S, Godse S, Thomas-Gooch S, Kumar A, Raji B. et al. Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles. Pharmaceuticals (Basel). 2022 15
178. Yang Z, Li Y, Wang Z. Recent Advances in the Application of Mesenchymal Stem Cell-Derived Exosomes for Cardiovascular and Neurodegenerative Disease Therapies. Pharmaceutics. 2022 14
179. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748-59
180. Yu Y, Li W, Mao L, Peng W, Long D, Li D. et al. Genetically engineered exosomes display RVG peptide and selectively enrich a neprilysin variant: a potential formulation for the treatment of Alzheimer's disease. J Drug Target. 2021;29:1128-38
181. Kooijmans SAA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol Res. 2016;111:487-500
182. Veerman RE, Güçlüler Akpinar G, Eldh M, Gabrielsson S. Immune Cell-Derived Extracellular Vesicles - Functions and Therapeutic Applications. Trends Mol Med. 2019;25:382-94
183. Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol Life Sci. 2015;72:1-10
184. Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ. et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336-41
185. Liu WZ, Ma ZJ, Li JR, Kang XW. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021;12:102
186. Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21:379-99
187. Rahbaran M, Zekiy AO, Bahramali M, Jahangir M, Mardasi M, Sakhaei D. et al. Therapeutic utility of mesenchymal stromal cell (MSC)-based approaches in chronic neurodegeneration: a glimpse into underlying mechanisms, current status, and prospects. Cell Mol Biol Lett. 2022;27:56
188. Urrutia DN, Caviedes P, Mardones R, Minguell JJ, Vega-Letter AM, Jofre CM. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS One. 2019;14:e0213032
189. Tsuchiya A, Terai S, Horiguchi I, Homma Y, Saito A, Nakamura N. et al. Basic points to consider regarding the preparation of extracellular vesicles and their clinical applications in Japan. Regen Ther. 2022;21:19-24
190. Bachurski D, Schuldner M, Nguyen PH, Malz A, Reiners KS, Grenzi PC. et al. Extracellular vesicle measurements with nanoparticle tracking analysis - An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J Extracell Vesicles. 2019;8:1596016
191. Gupta D, Zickler AM, El Andaloussi S. Dosing extracellular vesicles. Adv Drug Deliv Rev. 2021;178:113961
192. Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S. et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10
193. Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M. et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8:1609206
194. Zhang J, Li S, Li L, Li M, Guo C, Yao J. et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17-24
195. Almeria C, Kreß S, Weber V, Egger D, Kasper C. Heterogeneity of mesenchymal stem cell-derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions. Cell Biosci. 2022;12:51
196. Gimona M, Pachler K, Laner-Plamberger S, Schallmoser K, Rohde E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int J Mol Sci. 2017 18
197. McKee C, Chaudhry GR. Advances and challenges in stem cell culture. Colloids Surf B Biointerfaces. 2017;159:62-77
198. Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G. et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195-205
199. Yin Y, Han X, Li C, Sun T, Li K, Liu X. et al. The status of industrialization and development of exosomes as a drug delivery system: A review. Front Pharmacol. 2022;13:961127
200. Torikai H, Mi T, Gragert L, Maiers M, Najjar A, Ang S. et al. Genetic editing of HLA expression in hematopoietic stem cells to broaden their human application. Sci Rep. 2016;6:21757
201. Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N. et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell. 2019;24:566-78.e7
Author contact
![]() Corresponding authors: Dr. You Yin. Email: yinyou179com; Dr. Jie Gao. Email: jmsx2021edu.cn
Corresponding authors: Dr. You Yin. Email: yinyou179com; Dr. Jie Gao. Email: jmsx2021edu.cn
 Global reach, higher impact
Global reach, higher impact