13.3
Impact Factor
Theranostics 2023; 13(2):767-786. doi:10.7150/thno.79806 This issue Cite
Review
Radiopharmaceuticals heat anti-tumor immunity
1. Peking University-Tsinghua University Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing 100871, China
2. Beijing National Laboratory for Molecular Sciences, Radiochemistry and Radiation Chemistry Key Laboratory of Fundamental Science, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
3. Changping Laboratory, Beijing 102206, China
# Contributed equally to this work
Received 2022-11-1; Accepted 2022-11-1; Published 2023-1-1
Abstract
Radiopharmaceutical therapy (RPT) has proven to be an effective cancer treatment with minimal toxicity. With several RPT agents approved by FDA, the remarkable potential of this therapy is now being recognized, and the anti-tumor immunity induced by RPT is beginning to be noticed. This review evaluates the potential of RPT for immune activation, including promoting the release of danger associated-molecular pattern molecules that recruit inflammatory cells into the tumor microenvironment, and activating antigen-presenting cells and cytotoxic T cells. We also discuss the progress of combining RPT with immunotherapy to increase efficacy.
Keywords: anti-tumor immunity, radiopharmaceutical therapy (RPT), immunogenic cell death (ICD)
Introduction
Radiopharmaceutical therapy (RPT) is a novel radiotherapy modality defined by the delivery of radionuclides to tumors systemically or locoregionally in a targeted manner, showing strong anti-tumor efficacy [1, 2]. Almost all radionuclides used in RPT can be visualized by nuclear medicine based imaging techniques, providing non-invasive visualization of radiopharmaceuticals while ensuring precision delivery [3-6]. RPT has made significant progress in the past several years with the development of new radionuclides and vectors and the improvement of labeling efficiencies and targeted property [7-9]. These recent developments indicate that RPT is poised to emerge as an effective, safe, and economical therapeutic modality with tremendous potential. Unlike the external irradiation used in traditional radiotherapy, RPT delivers cytotoxic radiation directly to cancer cells or tumor microenvironments systemically or locally [1, 2, 10]. The radiation exposure from RPT is continuous, with the unique exponential decay spectrum depending on the characteristics of the radionuclide utilized, consisting of α or β particles, auger electrons, and gamma emissions of varying energies [11-14].
Radiobiology is fundamental to understanding the therapeutic capacity of RPT. There are accumulating investigations into the cytotoxic effects of RPT on tumors, however, direct cytotoxicity is not the only process accounting for tumor destruction. Growing evidence shows that ionizing radiation elicits anti-tumor effects that exceed cell killing, and immune activation plays an important role in response to radiation which contributes to tumor elimination. Radiotherapy could modulate the immunogenicity of tumor cells, and augment innate and adaptive immune responses against tumors, thereby decreasing immunosuppression and potentiating the responsiveness of tumors to radiation both in the tumor microenvironment (TME) and even at a systemic level [15, 16]. Irradiated tumor cells undergo a stressful death process, associated with the upregulation of immunomodulatory cell surface molecules, expansion of the cellular peptide pool, and the release of cytokines, which are also known as danger-associated molecular patterns (DAMPs) [17]. Then antigen-presenting cells are recruited into TME, subsequently enriching the T‐cell infiltrates, primes, and propagates the pre‐existing or newly infiltrating T cells, inducing anti-tumor effects [18]. Notably, current evidence indicates that radiotherapy can also support tumor cell survival via diverse mechanisms [19, 20]. The immune response induced by RPT may similar to ionizing radiation, but limited knowledge is available regarding the consequences of targeted RPT on the antitumor immune response.
Given that antitumor immunity may help to achieve the ultimate goal of the improvement of the therapeutic efficacy of RPT to cure cancer, there is a need to focus on the RPT-induced antitumor immune response and the synergistic effects with immunotherapy (IT) approaches. In this review, we discussed in detail the immune response activated by radionuclides with different emission properties, and present a brief overview of the interaction between RPT and the immune system (Figure 1). And we provide insight into recent data from pre-clinical and early-phase clinical trials that have investigated RPT in combination with immunotherapy, further highlighting limitations and future challenges.
Basic immune responses of radiotherapy
Usually, tumor cells can be eliminated by the innate and adaptive immune system leading to complete immunologic eradication of cancer, but a prerequisite for efficient elimination is the recognition of danger signals on tumor cells. It is possible that some cells could progress into an equilibrium phase and begin a chronic tug-of-war with immune system, in this state the immune system is still able to keep these cells under control. However, the final stage of interplay between the immune system and an emergent cancer is that uncontrolled tumor cells successfully escape the immunosurveillance, then grow progressively due to their reduced immunogenicity and the establishment of immunosuppressive tumor microenvironment [21, 22].
Even though this relationship between fundamental process of tumor growth and immune system has been known for a long time, it was once generally believed that there was no direct synergy between radiotherapy-induced local tumor regression and the immune system. Instead, radiotherapy was considered immunosuppressive due to bone marrow and lymphocytes being known to be radiosensitive and may be damaged under systemic irradiation [23].
Nevertheless, recent studies led to a paradigm shift in which radiotherapy can also achieve immunostimulatory effects. There are emerging and convincing hints that a contribution of complex radiotherapy-induced immune activation mechanisms can no longer be neglected, radiation can enhance both the priming and the effector phase of the immune response whether by directly inducing an immunogenic tumor cell death or by altering the tumor microenvironment [24, 25]. Undoubtedly, in addition to cell death induction, a productive anti-tumor immune response induced by irradiation is also key to its therapeutic success. Notably, some reports have observed that these immune responses would not only target local tumors but may also treat out-of-field metastases, which has been described as the abscopal effect [26-30].
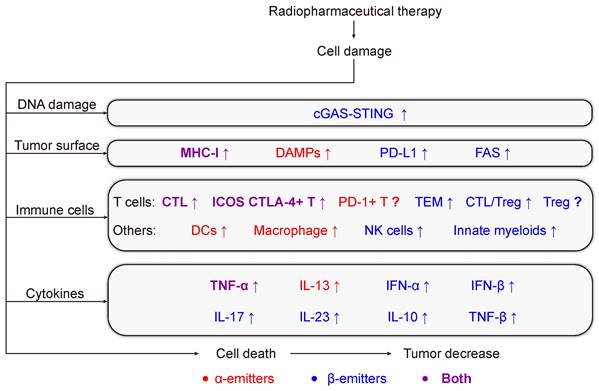
Schematic illustration of immunologic process induced by radiopharmaceuticals. Hsp: heat shock protein. CRT: calreticulin. HMGB1: high mobility group box 1. APCs: antigen-presenting cells. EM T cells: effector memory T cells. MDSCs: myeloid-derived suppressor cells. Treg: regulatory T cell. CTLs: cytotoxic T lymphocytes.
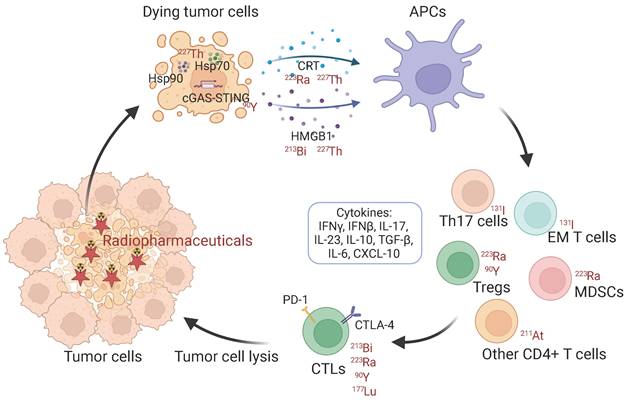
The main target of irradiation is the induction of DNA damage, abnormal DNA damage repair leading to the deficiency of DNA damage response (DDR) which has recently emerged as an important determinant of tumor immunogenicity [31]. DDR caused by radiotherapy could promote the antigenicity and adjuvanticity of targeted tumors. Radiation enhances mutability and genomic instability, increasing the degradation of existing proteins and new peptide production which results in an increase in intracellular peptide pool and thus new tumor-associated antigens (TAAs) [24]. These TAAs may be captured by antigen-presenting cells (APCs), which process TAAs into short peptides that are presented on the cell surface that diversifies the antigen presented, leading to an expanded repertoire of tumor-specific CD8+ T cells. After tumor antigen-specific T cells attack the tumor, other new antigens may be released and captured, which is called 'epitope spreading' [32], creating a positive feedback loop and evoking durable and adaptable immune responses against tumors. In addition, the tumor antigens after radiation exposure may vary significantly, depending on the cell types as well as the dose of radiation applied [33]. And the changes in the antigenic landscape have not been fully explored.
Meanwhile, activation of cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway that senses radiation-induced damaged DNA are capable of generating adjuvant activity for enhancing adaptive immune responses to tumor antigens released [34, 35]. These new antigens could act as an immune-activating danger signal for antigen-presenting cells (APCs) and thus contribute to adaptive immune system [36].
In particular, the immunogenic cell death (ICD) elicited by radiotherapy also links the DDR to anti-tumor immunity, which refers to cell death modalities that share the propensity to activate an immune response [25, 37]. ICD following radiotherapy triggers the emission of a plethora of mediators into the extracellular space as danger signals, which are termed DAMPs. DAMPs represent a large range of molecules and originate from different sources, including extracellular proteins such as fibronectin and biglycan, and intracellular proteins, such as high mobility group box 1 (HMGB1) [38, 39], heat-shock proteins [40], adenosine triphosphate (ATP) [41, 42], calreticulin [43, 44] and Il-1α [45, 46] that only released following stress or cell death. These DAMPs are recognized by macrophages and dendritic cells (DCs) triggered by different pathways including Toll-like receptors (TLRs), induce their maturation and promote cross-presentation of tumor antigens, stimulate the release of cytokines including IL-1β, IL-23, and CXCL-10 e.g., upregulate the co-stimulatory molecules, that in turn is conducive for the infiltration and chemotaxis of immune effectors. Activated natural killer cells (NKs) and cytotoxic T lymphocytes (CTLs, which are CD8+ T cells) possess the ability to kill tumor cells with the help of CD4+ T cells [46-49]. IFN-γ and TNF-α, the CTL signature cytokines, also have anti-tumor mediating properties [50, 51]. In addition, radiotherapy can upregulate multiple pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL-6, IL-8, IL-1α, and IL-1β, to induce an acute inflammatory reaction in tumors [46].
Consequently, accumulating evidence suggests that radiotherapy could kill tumor cells via induction of anti-tumor immune responses by acting as in situ vaccine. However, the dialogue between radiotherapy and immune responses is mostly unknown. Radiotherapy sometimes also plays a role in immunosuppression [52-54]. While extensive research concerning DNA damage and ICD following radiotherapy has been carried out, there is still a lack of data in pre-clinical and clinical studies about its effects on the immune system, with special regard to the impact of different radiation regiments.
Within radiation, RPT with high LET differs from X-ray radiation but shares common features of DNA damage [1, 55-57]. It is important to understand whether the impacts of RPT on the immune system follow the common characteristics of radiotherapy or whether there are specific response signaling pathways, which could guide the clinical treatment of RPT in combination with immunotherapy to improve anti-tumor efficacy. Data about the influence of RPT concepts on immunological consequences are scarce and not conclusive, and present knowledge will be reviewed in the following. Radionuclides are used to deliver radiation with different emission properties: α-particles or β-particles [1]. Here we present a summary of radiopharmaceutical-induced anti-tumor immune activation according to the types of radiation and the radionuclides (Table 1).
α-emitting radiopharmaceuticals propagate antitumor immunity
Physical properties of α-emitting radionuclides
An α-particle is a positively charged helium ion (2p, 2n, +2e) that is emitted from the nucleus of an unstable atom. Alpha particles are energetic with a typical kinetic energy of 5-8 MeV traveling at 5% of speed of light losing a large amount of energy (high linear energy transfer of about 100 keV/μm) in a short path of 50 to 80 μm in view of their electric charge and relatively large mass [58, 59]. Several studies have been performed to describe α emitter radiobiology and cell death mechanisms induced after α irradiation. Like other high linear energy transfer particles, α emitters induce more DNA double-strand breaks than γ or X-rays and provoke a cell cycle arrest in the G2 phase that is more marked than with γ rays [60-63]. Furthermore, radiobiologic effects associated with α radionuclides are advantageously less sensitive to dose rate, hypoxia, and cell cycle distribution than β particles or γ rays [64, 65].
Alpha-particle emitting radionuclides have also been applied for RPT, including 211At, 213Bi, 223Ra, 225Ac, 227Th e.g., [1]. The alpha emitters have tended to show particular promise and there is substantial interest in further developing these agents for therapy of cancers that are particularly difficult to treat. When properly targeted to disease sites, α particles may lead to cellular death through catastrophic clustered DSBs in the nucleus which is difficult to repair and threatens genome integrity, then may further influence multiple aspects of tumor immunogenicity and have immunomodulatory effects in the tumor microenvironment, making them compelling for RPT.
The influence on anti-tumor immune stimulation in pre-clinical in vitro and in vivo models.
| Radionuclide | t 1/2 | Range (mm) | Drug | Model (tumor cell line or indication) | Immune response | Ref |
|---|---|---|---|---|---|---|
| α | ||||||
| 213Bi | 45.6 min | 0.05 | 213Bi-BSA | Mouse vaccine (MC38) | T cells are essential for the antitumor effectDAMPs (Hsp70 and HMGB1) ↑DCs ↑ | [66] |
| 223Ra | 11.4 d | 0.05-0.08 | Xofigo | Cell (LNCaP, PC3, MDA-MB-231, ZR75-1, H441, H1703) | MHC-I and calreticulin ↑CD8+ T cell cytotoxic efficiency specific for MUC-1, brachyury, and CEA tumor antigens ↑ | [72] |
| Xofigo | Human (Prostate cancer) | PD-1 expressing EM CD8+ T cells ↓CD8+ T cells and their subsets (N.S.)CD27, CD28 or CTLA4-expressing T cells (N.S.)CD8+ T cells producing IFN-γ, TNF-α and IL-13 (N.S.) | [73] | |||
| - | Human (Prostate cancer) | T cells that expressed ICOS, TIM-3, PD-L1, and PD-1 ↑Treg cells and MDSC ↑ | [74] | |||
| - | Human (Prostate cancer) | Abscopal effect IL-6 ↑ | [75] | |||
| 227Th | 18.7 d | BAY 2287411 | Cell (OVCAR-3) | DAMP markers (calreticulin, HSP70, HSP90, and HMGB1) ↑ | [76] | |
| 211At | 7.2 h | 0.05 | [211At]MM4 | Mouse (GL26) | Tumor-associated macrophages ↑CD4+ T cells ↑ | [77] |
| 211At-ATE-MnO2-BSA | Mouse (4T1, CT26) | CD80+CD86+ DCs ↑ | [78] | |||
| β | ||||||
| 90Y | 64.1 h | 5.3 | 90Y-labeled anti-CEA mAb | Mouse (MC38-CEA+) | Fas ↑ | [81] |
| 90Y-NM600 | Cell (B78, MOC2) | IFNγ ↑ CTLA-4 of T cells ↑ | [83] | |||
| 90Y-NM600 | Mouse (EL4) | CD8+ T ↑ Treg cells ↓ | [82] | |||
| 90Y-NM600 | Mouse (B78) | CD11b+ and NK cells ↑ Effector T cells (CD8+) / suppressor Tregs (CD4+CD25+FOXP3+) ↑ Vcam1, Fas and IFNβ ↑ cGAS/STING pathway activation was required for antitumor efficacy | [84] | |||
| 90Y radioembolization | Human (Colorectal cancer) | low levels of tumor-infiltrating lymphocyte (TIL) infiltration in tumor cancer islands before and after Y90 radioembolization (N.S.) | [85] | |||
| 131I | 8 d | 0.8 | - | Human (Thyroid cancer) | Th17, Tc17 and Treg cells increased ↑IL-17, IL-23, IL-10 and TGF-β1 ↑ | [88] |
| 131I-Cat-CpG/ALG | Mouse (4T1, CT26) | Effector memory T cells ↑ TNF-α and IFN-γ ↑ | [89] | |||
| 153Sm | 46.5 h | 0.4 | 153Sm-EDTMP | Cell (LNCaP) | Surface molecules (Fas, CEA, MUC-1, MHC class I, and ICAM-1) ↑ Antigen-specific CTL-mediated killing ↑ | [91] |
| 177Lu | 6.6 d | 0.62 | 177Lu-DOTATATE | Mouse (NCI-H727) | FasL+ CD49b+ NK cells ↑ | [93] |
| 177Lu-EB-RGD | Mouse (MC38) | CD45+/PD-L1+ and cd11b+/PDL1+ cells ↑ | [94] | |||
| 177Lu-DOTA-diZD | Mouse (4T1) | CD4+ and CD8+ cells ↑ | [95] | |||
| 188Re | 16.9 h | 3.1 | 188Re-6D2 | Mouse (A2058) | Complement C3 ↑ | [96] |
| 18F | 109.7 min | 2.39 | 2-[18F]FDG | Cell/Mouse (MC38, CT26) | PD-L1 ↑ | [97] |
| 64Cu | 12.7 h | 2.5 | 64Cu-DOTA-EB-cRGDfK | Cell (MC38, CT26) | PD-L1 ↑ | [99] |
DAMPs: Damage-associated molecular patterns; DCs: Dendritic cells; MHC-1: Major histocompability complex-1; MUC-1: mucin-1; CEA: Carcinoma embryonic antigen; N.S.: No significance; MDSC: Myeloid-derived suppressor cell.
Immunologic effects of α-emitting radionuclides
213Bi
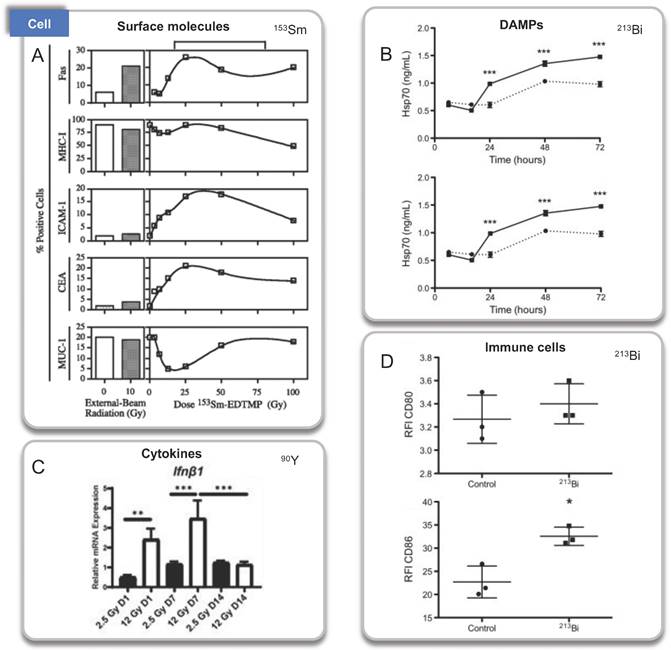
The first investigation to analyze propagating antitumor immunity of α particles was undertaken by J. B. Gorin et al (2014) [66]. They studied the immunogenicity of 213Bi-BSA on mouse MC38 adenocarcinoma. 213Bi emits both α (~92.7%) and β (~7.3%) particles with a relatively short half-life of 46 minutes. Irradiated MC38 cells were injected into immunocompetent C57Bl/6 mice in a vaccination approach, and the overall survival increased 2 months after vaccination from 16% in the control group to 88%. The lasting protective effect was indicated by tumor transplantation rechallenge, and the immunization with irradiated MC38 failed in nude mice. These results indicated that even long after vaccination, irradiating cancer cells with 213Bi can stimulate adaptive immunity mediated by tumor-specific T cells. Regarding the possible molecular mechanisms, they also showed that 213Bi-BSA of MC38 cells in vitro induced the release of DAMP, such as heat shock protein 70 (Hsp70) and HMGB1 through ELISA performed on a conditioned medium. In addition, the activation of co-cultured bone marrow-derived DCs was observed through the upregulation of costimulatory molecules (CD40, CD80, and CD86).
223Ra
223RaCl2 is the first approved targeted α-emitting radiopharmaceuticals by the U.S. Food and Drug Administration (FDA). It has been used in clinics since 2013 to treat patients with metastatic castration-resistant prostate cancer (mCRPC), but still limited preclinical and clinical studies of antitumor immune responses induced by 223Ra were discussed. 223Ra is a calcium mimetic and naturally targets the bone hydroxyapatite (Ca10(PO4)6(OH)2) matrix [67-70]. 223Ra decays to stable 207Pb with an emitted decay energy distribution of 93.5% α particle, <3.6% β- particle, and <1.1% γ radiation with a half-life of 11.43 days [71].
In 2016, A. S. Malamas et al. reported the immunogenic modulation in tumor cells (human prostate, breast, and lung carcinoma cells) exposed to sublethal doses of 223Ra in vitro [72]. 223Ra significantly enhanced T cell-mediated lysis of each tumor type (MDA-MB-231, ZR75-1, LNCaP, PC3, H1703, and H441 cells) by CD8+ CTLs specific for MUC-1, brachyury, and CEA tumor antigens. Immunofluorescence analysis revealed that the increase in CTL killing was accompanied by augmented protein expression of MHC-I and calreticulin. Kim JW et al. discussed immune responses of circulating peripheral blood mononuclear cells (PBMCs) of bone metastatic castration-resistant prostate cancer (mCRPC) patients after 223Ra radiation, fifteen patients received a course of 223Ra 50 kBq/kg [73]. PD-1 expressing EM CD8+ T cells decreased after one 223Ra treatment from 20.6% to 14.6%, while no significant change was observed in the frequencies of CD27, CD28, or CTLA4-expressing T cells. However, no significant change in the overall frequencies of CD8+ T cells including naïve, central memory and effector memory (EM) cells, and also the frequencies of CD8+ T cells producing IFN-γ, TNF-α, and IL-13. Another similar case was reported, a total of 35 mCRPC patients were screened with 223Ra administered intravenously every four weeks at a dose of 55 kBq/kg (a maximum of six injections) [74]. H. A. Jeroen et al. observed a decrease in absolute lymphocyte counts and an increase in the proportion of T cells that expressed costimulatory (ICOS) or inhibitory (TIM-3, PD-L1, and PD-1) checkpoint molecules. All memory and effector phenotypes within the CD4+ and the CD8+ subset appeared stable throughout 223Ra therapy. Moreover, the fraction of two immunosuppressive subsets (the regulatory T cells and the monocytic MDSCs) increased throughout the treatment which was contrary to Kim's results. They hypothesized that Tregs, M-MDSCs, and checkpoint-expressing T cells may be the reflection of the migration of (non-exhausted) effector T cells into the tumor.
Abscopal effects also be occurring with 223Ra-dichloride therapy. Kwee et al. reported observations in 2 patients that suggested 223RaCl2 abscopal effect with resultant favorable response in untargeted soft tissue disease [75]. Concomitant increases in plasma interleukin 6 were detected, suggesting that the response may be mediated by immune activation.
227Th
In 2019, Hagemann et al. presented the preclinical evaluation of a mesothelin (MSLN)-targeted thorium-227 conjugate, BAY 2287411, for the treatment of MSLN-expressing tumors [76]. On OVCAR-3 cells, they found that BAY 2287411 was able to upregulate the DAMPs in vitro, including calreticulin, HSP70, HSP90, and HMGB1 detected. However, additional studies are needed in mouse animal models to evaluate the potential immunostimulatory effects of BAY 2287411.
211At
In 2021, Hannah Dabagian et al. observed the enhanced recruitment of macrophages and CD4+ T cells to tumors treated with [211At]MM4 assessed by histopathology of a poor mouse responder in vivo [77]. But CD4+ T cells contain immunosuppressive T-regulatory cells, so more evidence is needed to demonstrate the pro-inflammatory processes. Jiajia Zhang et al. designed the 211At labeled Mn-based radiosensitizer (211At-ATE-MnO2-BSA), which increased the proportion of CD80+CD86+ DCs on mice bearing CT26 tumors, compared to the group of free 211At treatment or MnO2-BSA treatment [78].
β-emitting radiopharmaceuticals propagate antitumor immunity
Physical properties of β-emitting radionuclides
β-particles are electrons emitted from the nucleus, which are the most frequently used emission type for RPT agents. β-particle have a longer path length in tissue (1-10 mm), but a lower LET (0.2 keV/μM), which leads to less complex cell damage and is more readily repaired [79, 80]. The β-particle emitters yttrium-90, and iodine-131, samarium-153, and lutetium-177, have been introduced and are commonly used [1].
Immunologic effects of β-emitting radionuclides
90Y
Mala Chakraborty et al. studied 90Y-labeled COL-1, using CEA-transgenic mice transplanted with MC38-CEA+ tumor cells. COL-1 (a murine IgG2a) is a murine antibody specific for CEA (human carcinoembryonic antigen). They found that cell-surface expression of Fas was upregulated in irradiated than in nonirradiated MC38-CEA+ tumor cells, and peaked at the treatment of 50 and 100 μCi 90Y-labeled COL-1. Through MC38-CEA-DN1 tumors (Fas nonfunctional) models, they confirmed that increased survival treatment with 90Y-labeled COL-1 was mediated by engagement of the Fas/Fas ligand pathway. However, in MC38 cells, the expression of Fas was not increased, showing the importance of retaining radiolabeled antibodies in the phenotypic changes of tumor cells [81].
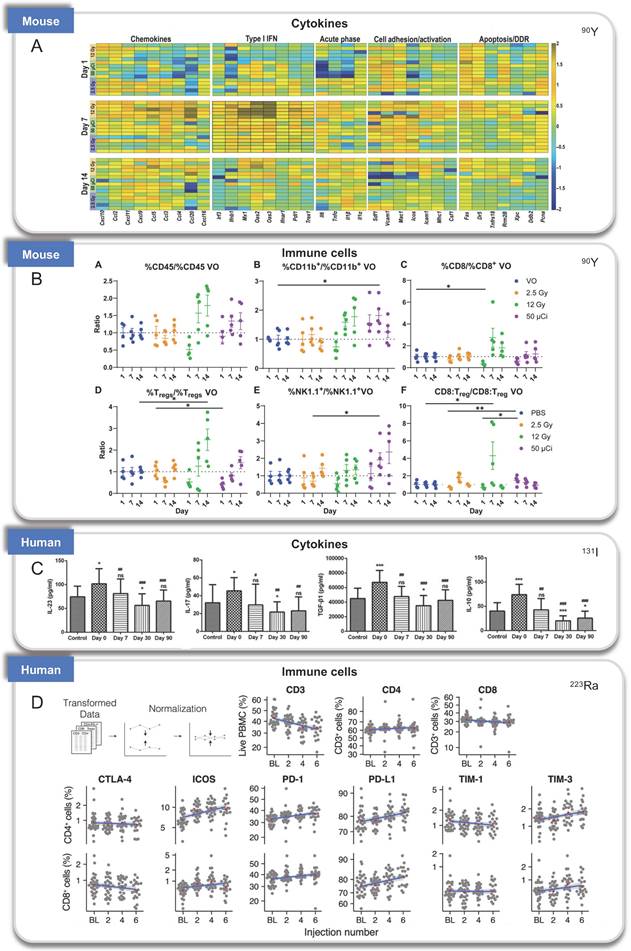
NM600 is an alkylphosphocholine analog that exhibits preferential uptake and accumulation in nearly all tumor types. 90Y-NM600 is delivered to tumor microenvironments (TME) for tumor therapeutic, and three groups studied the immune effect of 90Y-NM600 [82]. Justin Jagodinsky et al. treated B16 or MOC2 tumor cells by 90Y-NM600 (140 µCi), and splenocytes were added three days after irradiation. By flow cytometry one day later, they found when co-cultured with 90Y-NM600-treated tumor cells, live CD4 and CD8 cells number and the expression of IFN-γ of CD8+ T cells increased, at the same time, CTLA-4 on T cells, an inhibitory immune signal, also increased compared to those co-cultured with untreated control tumor cells. In addition, using STING KO cells, they determined that the activation of IFN-γ production is independent of STING pathway [83]. Reinier Hernandez et al. established mice bearing T-cell NHL tumors treated with 90Y-NM600 (9.25 MBq) which experienced tumor growth inhibition and extended survival with immune memory. By immunohistochemistry staining, increased CD8+ T cells and decreased Foxp3+ regulatory T cells at day 6 were discovered. And tumors did not grow 10 days after re-inoculation of tumor cells. Besides, they transplanted adoptive T cells from radiated mice to Rag2 KO (immunocompromised) mice, the tumor tissues of Rag2 KO mice achieved sustained complete response, suggesting the tumor suppression was dependent on T-cells activation [82]. Furthermore, Ravi Patel et al. investigated immunomodulatory effects of 90Y-NM600 in vivo by C57Bl/6 mice bearing B78 flank tumors treated with 50 μCi 90Y-NM600. They observed that the ratio of effector T cells (CD8+) to suppressor Tregs (CD4+CD25+FOXP3+), expression of Vcam1, Fas, and IFNβ, increased at day 1 after treatment with a modest decrease in Il6. Activation of the cGAS/STING pathway was found to be required for antitumor efficacy by STING KO model. Besides, innate myeloid (CD11b+) and NK cells were significantly increased on day 7 after radiation [84].
However, before and after 90Y radioembolization, low levels of tumor‐infiltrating lymphocyte (TIL) infiltration were observed in tumor tissue of patients with metastatic colorectal cancer, suggesting the lack of immunomodulatory responses to 90Y radioembolization in this clinical pilot feasibility study [85].
131I
131I radioiodine has been used for many years to treat thyroid cancers [86]. Previous studies have revealed that T helper cells are an important immunological mechanism in the pathogenesis of differentiated thyroid cancer (DTC) [87]. Lixia Zhang et al. examined the distribution of Th17, Tc17, and Treg cells in patients with DTC before and after 131I therapy, they discovered that at 90 days following 131I therapy, the numbers of Th17, Tc17, and Treg cells, as well as the levels of related cytokines (IL-17, IL-23, IL-10, and TGF-β1) decreased and returned to the similar values as detected in healthy control patients [88].
Yu Chao et al. designed an in situ gelation strategy to trap a 131I within sodium alginate (ALG), catalase (Cat), and CpG oligonucleotide, 131I-Cat/CpG/ALG. In the 131I-Cat-CpG/ALG treatment group, more effector memory T cells (TEM), and higher serum levels of TNF-α and IFN-γ were observed following tumor rechallenging, indicating long-term protection of immunological memory induced by 131I-Cat-CpG/ALG [89]. It is worth noting that substances other than 131I in the hydrogel may play a role in immune activation, so we must be cautious that the immunity caused by 131I-Cat-CpG/ALG may not completely represent the effect of 131I.
153Sm
153Sm lexidronam is a chelated complex of a radioisotope of the element samarium with EDTMP that binds avidly to hydroxyapatite in bone, has been approved for the treatment of pain associated with bone cancer in 1997 [90]. Mala Chakraborty et al. explored the phenotype of tumor cells and T cell-mediated killing. Using 10 human tumor cell lines exposed to 153Sm-EDTMP, at least two of the five surface molecules (Fas, CEA, MUC-1, MHC class I, and ICAM-1) on each cell line were upregulated. In addition, treatment of LNCaP cells with 153Sm-EDTMP functionally increases antigen-specific CTL-mediated killing, suggesting an induced immune response [91].
177Lu
In 2018, FDA approved lutetium 177Lu-DOTATATE (Lutathera) for the treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). And 177Lu-PSMA-617 (Pluctivo) is now approved in 2022 for the treatment of patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have previously been treated with an androgen-receptor pathway inhibitor and taxane-based chemotherapy. It is promising to understand the immune response induced by 177Lu.
177Lu-DOTATATE is a type of peptide receptor radionuclide therapy (PRRT) for the treatment of neuroendocrine tumor (NET) patients approved by the FDA approval in 2018 [92]. By using NCI-H727 cells xenograft model of NETs, Yin W et al. showed that 177Lu-DOTATATE PRRT led to increased infiltration of CD86+ cells and CD49b+/FasL+ NK cells in tumor tissues, which is capable of tumor killing [93]. Haojun Chen et al. treated MC38 tumor-bearing C57BL/6 mice with 18.5 MBq 177Lu-EB-RGD, which contained Arg-Gly-Asp (RGD) sequence specifically targets the cell surface receptor integrin αvß3. On days 4 and 7, the percentage of CD45+/PD-L1+ and CD11b+/PD-L1+ cells increased at least twofold, indicating that 177Lu-EB-RGD led to an acute increase in PD-L1 expression on T cells [94]. Additionally, 177Lu-DOTA-diZD induced immune response was studied [95], which is a high-affinity vascular endothelial growth factor receptor (VEGFR)-targeted agent labeled with 177Lu by 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) chelator. In 4T1-bearing mice treated with 177Lu-DOTA-diZD, the population of CD4+ and CD8+ cells increased, suggesting that 177Lu-DOTA-diZD modulates the tumor microenvironment.
188Re
Treated with 1.5 mCi 188Re-6D2 which binds to melanin, the tumors of A2058 human melanoma xenograft model were suppressed and the pronounced presence of complement C3 was discovered, which was able to activate the host immune system [96].
18F
In addition, positron emission tomography (PET) imaging tracer 2-[18F]FDG may also be an immunomodulator. After co-incubation with 2-[18F]FDG (1.85 MBq/mL), the proportions of PD-L1+ cells of CT26, MC38, 4T1, and B16F10 tumor cells were significantly increased, which was induced by DNA damage via STAT1/3-IRF1 pathway. And the upregulation of PD-L1 expression has also been demonstrated in CT26 and MC 38 tumor-bearing mice models [97], which may make tumor cells more sensitive to anti-PD-L1 antibody treatment.
64Cu
64Cu decays in three ways: β+ (17%, Emax = 655 KeV), β- (39%, Emax = 573 KeV) and electron capture (44%) [98]. 64Cu-based radioligands have been developed for PET imaging, while 64Cu might be a potential therapeutic radionuclide. Similar to 2-[18F]FDG, 370 kBq of 64Cu-DOTA-EB-cRGDfK also upregulated PD-L1 expression in CT26 and MC38 cells [99].
See Figure 2-4 and Table 2 for a summary of the RPT-induced effects based on the activated immune process.
Combination of immunotherapy with RPT to increase anti-tumor efficacy
Pre-clinical studies with combined strategies to enhance tumor killing effects
Although according to the above summary, nuclear medicine can activate the immune system to enhance anti-tumor effects, there are not many reports or clinical data. Additionally, only a small percentage of patients achieve complete response through just radiotherapy, a common point seems to be the fact that radiation probably can only amplify a pro-immunogenic phenotype and can hardly change by itself a net immune-suppressing environment into an immune-stimulating one. Thus, future research could focus on defining optimal RPT protocols on one hand, and optimal combination approaches on the other hand to achieve greater therapeutic effects than the respective monotherapies and lower dosages or numbers of cycles required, in turn, reducing unwanted toxicities. Several preclinical investigations have suggested the combination of radiation and immune checkpoint inhibitors (ICI) promotes response and immunity [100-102]. To improve the anti-tumor effect of nuclear medicine and better take advantage of nuclear medicine in inducing an anti-tumor immune response, radioimmunotherapy has proven to be a useful tool.
Immunologic processes activated by RPT confirmed by cell experiments. A. Surface molecules, 153Sm; B. DAMPs, 213Bi; C: Cytokines, 90Y. D. Immune cells, 213Bi. Panel A is adapted with permission from [91], copyright 2021 by the American Association for Cancer Research. Panel B and D is adapted with permission from [66], copyright 2021 Elsevier, Inc. Panel C from [83].
At present, it is clinically applied immunotherapy target ICI with programmed death 1 (PD-1) [103] and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) [104, 105], expressed in T cell surface negative direction The T cell function is regulated so that the tumor tissue is immunized. The inhibitors of these two targets can activate the immune system to generate significant improvements in disease outcomes for various cancers. PD-1 inhibitors mainly act on the effect phase of T cells. T cells will express PD-1 after being activated, and tumors evade the monitors of immune systems by expressing PD-L1 which is the ligand of PD-1, thus the anti-tumor activities of T cells are often inhibited [103]. The PD-1 inhibitor can block the binding of PD-L1 and PD-1, re-release the anti-tumor capacity of tumor suppression. Nivolumab [106] and pembrolizumab [107, 108], Both PD-1 Inhibitors, have been approved to treat patients with advanced or metastatic melanoma and patients with metastatic, refractory non-small cell lung cancer. PD-L1 inhibitors Atezolizumab [109], Durvalumab [110] and Avelumab [111] have been approved to treat non-small cell lung cancer, locally advanced or metastatic urinary tract cancer, Merkel cell carcinoma. The CTLA-4 inhibitor mainly acts on the early development stage of T cells, by combining CTLA-4 on the surface of T cells, inhibiting the antigen presenting cells on T cell function, indirectly promoting the activation and proliferation of T cells. Ipilimumab, an inhibitor of CTLA-4, has been approved for the treatment of advanced or unresectable melanoma [112, 113]. Although immune checkpoint inhibitor treatment may be effective initially, many patients will eventually relapse and develop tumor progression, and only part of the patients can achieve complete response [114].
Immunologic processes activated by RPT found in preclinical and clinical studies. (A-B). A. Cytokines and B. immune cells induced by 90Y in mouse models; C. Cytokines induced by 131I in patients; D: Infiltration of immune cells treated by 223Ra in patients. Panel A and B is adapted with permission from [84], copyright 2021 American Association for the Advancement of Science. Panel C is adapted with permission from [88], copyright Spandidos Publications 2021. Panel D is adapted with permission from [131], copyright 2007 - 2021 Frontiers Media S.A.
RPT-induced anti-tumor immune response. RPT causes DNA damage, induces the expression of tumor antigens, recruits immune cells, enhances the secretion of cytokines in the tumor environment, and kills tumor cells. Red and blue present α-emitters or β-emitters respectively. Purple represents molecules, signaling pathways, or cells that are involved in common.
Summary of described immune effects by RPT.
| Classification | Description | Model (tumor cell line or indication) | Radionuclide | Ref |
|---|---|---|---|---|
| DAMP | Calreticulin, HSP70, HSP90, and HMGB1 ↑ | Cells (MC38, MDA-MB-231, ZR75-1, LNCaP, PC3, H1703, H441, OVCAR-3) | 223Ra 227Th 213Bi | [66, 72, 76] |
| Tumor surface | Fas, ICAM-1 ↑ | Cells (MC38-CEA+)/Mouse (B78) | 153Sm 90Y | [81, 84] |
| MHC-I ↑ | Cells (MC38, MDA-MB-231, ZR75-1, LNCaP, PC3, H1703, H441, OVCAR-3) | 233Ra 153Sm | [72, 91] | |
| Other TAAs (PSA, CEA and MUC-1) ↑ | Cells (LNCaP) | 153Sm | [91] | |
| PD-L1 ↑ | Cells/Mouse (MC38, CT26) | 18F 64Cu | [97, 99] | |
| T cells | T cells are essential for the antitumor effect | Mouse (MC38) | 213Bi | [66] |
| CTL ↑ | Mouse (EL4) | 90Y | [82] | |
| T cell cytotoxic efficiency ↑ | Cells (MC38, MDA-MB-231, ZR75-1, LNCaP, PC3, H1703, H441, OVCAR-3)/Mouse (MC38) | 213Bi 223Ra | [66, 72] | |
| Costimulatory molecules (ICOS and CTLA-4) ↑ | Cells (MC38-CEA+)/Human (Prostate cancer) | 223Ra 90Y | [74, 81] | |
| Effector memory T cells ↑ | Mouse (4T1, CT26) | 131I | [89] | |
| Th17 and Tc17 ↑ | Human (Thyroid cancer) | 131I | [88] | |
| CTL/Treg ↑ | Mouse (B78) | 90Y | [84] | |
| Treg ↓ | Mouse (EL4) | 90Y | [82] | |
| Treg ↑ | Human (Prostate cancer, thyroid cancer) | 223Ra 131I | [74, 88] | |
| Inhibitory molecules (PD-1) ↓ | Human (Prostate cancer) | 223Ra | [73] | |
| inhibitory molecules (PD-1 and Tim-3) ↑ | Mouse (MC38)/Human (Prostate cancer) | 223Ra 177Lu | [74, 94] | |
| Cytokine | IFNγ, IFNβ, IL-17, IL-23, IL-10, TGF-β1, IL-6, CXCL-10↑ | Mouse (B78, MOC2, 4T1, CT26)/Human (Thyroid cancer) | 90Y 131I | [83, 88, 89] |
| Other immune cells | DC, Innate myeloid, NK cells, and MDSC ↑ | Mouse (MC38, B78)/Human (Prostate cancer) | 213Bi 90Y 223Ra | [66, 74, 84] |
| Complement | Complement C3 ↑ | Mouse (A2058) | 188Re | [96] |
| cGAS/STING | cGAS/STING ↑ | Mouse (B78) | 90Y | [84] |
MHC-1: Major histocompability complex-1; TAAs: Tumor-associated antigens; PSA: Prostate-specific antigen; CEA: Carcinoma embryonic antigen; MUC-1: mucin-1; CTL: cytotoxic T lymphocyte; DC: Dendritic cell; NK: Natural killer; MDSC: Myeloid-derived suppressor cell.
Thus, the use of RPT activation of immune systems while inhibiting tumor immune escape may be more efficient than a separate treatment to effectively inhibit tumor growth. As of now, there have been limited pre-clinical attempts to improve RPT outcomes through combinations with immunotherapy. Here, we present a review of the combination strategies of TRT with immunotherapy reported in the literature to date.
Johannes Czernin et al. found a synergistic anti-tumor effect used in nuclear drugs and PD-1 inhibitors. C57BL/6-mice bearing syngeneic RM1-PGLS tumors were treated with 30 kBq 225Ac-PSMA617 (on day 12), 10 mg/kg Anti-PD-1 Antibody (on days 13, 16, 20, and 23), or Both. Combining PSMA-RPT and anti-PD-1 significantly improved disease control compared with either monotherapy. Time to progression was extended to 47.5 days (isotype control, 25 days; 225Ac-PSMA617, 30 days; Anti-PD-1, 33.5 days), and survival to 51.5 days (isotype control, 28 days; 225Ac-PSMA617, 32 days; Anti-PD-1, 37 days) [115].
Hannah Dabagian et al. investigated the effects of 36 MBq/kg [211At]MM4 administered on day 11 in combination with 200 μg anti-PD-1 administered on days 8, 11, and 14 in a syngeneic mouse model of glioblastoma using GL26 glioblastoma cells in C57BL/6J mice. The average best tumor responses for combination, anti-PD-1, and [211At]MM4 were 100%, 83.6%, and 58.2% decrease in tumor volume, respectively. Average progression free intervals for combination, anti-PD-1, [211At]MM4, and control groups was 65, 36.4, 23.2, and 3 days, respectively. The percentages of disease-free mice at the end of the study for combination and anti-PD-1 were 100% and 60%, while [211At]MM4 and control groups were both 0%. In summary, combination therapy was more effective than either single agent in all response categories analyzed, highlighting the potential for PARP targeted alpha-therapy to enhance PD-1 immune-checkpoint blockade [77].
Patrycja Guzik and others explored the anti-tumor treatment of 177Lu nuclear medicine combined with an anti-CTLA-4 antibody. NF9006 tumor-bearing mice received 177Lu-DOTA-folate (5 MBq; 3.5 Gy absorbed tumor dose), anti-CTLA-4 antibody (3 × 200 μg), or both agents. They found 177Lu-DOTA-folate only or ICI only had only a minor effect on tumor growth and did not increase the median survival time (23 days and 19 days, respectively) as compared with untreated controls (12 days). However, tumors treated with combination therapy decreased and median survival time of mice increased (> 70 days). Thus, the application of 177Lu with anti-CTLA-4 immunotherapy had a positive effect on the anti-tumor efficiency [116].
Since PD-1/PD-L1 and CTLA-4 possessed different mechanisms of action, the combination therapy using these two inhibitors has also been studied and applied [117, 118]. Scientists also explored the anti-tumor effects of nuclear medicine cooperated with these two inhibitors at the same time. They reported the anti-tumor effect studies of 30 MBq 177Lu-LLP2A treatment, alone and combined with immune checkpoint inhibitors (200 μg anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies on days 9, 12, and 15 after tumor cell injection), in B16F10 tumor-bearing mice. They discovered that the survival of RPT alone was comparable to the dual-ICI anti-PD-1 + anti-CTLA-4 or anti-PD-L1 + anti -CTLA-4, whereas RPT + ICIs significantly enhanced survival. At the same time, TUNEL staining also indicated more apoptosis signals in RPT + ICI groups [119].
In addition to the combination strategy, radioisotope-labeled monoclonal antibodies which target immune checkpoints such as PD-L1 may also be effective cancer treatment strategies, because immune checkpoint-associated antibodies linked to cytotoxic nuclides not only have the function of immune modulation as an ICI, but only can selectively bind tumor antigens and release cytotoxic radiation. 213Bi-anti-hPD-L1 mAb was developed by Marisa Capitao et al [120]. Using M113PD-L1+ melanoma xenograft model, delayed tumor growth was observed in mice treated with 125 kBq/g 213Bi-Anti-hPD-L1 mAb, while no significant change was found in PD-L1 negative M113WT melanoma xenograft model with the same treatment. Improving tumor targeting is a big challenge for designing radioisotope-labeled monoclonal antibodies, with high tumor uptake, long tumor retention and low uptake in other major organs. Jingyun Ren et al. screened the antibody by systematic PET imaging study and labeled it with the 177Lu for RIT, denoted as 177Lu-DOTA-Y003 [121]. With the treatment of 11.1 MBq of 177Lu-DOTA-Y003, slower tumor growth was shown in MC38-bearing mouse model than the control group, and the survival was prolonged. Although single 131I-αPD-L1 alone showed limited tumor suppression developed by Xuejun Wen et al., 131I-αPD-L1 could induce PD-L1 expression and further increase the uptake of αPD-L1 mAb in CT26 and MC38 tumors [122]. Moreover, the combination treatment of 11.1 MBq of 131I-αPD-L1 + 200 μg of αPD-L1 mAb prolonged the survival of mice, of note, cures half of the tumor-bearing mice.
Altogether, these data exhibited that radioisotope-labeled immune checkpoint antibodies have the potential to enhance the efficacy of cancer immunotherapy. Furthermore, since some RPTs have been shown to potentially upregulate PD-L1 expression, this phenomenon could be exploited for the combination therapy in an appropriate time window.
Harnessing RT to Improve Responses to Immunotherapy
Some works have also helped to explain why the combination strategy is better than monotherapy in the terms of anti-tumor efficacy (Figure 5).
Anti-tumor immune response activated by RPT combined with immunotherapy. A. 90Y-NM600 combined with anti-CTLA-4 therapy. B. [177Lu]LuCl3 combined with anti-PD-L1 and anti-CTLA-4 therapy. Panel A is adapted with permission from [84], copyright 2021 American Association for the Advancement of Science. Panel B is adapted with permission from [132], copyright 1996-2021 MDPI (Basel, Switzerland).
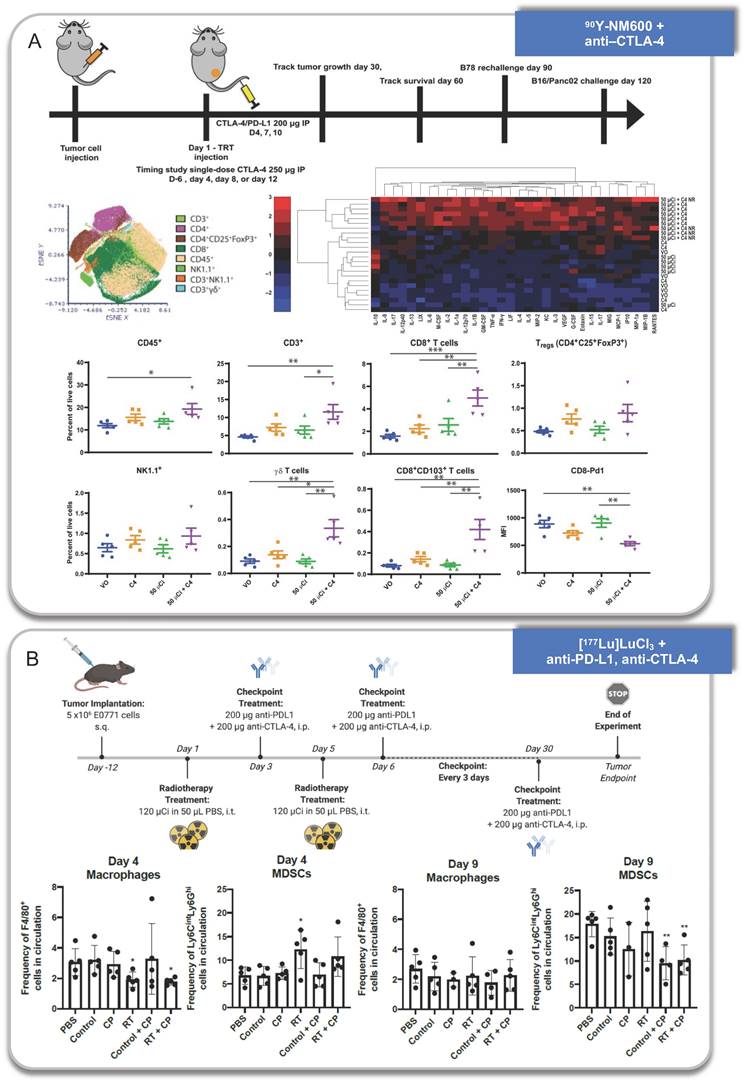
Jiajia Zhang et al. proved that the tumor growths were inhibited in 211At-ATE-MnO2-BSA group, while 211At-ATE-MnO2-BSA plus anti-PD-L1 treatment exhibited a prolonged survival rate. In the model of distal tumors, the intratumoral CD8+ T cells increased upon the treatment of 211At-ATE-MnO2-BSA plus anti-PD-L1 with no obvious changes of Tregs. Also, the level of TNF-α and IFN-γ increased by the synergistic treatment [78]. In addition, after 28 days of combinational treatment, the secondary tumors were inoculated on the opposite side of the initial tumors, and found that the proportion of effector memory CD4+ T and CD8+ T cells increased in spleens, suggesting the generation of immune memory.
Xuejun Wen et al. proved that 2-[18F]FDG treatment could upregulate the expression of PD-L1 on MC38 tumors, and the combination of 2-[18F]FDG (37 MBq) and anti-PD-L1 mAb improved the anti-tumor efficacy and prolonged the overall survival [97]. Upon treatment, the increase in the infiltration of the effector memory T (TEM) cells in the spleen was observed, suggesting the enhancement of immunologic memory. Additionally, the numbers of intratumoral CD4+ Th1 (which helps the macrophage or CD8+ T cells mediated immunity) and CD8+ CTLs increased with M1-like macrophages infiltration following the combination treatment, while immunosuppressive regulatory T cells (Treg) and M2-like macrophages showed a decrease. Besides, the proinflammatory cytokines including TNFa, IFNγ, and IL6 increased in serum, and maintained for at least 7 days.
64Cu-DOTA-EB-cRGDfK may also increase the expression of PD-L1 in MC38 tumors, and the sequential treatment group of MC38-bearing mice with 64Cu-DER (18.5 MBq) followed by αPD-L1 mAb (10 mg/kg) with a 4 h interval displayed complete cure within 30 days [99]. Moreover, in the infiltration of CD8+ CTLs (CD45+CD8+IFN-γ+) and CD4+ Th1 (CD45+CD4+IFN-γ+) cells in tumors, the ratios of CD8+ CTL/Treg and CD4+ Teff (CD4+ T effector cell)/Treg in tumors, and the proportion of TEM cells (CD8+CD44highCD62Llow and CD4+CD44highCD62Llow) in spleen increased 7 days after the combination treatment.
Haojun Chen et al. discovered in MC38 bearing C57BL/6 mice, 177Lu-EB-RGD (18.5 MBq) combined with anti-PD-L1 antibody (10 mg/kg) synergistically enhances anti-tumor immunity by stimulating CD8+ T cell infiltration, improving tumor control, overall survival and protecting against tumor rechallenge. Besides, in both concurrent (day 4 and 7) or sequential (day 14) combined therapy, the level of CD8+ T cells is higher than that in mice treated [94].
Ravi Patel et al. observed increased survival with the combination of 50 or 100 μCi 90Y-NM600 and anti-CTLA-4, and the combination strategy induced distant tumor response in B87 tumor models. They found CD45+ immune cells, CD3+ T cells, CD8+ effector T cells, CD8+ CD103+ tissue resident effector memory T cells, and γδ T cells increased and PD-1 decreased which suggested reduced immune exhaustion at day 25 after treatment. Meanwhile, most cytokines also increased in the TME, particularly in responding mice. One exception was IL-10, which was reduced in the TME, consistent with a reduction of infiltrating Tregs or suppressive monocyte populations. They further found tumor-infiltrating lymphocytes (TILs) isolated from combination-treated animals produced more IFN-γ upon stimulation. More importantly, an increase in clonal expansion of T cells was also observed through deep TCR-β sequencing [84].
Based on Yu Chao's experimental design, 131I was used to label Cat, and combined with CTLA-4 inhibitors to explore the remote effects and mechanisms [89]. After local injection of the 131I-Cat/ALG hybrid solution into tumors, Ca2+ triggers rapid gelation of ALG, and by introducing immune-adjuvant CpG, local treatment with a mixture of 131I-Cat/CpG/ALG could trigger stronger systemic anti-tumor immune responses. They used 131I-Cat/CpG/ALG hybrid solution plus CTLA-4 checkpoint-blockade therapy in distant CT26 tumors, a remarkable synergistic effect to eliminate distant metastatic tumors was found, with increased CD8+ CTL infiltration and decreased Treg cells in the distant tumors. At 20 days post different treatments, TNF-α and IFN-γ increased in mice sera which play crucial roles in the cytotoxic functions of CTLs. And this combination therapy also provided long-term immune memory protection for treated mice. Through T cell blocking experiments using anti-CD4 and anti-CD8 antibodies, both CD4+ and CD8+ T cells were indicated important to the abscopal effect.
In addition to using immune checkpoint inhibitors, Mala Chakraborty and others have also tried recombinant anticancer vaccine, they treated CEA-Tg mice with a combination therapy of CEA/TRICOM vaccine and 90Y-labeled anti-CEA bearing mAb in MC38 mice. Overall survival was improved compared to vaccine or 90Y-labeled anti-CEA mAb only, with increased tumor-infiltrating CEA-specific CD8+ T cells and IFN-γ [81].
Although some clinical trials have yielded promising results, others have shown no clear survival benefit from particular combination treatments. 3.7 MBq 177Lu-DOTA-diZD combined with anti-PD1 mAb treatment did not improve the survival of mice with TNBC [95].
See Table 3-5 for a summary of pre-clinical studies, clinical study, and retrospective study of a combination strategy.
Pre-clinical studies of combination strategy
| Radiopharmaceutical | Dose | Combination strategy | Indication | Therapeutic outcome | Immune response | Ref |
|---|---|---|---|---|---|---|
| ↑ | ||||||
| 225Ac-PSMA-617 | 30 kBq | Anti-PD-1 mAb | Prostate cancer | Reduces tumor burdenImproves TTP and survival | - | [115] |
| [211At]MM4 | 36 MBq/kg | Anti-PD-1 mAb | Glioma | Decreasing tumor burden | - | [77] |
| 213Bi-anti-hPD-L1 mAb | 125 kBq/g | - | Melanoma | Increased tumor growth delay | - | [120] |
| 177Lu-DOTA-Y003 | 11.1 MBq | - | Colon cancer | Improve anti-tumor efficacyProlong overall survival | - | [121] |
| 131I-αPD-L1 | 11.1 MBq | Anti-PD-L1 mAb | Colon cancer | Improve anti-tumor efficacyProlong overall survival | - | [122] |
| 177Lu-DOTA-folate | 5 MBq | Anti-CTLA-4 mAb | Breast tumor | Improve anti-tumor efficacyProlong overall survival | - | [116] |
| 177Lu-DOTA-PEG4-LLP2A | 30 MBq | Anti-PD-L1 mAb, anti-PD-1 mAb, anti-CTLA-4 mAb | Melanoma | Improved overall survival | - | [119] |
| 211At-ATE-MnO2-BSA | 15 μCi | Anti-PD-L1 mAb | Colon cancer | Improve anti-tumor efficacyProlong overall survival | CD8+ T cells infiltration, effector memory CD4+ T and CD8+ T cells, TNF-α, IFN-γ ↑ | [78] |
| 2-[18F]FDG | 37 MBq | Anti-PD-L1 mAb | Colon cancer | Improve anti-tumor efficacyProlong overall survival | Effector memory T cells, CD4+ Th1, CD8+ TCLs, M1-like macrophages infiltration ↑ Tregs, M2-like macrophages infiltration↓ TNFa, IFNγ, and IL6 ↑ | [97] |
| 64Cu-DOTA-EB-cRGDfK | 18.5 MBq | Anti-PD-L1 mAb | Colon cancer | Improve anti-tumor efficacyProlong overall survival | CD8+ CTLs and CD4+ Th1 cells infiltration, CD8+ CTL/Treg, CD4+ Teff/Treg, TEM cells ↑ | [99] |
| 177Lu-EB-RGD | 18.5 MBq | Anti-PD-L1 mAb | Colon cancer | Improve anti-tumor efficacyProlong overall survival | CD8+ T cells infiltration ↑ | [94] |
| 90Y-NM600 | 7 MBq | Anti-CTLA-4 mAb | Melanoma | Improved overall survival | CD45+ immune cells, CD3+ T cells, CD8+ effector T cells, CD8+CD103+ tissue resident effector memory T cells, and γδ T cells ↑ PD-1 ↓IL-10 ↓Clonal expansion of T cells ↑IFNγ ↑ | [84] |
| 131I-Cat/ALG hybrid solution | 50 μCi | Immune-adjuvant CpG, anti-CTLA-4 mAb | Breast cancer | Long-term immune memory protectionAbscopal effectInhibited tumor metastases | CD8+ CTL infiltration in the distant tumors ↑ Treg in the distant tumors ↓The CTL/Treg ratio ↑TNF-α and IFN-γ ↑Both CD4+ and CD8+ T cells are important | [89] |
| 90Y-labeled anti-CEA mAb | 6 MBq | CEA/TRICOM vaccine | Colon cancer | Improved overall survival | Tumor-infiltrating CEA-specific CD8+ T cells ↑IFN-γ ↑ | [81] |
| N.S. | ||||||
| 177Lu-DOTA-diZD | 3.7 MBq | Anti-PD1 mAb | TNBC | Not improve the survival | - | [95] |
TTP: Time to progression; MDSCs: Myeloid-derived suppressor cell; CTL: cytotoxic T lymphocyte; CEA: Carcinoma embryonic antigen.
Clinical studies of combination strategy
| Radiopharmaceutical | Combine drug | Clinical trail | Indication | Phase | Estimated/ completion date | Participants |
|---|---|---|---|---|---|---|
| Complete | ||||||
| 90Y glass microspheres | Ipilimumab | NCT01730157 | Uveal melanoma with liver metastases | Phase 1 | Feb-16 | 6 |
| 153Sm-EDTMP | PSA-TRICOM | NCT00450619 | mCRPC | Phase 2 | Jan-17 | 44 |
| 223Ra, Xofigo | Sipuleucel-T | NCT02463799 | Asymptomatic or minimally symptomatic bone-mCRPC | Phase 2 | Dec-19 | 32 |
| 223Ra dichloride | Atezolizumab | NCT02814669 | mCRPC | Phase 1 | Jul-19 | 45 |
| 177Lu-DOTATATE | Nivolumab | NCT03325816 | Extensive-stage small cell lung cancer | Phase I /2 | Aug-20 | 9 |
| Ongoing | ||||||
| 177Lu-PSMA-617 | Pembrolizumab | NCT03805594 | mCRPC | phase Ib | Apr-24 | 16 |
| 177Lu-PSMA | Pembrolizumab | NCT03658447 | Prostate cancer | Phase I/2 | Dec-22 | 37 |
| 177Lu-DOTATATE | Avelumab | NCT04261855 | Merkel cell carcinoma | Phase I/2 | Jan-24 | 65 |
| 223Ra, Xofigo | Pembrolizumab | NCT03996473 | NSCLC with bone metastases | Phase I/2 | May-23 | 164 |
| 223Ra | Pembrolizumab | NCT03093428 | mCRPC | Phase 2 | Jun-24 | 45 |
| 223Ra dichloride | Avelumab | NCT04071236 | Advanced prostate cancer | Phase I/2 | Jan-23 | 24 |
| 223Ra | Nivolumab | NCT04109729 | mCRPC | Phase I/2 | Jun-24 | 36 |
| 225Ac-J591 | Pembrolizumab | NCT04946370 | mCRPC | Phase I/2 | Jun-28 | 76 |
| 90Y radioembolization, 177Lu-DOTATATE | Pembrolizumab | NCT03457948 | Neuroendocrine tumors and liver metastases | Phase 2 | Mar-24 | 32 |
| 90Y radioembolization | Nivolumab | NCT03033446 | Hepatocellular carcinoma | Phase I/2 | Dec-22 | 40 |
| 90Y glass microspheres | Nivolumab | NCT02837029 | Advanced liver cancer | Phase 1 | Jul-23 | 35 |
| 90Y radioembolization | Durvalumab | NCT04108481 | Metastatic colorectal cancer | Phase I/2 | Dec-25 | 18 |
| 90Y radioembolization | Pembrolizumab | NCT03099564 | Hepatocellular carcinoma | Phase 1 | Jan-23 | 30 |
| 90Y radioembolization | Ipilimumab, Nivolumab | NCT02913417 | Uveal melanoma with liver metastases | Phase I/2 | Jun-23 | 26 |
| 131I | Durvalumab | NCT03215095 | Recurrent/metastatic thyroid cancers | Phase 1 | Jul-23 | 11 |
mCRPC: Metastatic castrate-resistant prostate cancer; NSCLC: Non-small cell lung cancer.
Clinical trial and retrospective study of combination strategy
| Radiopharmaceutical | Combine drugs | Indication | Phase/ patients | Therapeutic outcomes | Ref |
|---|---|---|---|---|---|
| Clinical Trial | |||||
| 177Lu-PSMA-617 | Pembrolizumab | mCRPC | phase Ib | Durable responses in a subset of mCRPC without high mutational burden or microsatellite instability, suggesed a possible immunogenic priming effect of radioligand therapy. | [133] |
| 223Ra dichloride | Atezolizumab | mCRPC | Phase 1 | This Phase 1b study did not seem to show clinical benefit from combination. | [134] |
| 177Lu-PSMA | Pembrolizumab | mCRPC | Phase I/2 | The combination of anti-PD-1 and RNT synergistically reduces tumour burden and improves the time to progression and overall survival. | [135] |
| 177Lu- DOTATATE | Nivolumab | Neuroendocrine tumors of the lung | Phase 1 | The combination was well tolerated with most TRAEs with initial signs of antitumor activity. | [136] |
| 153Sm-EDTMP | PSA-TRICOM | mCRPC | Phase 2 | The primary endpoint was the proportion of patients without radiographic disease progression at 4 months. There was no statistical difference in the primary endpoint. | [137] |
| 223Ra, Xofigo | sipuleucel-T | Bone- mCRPC | Phase 2 | Patients in the combination arm were more likely to have a >50% PSA decline, longer PFS and OS, but the paradoxically lower immune responses observed. | [138] |
| 90Y radioembolization | DurvalumabTremelimumab | MSS CRC | Phase 2 | Limited benefits of radiation on promoting antitumor immune response in MSS CRC. | [85] |
| Retrospective study | |||||
| 177Lu-PSMA | Pembrolizumab | mCRPC | 1 patient | The combination strategy might be well tolerated in single patients. | [139] |
| 90Y radioembolization | Nivolumab | Advanced HCC | 1 patient | The combination served to increase their response rate and depth of response in HCC. | [140] |
| 90Y radioembolization | NivolumabIpilimumab | HCC | 26 patients | The combination strategy appeared safe, with no incidence of early toxicity or mortality in HCC patients. | [141] |
| 90Y radioembolization | Pembrolizumab Ipilimumab Nivolumab | Unresectable hepatic metastases from UM | 11 patients | The combination strategy is safe and effective and may improve hPFS and OS in patients with hepatic metastases from UM. | [142] |
mCRPC: Metastatic castrate-resistant prostate cancer; TRAEs: Treatment-related adverse events; MSS: Microsatellite stable metastatic; CRC: Colorectal cancer; HCC: Hepatocellular carcinoma; PSA: Prostate-specific antigen; PFS: Progression-free survival; OS: Overall survival; UM: Uveal melanoma.
Challenges and perspectives
In summary, although still anecdotal, evidence is emerging to support the concept that local radiation therapy and immuno-therapy can successfully synergize and produce a therapeutically effective antitumor immune response, even in metastatic cancer. It is the very beginning of a novel field. More research is warranted to define the many mechanisms underlying the crosstalk with the immune system and to establish how best to harness ionizing radiation in this new role.
Whether as a direct inducer of immunogenic cell death or in its application as a simple adjuvant to more complex immunotherapy manipulations, radiation therapy is once again playing a central role in the management of cancer at any stage and the ever-lasting quest for cancer cure. However, due to the limited understanding of the phenomenon and mechanism of RPT-induced anti-tumor immunity, and the existing research has continued the same direction as traditional radiotherapy, the similarities and uniqueness of RPT compared with traditional radiotherapy in immunity are still unknown, and need further exploration.
Aside from this, for the combination of RPT and IT, improved preclinical model systems for RPT-dose definition are needed, as well as a detailed and easy-to-perform immune monitoring of patients. The aims of innovative RPT in multimodal radioimmunotherapy concepts are diverse: assure local tumor control, stop the proliferation of tumor cells, induce tumor shrinkage, induce tumor cell death, alter the tumor cell phenotype, induce ICD together with an immune stimulatory microenvironment, foster infiltration of immune cells into the tumor with consecutive activation of the latter, converse immune suppressive conditions in activation ones, generate increased tumor antigen pool and neoantigens, induce specific and long-lasting anti-tumor immune responses against the primary tumor and metastases. But these are not mere wishes; every single aim of traditional radiotherapy is already achievable under distinct conditions. The big challenge will be to understand these conditions induced by RPT and to exploit them for a very personalized combination strategy in the future.
Even though extensive research is carried out in the field of radiation oncology, most clinical studies only consider the effects of radiation on the local tumor tissue. The influence of dose, fractionation and timing particularly about immune activation is not been satisfactorily investigated so far. However, this is of particular interest, since recent studies, including additive immune therapy approaches, showed that not every therapy combination of classical RPT concepts and IT is equally successful, the heterogeneous influences on the immune system of today's RPT schemes need more attention.
Though some evidence on PRT-induced immune activation, particularly in preclinical mouse models, have been reporte, differences in the doses and duration may lead to discordant immune activation between preclinical and clinical exploration. In the case of 177Lu-DOTATATE, treatment of mice with 30-40 MBq (1500-2000 MBq/kg for 20 g mice, single dose) caused increased infiltration of CD86+ APC and FasL-expressing NK cells [93], yet the clinical use of 7.4 GBq (92.5 MBq/kg for 80 kg patients, 4 doses every 8 weeks) may cause neutropenia, thrombocytopenia, and lymphocytopenia (1%, 2%, and 9% of patients in the 177Lu-DOTATATE group, respectively) [123]. Although these hematological events were transient, the hematological toxicity caused by RPT remains unknown whether it inhibits the activation process of immunity.
Beyond this, the long-term adverse events to immune cells are often unpredictable. In clinical practice, bone marrow is the critical target, with the reduced bone marrow reserve, and more infrequently, myelodysplastic syndrome (MDS) which was observed in approximately 2% of patients treated with 177Lu-DOTATATE [124]. Even though the overall incidence of PRRT-induced myelosuppression is acceptable, it suggests to us that a complex relationship between RPT procedure and immune activation exists that requires more experimental evidence.
Reducing or avoiding damage to the immune cells and bone marrow from RPT may allow for a more rapid response of immunity. Therefore, it is of great interest to explore the interaction between short-term hematologic toxicity and immunity, the mechanisms of long-term toxicity, and methods to protect immune system. Up to now, chemical perturbing tools based on radiotherapy and PET probes have been developed to regulate biological effects [125-130], which may potentiate RPT by balancing toxicity and efficacy via RPT-mediated controlled drug release in vivo.
To this end, combined, multi-modal treatment regimens have to be developed with the capacity to induce immunogenic forms of tumor cell death and concomitantly activate the immune system. The close collaboration of clinical radiation oncologists, surgeons, radiobiologists, molecular oncologists, and immunologists is indispensable to develop and optimize the personalized therapeutic regime with the highest benefit for each individual patient.
Abbreviations
RPT: radiopharmaceutical therapy; ICD: immunogenic cell death; TME: tumor microenvironment; DAMPs: danger-associated molecular patterns; IT: immunotherapy; DDR: DNA damage response; TAAs: tumor-associated antigens; APCs: antigen-presenting cells; cGAS-STING: cyclic GMP-AMP synthase-stimulator of interferon genes; HMGB1: high mobility group box 1; DCs: dendritic cells; TLRs: Toll-like receptors; NKs: natural killer cells; CTLs: cytotoxic T lymphocytes; TNF-α: tumor necrosis factor-α; Hsp70: heat shock protein 70; mCRPC: metastatic castration-resistant prostate cancer; PBMCs: peripheral blood mononuclear cells; TEM: effector memory T cells; ICOS: expressed costimulatory; TIL: tumor‐infiltrating lymphocyte; DTC: differentiated thyroid cancer; GEP-NETs: gastroenteropancreatic neuroendocrine tumors; PRRT: peptide receptor radionuclide therapy; PET: positron emission tomography; ICI: immune checkpoint inhibitors; PD-1: programmed death 1; CTLA-4: cytotoxic T-lymphocyte-associated antigen 4; Treg: regulatory T cells; MDS: myelodysplastic syndrome; MHC-1: Major histocompability complex-1; MUC-1: mucin-1; CEA: Carcinoma embryonic antigen; MDSC: Myeloid-derived suppressor cell.
Acknowledgements
This study was funded by the Beijing Municipal Natural Science Foundation (Grant No. Z200018), the National Nature Science Foundation of China (Grant No. U1867209), the Ministry of Science and Technology of the People's Republic of China (Grant Nos. 2021YFA1601400 and 2017YFA0506300) and Changping Laboratory under the project number (2022C-07-01), the Special Foundation of Beijing Municipal Education Commission (Grant No. 3500-12020123), the Central Guidance for Local Science and Technology Development Projects (No. 202138-03), Li Ge-Zhao Ning Life Science Youth Research Foundation (LGZNQN202004) to Z.L. We thank the facility support from the Analytical Instrumentation Center of Peking University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020;19:589-608
2. Dolgin E. Drugmakers go nuclear, continuing push into radiopharmaceuticals. Nat Biotechnol. 2021;39:647-9
3. Wadsak W, Mitterhauser M. Basics and principles of radiopharmaceuticals for PET/CT. Eur J Radiol. 2010;73:461-9
4. Li J, Shi Y, Zhang Z, Liu H, Lang L, Liu T. et al. A Metabolically Stable Boron-Derived Tyrosine Serves as a Theranostic Agent for Positron Emission Tomography Guided Boron Neutron Capture Therapy. Bioconjugate Chemistry. 2019;30:2870-8
5. Chen J, Li C, Hong H, Liu H, Wang C, Xu M. et al. Side Chain Optimization Remarkably Enhances the in Vivo Stability of 18F-Labeled Glutamine for Tumor Imaging. Molecular Pharmaceutics. 2019;16:5035-41
6. Li Z, Kong Z, Chen J, Li J, Li N, Yang Z. et al. 18F-Boramino acid PET/CT in healthy volunteers and glioma patients. European Journal of Nuclear Medicine and Molecular Imaging. 2021;48:3113-21
7. Chen J, Wang J, Xu M, Jia X, Song G, Liu Z. Production of positron-emitting radionuclide yttrium-86 with a computer-aided design target for positron emission tomography. Nucl Med Biol. 2022;108-109:54-60
8. Chen J, Xu M, Liu Y, Duan D, Han Y, Liu Z. Isolation of 212Pb from natural thorium for targeted alpha-therapy. Chinese Chemical Letters. 2022;33:3474 -
9. Xu M, Zhang P, Ding J, Chen J, Huo L, Liu Z. Albumin Binder-Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J Nucl Med. 2022;63:952-8
10. Herrmann K, Schwaiger M, Lewis JS, Solomon SB, McNeil BJ, Baumann M. et al. Radiotheranostics: a roadmap for future development. Lancet Oncol. 2020;21:e146-e56
11. Zhang P, Xu M, Ding J, Chen J, Zhang T, Huo L. et al. Fatty acid-conjugated radiopharmaceuticals for fibroblast activation protein-targeted radiotherapy. Eur J Nucl Med Mol Imaging. 2022;49:1985-96
12. Cui X-Y, Liu Y, Wang C, Wen Z, Li Y, Tang H. et al. China's radiopharmaceuticals on expressway: 2014-2021. Radiochimica Acta. 2022;110:765-84
13. Li J, Sun Q, Lu C, Xiao H, Guo Z, Duan D. et al. Boron encapsulated in a liposome can be used for combinational neutron capture therapy. Nature Communications. 2022;13:2143
14. Hatcher-Lamarre JL, Sanders VA, Rahman M, Cutler CS, Francesconi LC. Alpha emitting nuclides for targeted therapy. Nuclear Medicine and Biology. 2021;92:228-40
15. Gaipl US, Multhoff G, Scheithauer H, Lauber K, Hehlgans S, Frey B. et al. Kill and spread the word: stimulation of antitumor immune responses in the context of radiotherapy. Immunotherapy. 2014;6:597-610
16. Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S. et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res. 2015;21:3727-39
17. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1-5
18. Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H. et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70:2697-706
19. Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31:363-72
20. Sia J, Szmyd R, Hau E, Gee HE. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front Cell Dev Biol. 2020;8:41
21. Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ. et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903-7
22. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16-25
23. Campbell AC, Hersey P, MacLennan IC, Kay HE, Pike MC. Immunosuppressive consequences of radiotherapy and chemotherapy in patients with acute lymphoblastic leukaemia. Br Med J. 1973;2:385-8
24. Ozpiskin OM, Zhang L, Li JJ. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics. 2019;9:1215-31
25. Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25:11-7
26. Brix N, Tiefenthaller A, Anders H, Belka C, Lauber K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol Rev. 2017;280:249-79
27. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862-70
28. Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293-5
29. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925-31
30. Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA. et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5:404-7
31. Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5:60
32. Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nature Reviews Immunology. 2002;2:85-95
33. Gulley JL, Madan RA, Pachynski R, Mulders P, Sheikh NA, Trager J. et al. Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment. J Natl Cancer Inst. 2017 109
34. Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390-4
35. Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A. et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843-52
36. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516-23
37. Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P. et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11:1013
38. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331-42
39. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-5
40. Multhoff G, Pockley AG, Schmid TE, Schilling D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett. 2015;368:179-84
41. Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G. et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855-8
42. Trabanelli S, Ocadlíková D, Gulinelli S, Curti A, Salvestrini V, Vieira RP. et al. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J Immunol. 2012;189:1303-10
43. Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403-16
44. Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100-4
45. Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y. et al. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci U S A. 2004;101:2434-9
46. Di Maggio FM, Minafra L, Forte GI, Cammarata FP, Lio D, Messa C. et al. Portrait of inflammatory response to ionizing radiation treatment. J Inflamm (Lond). 2015;12:14
47. Chen J, Liu X, Zeng Z, Li J, Luo Y, Sun W. et al. Immunomodulation of NK Cells by Ionizing Radiation. Front Oncol. 2020;10:874
48. Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol. 2012;2:95
49. Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H. et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558-66
50. Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132-9
51. Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-γ mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. 2013;182:2345-54
52. Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS. et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81:1128-35
53. McFarland HI, Puig M, Grajkowska LT, Tsuji K, Lee JP, Mason KP. et al. Regulatory T cells in γ irradiation-induced immune suppression. PLoS One. 2012;7:e39092
54. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365-79
55. Cornelissen B, Darbar S, Kersemans V, Allen D, Falzone N, Barbeau J. et al. Amplification of DNA damage by a γH2AX-targeted radiopharmaceutical. Nucl Med Biol. 2012;39:1142-51
56. Pereira E, do Quental L, Palma E, Oliveira MC, Mendes F, Raposinho P. et al. Evaluation of Acridine Orange Derivatives as DNA-Targeted Radiopharmaceuticals for Auger Therapy: Influence of the Radionuclide and Distance to DNA. Sci Rep. 2017;7:42544
57. O'Neill E, Kersemans V, Allen PD, Terry SYA, Torres JB, Mosley M. et al. Imaging DNA Damage Repair In Vivo After (177)Lu-DOTATATE Therapy. J Nucl Med. 2020;61:743-50
58. Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR. et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311-28
59. Parker C, Lewington V, Shore N, Kratochwil C, Levy M, Lindén O. et al. Targeted Alpha Therapy, an Emerging Class of Cancer Agents: A Review. JAMA Oncol. 2018;4:1765-72
60. Bannik K, Madas B, Jarzombek M, Sutter A, Siemeister G, Mumberg D. et al. Radiobiological effects of the alpha emitter Ra-223 on tumor cells. Sci Rep. 2019;9:18489
61. Baidoo KE, Yong K, Brechbiel MW. Molecular pathways: targeted α-particle radiation therapy. Clin Cancer Res. 2013;19:530-7
62. Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. Impact of α-targeted radiation therapy on gene expression in a pre-clinical model for disseminated peritoneal disease when combined with paclitaxel. PLoS One. 2014;9:e108511
63. Asaithamby A, Hu B, Chen DJ. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proc Natl Acad Sci U S A. 2011;108:8293-8
64. Kassis AI. Therapeutic radionuclides: biophysical and radiobiologic principles. Semin Nucl Med. 2008;38:358-66
65. Runge R, Oehme L, Kotzerke J, Freudenberg R. The effect of dimethyl sulfoxide on the induction of DNA strand breaks in plasmid DNA and colony formation of PC Cl3 mammalian cells by alpha-, beta-, and Auger electron emitters (223)Ra, (188)Re, and (99m)Tc. EJNMMI Res. 2016;6:48
66. Gorin JB, Ménager J, Gouard S, Maurel C, Guilloux Y, Faivre-Chauvet A. et al. Antitumor immunity induced after α irradiation. Neoplasia. 2014;16:319-28
67. Joung JY, Ha YS, Kim IY. Radium Ra 223 dichloride in castration-resistant prostate cancer. Drugs Today (Barc). 2013;49:483-90
68. Kluetz PG, Pierce W, Maher VE, Zhang H, Tang S, Song P. et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2014;20:9-14
69. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213-23
70. Castello A, Macapinlac HA, Lopci E, Santos EB. Prostate-specific antigen flare induced by (223)RaCl(2) in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:2256-63
71. Henriksen G, Breistøl K, Bruland Ø S, Fodstad Ø, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002;62:3120-5
72. Malamas AS, Gameiro SR, Knudson KM, Hodge JW. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas' sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget. 2016;7:86937-47
73. Kim JW, Shin MS, Kang Y, Kang I, Petrylak DP. Immune Analysis of Radium-223 in Patients With Metastatic Prostate Cancer. Clin Genitourin Cancer. 2018;16:e469-e76
74. Creemers JHA, van der Doelen MJ, van Wilpe S, Hermsen R, Duiveman-de Boer T, Somford DM. et al. Immunophenotyping Reveals Longitudinal Changes in Circulating Immune Cells During Radium-223 Therapy in Patients With Metastatic Castration-Resistant Prostate Cancer. Front Oncol. 2021;11:667658
75. Kwee SA, Lim J, Coel MN. Soft Tissue Response on 18F-Fluorocholine PET/CT in Metastatic Castrate-Resistant Prostate Cancer Treated With 223Ra-Dichloride: A Possible Abscopal Effect? Clin Nucl Med. 2017;42:868-71
76. Hagemann UB, Ellingsen C, Schuhmacher J, Kristian A, Mobergslien A, Cruciani V. et al. Mesothelin-Targeted Thorium-227 Conjugate (MSLN-TTC): Preclinical Evaluation of a New Targeted Alpha Therapy for Mesothelin-Positive Cancers. Clin Cancer Res. 2019;25:4723-34
77. Dabagian H, Taghvaee T, Martorano P, Martinez D, Samanta M, Watkins CM. et al. PARP Targeted Alpha-Particle Therapy Enhances Response to PD-1 Immune-Checkpoint Blockade in a Syngeneic Mouse Model of Glioblastoma. ACS Pharmacol Transl Sci. 2021;4:344-51
78. Zhang J, Li F, Yin Y, Liu N, Zhu M, Zhang H. et al. Alpha radionuclide-chelated radioimmunotherapy promoters enable local radiotherapy/chemodynamic therapy to discourage cancer progression. Biomaterials Research. 2022;26:44
79. Dekempeneer Y, Keyaerts M, Krasniqi A, Puttemans J, Muyldermans S, Lahoutte T. et al. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin Biol Ther. 2016;16:1035-47
80. King AP, Lin FI, Escorcia FE. Why bother with alpha particles? Eur J Nucl Med Mol Imaging. 2021
81. Chakraborty M, Gelbard A, Carrasquillo JA, Yu S, Mamede M, Paik CH. et al. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol Immunother. 2008;57:1173-83
82. Hernandez R, Walker KL, Grudzinski JJ, Aluicio-Sarduy E, Patel R, Zahm CD. et al. (90)Y-NM600 targeted radionuclide therapy induces immunologic memory in syngeneic models of T-cell Non-Hodgkin's Lymphoma. Commun Biol. 2019;2:79
83. Jagodinsky JC, Jin WJ, Bates AM, Hernandez R, Grudzinski JJ, Marsh IR. et al. Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy. Theranostics. 2021;11:6120-37
84. Patel RB, Hernandez R, Carlson P, Grudzinski J, Bates AM, Jagodinsky JC. et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci Transl Med. 2021 13
85. Wang C, Park J, Ouyang C, Longmate JA, Tajon M, Chao J. et al. A Pilot Feasibility Study of Yttrium-90 Liver Radioembolization Followed by Durvalumab and Tremelimumab in Patients with Microsatellite Stable Colorectal Cancer Liver Metastases. Oncologist. 2020;25:382-e776
86. Higashi T, Kudo T, Kinuya S. Radioactive iodine (131I) therapy for differentiated thyroid cancer in Japan: current issues with historical review and future perspective. Ann Nucl Med. 2012;26:99-112
87. Simonovic SZ, Mihaljevic O, Majstorovic I, Djurdjevic P, Kostic I, Djordjevic OM. et al. Cytokine production in peripheral blood cells of patients with differentiated thyroid cancer: elevated Th2/Th9 cytokine production before and reduced Th2 cytokine production after radioactive iodine therapy. Cancer Immunol Immunother. 2015;64:75-82
88. Zhang L, Chen J, Xu C, Qi L, Ren Y. Effects of iodine-131 radiotherapy on Th17/Tc17 and Treg/Th17 cells of patients with differentiated thyroid carcinoma. Exp Ther Med. 2018;15:2661-6
89. Chao Y, Xu L, Liang C, Feng L, Xu J, Dong Z. et al. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat Biomed Eng. 2018;2:611-21
90. Maini CL, Bergomi S, Romano L, Sciuto R. 153Sm-EDTMP for bone pain palliation in skeletal metastases. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S171-8
91. Chakraborty M, Wansley EK, Carrasquillo JA, Yu S, Paik CH, Camphausen K. et al. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res. 2008;14:4241-9
92. Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP. et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124-30
93. Wu Y, Pfeifer AK, Myschetzky R, Garbyal RS, Rasmussen P, Knigge U. et al. Induction of Anti-Tumor Immune Responses by Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE in a Murine Model of a Human Neuroendocrine Tumor. Diagnostics (Basel). 2013;3:344-55
94. Chen H, Zhao L, Fu K, Lin Q, Wen X, Jacobson O. et al. Integrin α(v)β(3)-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics. 2019;9:7948-60
95. Huang Y, Yang Z, Li F, Zhao H, Li C, Yu N. et al. (64)Cu/(177)Lu-DOTA-diZD, a Small-Molecule-Based Theranostic Pair for Triple-Negative Breast Cancer. J Med Chem. 2021;64:2705-13
96. Jandl T, Revskaya E, Jiang Z, Bryan RA, Casadevall A, Dadachova E. Complement-dependent cytotoxicity of an antibody to melanin in radioimmunotherapy of metastatic melanoma. Immunotherapy. 2013;5:357-64
97. Wen X, Shi C, Zeng X, Zhao L, Yao L, Liu Z. et al. A Paradigm of Cancer Immunotherapy Based on 2-[18F]FDG and Anti-PD-L1 mAb Combination to Enhance the Antitumor Effect. Clin Cancer Res. 2022;28:2923-37
98. Gutfilen B, Souza SA, Valentini G. Copper-64: a real theranostic agent. Drug Des Devel Ther. 2018;12:3235-45
99. Wen X, Zeng X, Liu J, Zhang Y, Shi C, Wu X. et al. Synergism of (64)Cu-Labeled RGD with Anti-PD-L1 Immunotherapy for the Long-Acting Antitumor Effect. Bioconjug Chem. 2022;33:2170-9
100. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373-7
101. Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15:477-94
102. Yan Y, Kumar AB, Finnes H, Markovic SN, Park S, Dronca RS. et al. Combining Immune Checkpoint Inhibitors With Conventional Cancer Therapy. Front Immunol. 2018;9:1739
103. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239-45
104. Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852-63
105. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-64
106. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-39
107. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-33
108. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F. et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-92
109. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N. et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-301
110. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-29
111. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT. et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1103-15
112. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23
113. Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-26
114. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306
115. Czernin J, Current K, Mona CE, Nyiranshuti L, Hikmat F, Radu CG. et al. Immune-Checkpoint Blockade Enhances (225)Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer. J Nucl Med. 2021;62:228-31
116. Guzik P, Siwowska K, Fang HY, Cohrs S, Bernhardt P, Schibli R. et al. Promising potential of [(177)Lu]Lu-DOTA-folate to enhance tumor response to immunotherapy-a preclinical study using a syngeneic breast cancer model. Eur J Nucl Med Mol Imaging. 2021;48:984-94
117. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM. et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-33
118. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD. et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535-46
119. Choi J, Beaino W, Fecek RJ, Fabian KPL, Laymon CM, Kurland BF. et al. Combined VLA-4-Targeted Radionuclide Therapy and Immunotherapy in a Mouse Model of Melanoma. J Nucl Med. 2018;59:1843-9
120. Capitao M, Perrin J, Simon S, Gouard S, Chouin N, Bruchertseifer F. et al. Anti-Tumor Efficacy of PD-L1 Targeted Alpha-Particle Therapy in a Human Melanoma Xenograft Model. Cancers (Basel). 2021 13
121. Ren J, Xu M, Chen J, Ding J, Wang P, Huo L. et al. PET imaging facilitates antibody screening for synergistic radioimmunotherapy with a (177)Lu-labeled αPD-L1 antibody. Theranostics. 2021;11:304-15
122. Wen X, Zeng X, Cheng X, Zeng X, Liu J, Zhang Y. et al. PD-L1-Targeted Radionuclide Therapy Combined with αPD-L1 Antibody Immunotherapy Synergistically Improves the Antitumor Effect. Mol Pharm. 2022;19:3612-22
123. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-35
124. Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M. et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5-19
125. Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421-6
126. Fu Q, Li H, Duan D, Wang C, Shen S, Ma H. et al. External-Radiation-Induced Local Hydroxylation Enables Remote Release of Functional Molecules in Tumors. Angewandte Chemie International Edition. 2020;59:21546-52
127. Ding Z, Guo Z, Zheng Y, Wang Z, Fu Q, Liu Z. Radiotherapy Reduces N-Oxides for Prodrug Activation in Tumors. Journal of the American Chemical Society. 2022;144:9458-64
128. Guo Z, Hong H, Zheng Y, Wang Z, Ding Z, Fu Q. et al. Radiotherapy-Induced Cleavage of Quaternary Ammonium Groups Activates Prodrugs in Tumors. Angewandte Chemie International Edition. 2022 61
129. Wang C, Hong H, Chen M, Ding Z, Rui Y, Qi J. et al. A Cationic Micelle as In Vivo Catalyst for Tumor-Localized Cleavage Chemistry. Angewandte Chemie International Edition. 2021;60:19750-8
130. Duan D, Dong H, Tu Z, Wang C, Fu Q, Chen J. et al. Desilylation Induced by Metal Fluoride Nanocrystals Enables Cleavage Chemistry In Vivo. Journal of the American Chemical Society. 2021;143:2250-5
131. Creemers JHA, van der Doelen MJ, van Wilpe S, Hermsen R, Duiveman-de Boer T, Somford DM. et al. Immunophenotyping Reveals Longitudinal Changes in Circulating Immune Cells During Radium-223 Therapy in Patients With Metastatic Castration-Resistant Prostate Cancer. Frontiers in Oncology. 2021 11
132. Vito A, Rathmann S, Mercanti N, El-Sayes N, Mossman K, Valliant J. Combined Radionuclide Therapy and Immunotherapy for Treatment of Triple Negative Breast Cancer. International Journal of Molecular Sciences. 2021;22:4843
133. Aggarwal RR, Sam SL, Koshkin VS, Small EJ, Feng FY, Kouchkovsky Id. et al. Immunogenic priming with 177Lu-PSMA-617 plus pembrolizumab in metastatic castration resistant prostate cancer (mCRPC): A phase 1b study. Journal of Clinical Oncology. 2021;39:5053 -
134. Fong L, Morris MJ, Sartor O, Higano CS, Pagliaro L, Alva A. et al. A Phase Ib Study of Atezolizumab with Radium-223 Dichloride in Men with Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2021;27:4746-56
135. Sandhu S, Joshua A, Emmett L, Spain L, Horvath L, Crumbaker M. et al. 577O PRINCE: Interim analysis of the phase Ib study of 177Lu-PSMA-617 in combination with pembrolizumab for metastatic castration resistant prostate cancer (mCRPC). Annals of Oncology. 2021;32:S626-S7
136. Kim C, Liu SV, Subramaniam DS, Torres T, Loda M, Esposito G. et al. Phase I study of the (177)Lu-DOTA(0)-Tyr(3)-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J Immunother Cancer. 2020 8
137. Heery CR, Madan RA, Bilusic M, Kim JW, Singh NK, Rauckhorst M. et al. A phase II randomized clinical trial of samarium-153 EDTMP (Sm-153) with or without PSA-TRICOM vaccine in metastatic castration-resistant prostate cancer (mCRPC) after docetaxel. Journal of Clinical Oncology. 2013;31:102 -
138. Marshall CH, Fu W, Wang H, Park JC, DeWeese TL, Tran PT. et al. Randomized Phase II Trial of Sipuleucel-T with or without Radium-223 in Men with Bone-metastatic Castration-resistant Prostate Cancer. Clin Cancer Res. 2021;27:1623-30
139. Prasad V, Zengerling F, Steinacker JP, Bolenz C, Beer M, Wiegel T. et al. First Experiences with (177)Lu-PSMA Therapy in Combination with Pembrolizumab or After Pretreatment with Olaparib in Single Patients. J Nucl Med. 2021;62:975-8
140. Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 Radioembolization Combined with a PD-1 Inhibitor for Advanced Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2018;41:1799-802
141. Zhan C, Ruohoniemi D, Shanbhogue KP, Wei J, Welling TH, Gu P. et al. Safety of Combined Yttrium-90 Radioembolization and Immune Checkpoint Inhibitor Immunotherapy for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2020;31:25-34
142. Zheng J, Irani Z, Lawrence D, Flaherty K, Arellano RS. Combined Effects of Yttrium-90 Transarterial Radioembolization around Immunotherapy for Hepatic Metastases from Uveal Melanoma: A Preliminary Retrospective Case Series. J Vasc Interv Radiol. 2018;29:1369-75
Author contact
![]() Corresponding author: Zhibo Liu: zbliuedu.cn.
Corresponding author: Zhibo Liu: zbliuedu.cn.
 Global reach, higher impact
Global reach, higher impact