13.3
Impact Factor
Theranostics 2023; 13(2):685-703. doi:10.7150/thno.73568 This issue Cite
Research Paper
Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine
1. Translational Medical Center for Stem Cell Therapy & Institutes for Regenerative Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200123, China.
2. Department of Thoracic Cardiovascular Surgery, The Eighth Affiliated Hospital of Sun Yat-sen University, Shenzhen, Guangdong 518033, China.
3. Department of Cardiovascular and Thoracic Surgery, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, China.
4. Department of Radiology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, China.
5. Guangdong Cardiovascular Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong 510100, China.
6. Research Institute of Heart Failure, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, China.
7. CAS Key Laboratory of Tissue Microenvironment and Tumor, Laboratory of Molecular Cardiology, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences (CAS), CAS, Shanghai 200031, China.
8. Shanghai Institute of Stem Cell Research and Clinical translation, Shanghai East Hospital, Tongji University, Shanghai 200120, China.
#Co-first authors.
Abstract
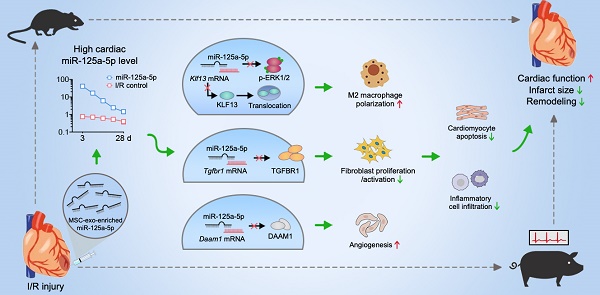
Rationale: Clinical application of mesenchymal stem cells (MSCs) and MSC-derived exosomes (MSC-Exos) to alleviate myocardial ischemia/reperfusion (I/R) injury is compromised by the low cell engraftment rate and uncontrolled exosomal content. As one of their active ingredients, single-component microRNA therapy may have more inherent advantages. We sought to find an ideal microRNA candidate and determine whether it could reproduce the cardioprotective effects of MSCs and MSC-Exos.
Methods: Cardiac function and myocardial remodeling in MSC, MSC-Exo, or microRNA oligonucleotide-treated mouse hearts were investigated after I/R injury. The effects of microRNA oligonucleotides on cardiac cells (macrophages, cardiomyocytes, fibroblasts, and endothelial cells) and their downstream mechanisms were confirmed. Large animals were also employed to investigate the safety of microRNA therapy.
Results: The results showed that microRNA-125a-5p (miR-125a-5p) is enriched in MSC-Exos, and intramyocardial delivery of their modified oligonucleotides (agomir) in mouse I/R myocardium, as well as MSCs or MSC-Exos, exerted obvious cardioprotection by increasing cardiac function and limiting adverse remodeling. In addition, miR-125a-5p agomir treatment increased M2 macrophage polarization, promoted angiogenesis, and attenuated fibroblast proliferation and activation, which subsequently contributed to the improvements in cardiomyocyte apoptosis and inflammation. Mechanistically, Klf13, Tgfbr1, and Daam1 are considered the targets of miR-125a-5p for regulating the function of macrophages, fibroblasts, and endothelial cells, respectively. Similar results were observed following miR-125a-5p agomir treatment in a porcine model, with no increase in the risk of arrhythmia or hepatic, renal, or cardiac toxicity.
Conclusions: This targeted microRNA delivery presents an effective and safe strategy as a stem cell and exosomal therapy in I/R cardiac repair.
Keywords: myocardial ischemia/reperfusion, macrophage polarization, fibrosis, angiogenesis, swine
 Global reach, higher impact
Global reach, higher impact