13.3
Impact Factor
Theranostics 2023; 13(2):659-672. doi:10.7150/thno.78773 This issue Cite
Research Paper
Application of the mineral-binding protein fetuin-A for the detection of calcified lesions
1. Helmholtz-Institute for Biomedical Engineering, RWTH Aachen University Hospital, Aachen, Germany.
2. IZKF - Interdisciplinary Center for Clinical Research, RWTH Aachen University Hospital, Aachen, Germany.
3. Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University Medical Centre, Maastricht, The Netherlands.
4. Department of Nuclear Medicine, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
5. Department of Radiology and Nuclear Medicine, Maastricht University Medical Centre, Maastricht, The Netherlands.
6. Department of Internal Medicine I, Cardiology, Medical Faculty, RWTH Aachen University Hospital, Aachen, Germany.
Abstract
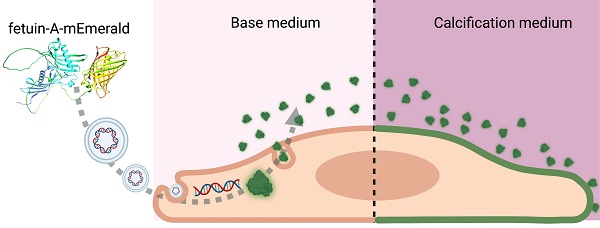
Rationale: Calcium plays an essential role in the biology of vertebrates. Calcium content in body fluids is maintained within a narrow physiologic range by feedback control. Phosphate is equally important for metabolism and is likewise controlled, albeit over a wider range. This results in a nearly supersaturated state of calcium phosphate in body liquids driving mineral precipitation in soft tissues, which is actively prevented by calcification inhibitors. The hepatic plasma protein fetuin-A is a circulating mineralization inhibitor regulating calcium phosphate crystal growth and calcified matrix metabolism. Ectopic mineralization is associated with many pathological conditions aggravating their outcome. Current diagnostic methods lack sensitivity towards microcalcifications representing the initial stages of the process. Given the irreversibility of established calcifications, novel diagnostic tools capable of detecting nascent calcium phosphate deposits are highly desirable.
Methods: We designed fluorescent fusion proteins consisting of fetuin-A coupled to a green or red fluorescent protein derivate, mEmerald or mRuby3, respectively. The proteins were expressed in mammalian cell lines. Sequence optimization resolved folding issues and increased sensitivity of mineral binding. Chimeric proteins were tested for their ability to detect calcifications in cell cultures and tissue sections retrieved from calcification-prone mice.
Results: We employed novel genetically labeled fetuin-A-based fluorescent proteins for the detection of ectopic calcifications. We show that fetuin-A-based imaging agents are non-toxic and suitable for live imaging of microcalcifications beyond the detection limit of conventional staining techniques. The ability of fetuin-A to preferentially bind nascent calcium phosphate crystals allowed the resolution of histopathological detail of early kidney damage that went previously undetected. Endogenous expression of fetuin-A fluorescent fusion proteins allowed extended live imaging of calcifying cells with unprecedented sensitivity and specificity.
Conclusion: Ectopic microcalcifications represent a major clinical concern lacking effective diagnostic and treatment options. In this paper, we describe novel highly sensitive fetuin-A-based fluorescent probes for imaging microcalcifications. We show that fusion proteins consisting of a fetuin-A mineral binding moiety and a fluorescent protein are superior to the routine methods for detecting calcifications. They also surpass in continuous live cell imaging the chemically fluorescence labeled fetuin-A, which we established previously.
Keywords: ectopic calcification, fetuin-A, calcification imaging, fluorescent proteins
 Global reach, higher impact
Global reach, higher impact