13.3
Impact Factor
Theranostics 2023; 13(2):560-577. doi:10.7150/thno.79227 This issue Cite
Research Paper
Hippo pathway activation mediates chemotherapy-induced anti-cancer effect and cardiomyopathy through causing mitochondrial damage and dysfunction
1. Department of Physiology and Pathophysiology, School of Basic Medical Sciences, Xi'an Jiaotong University Health Science Center, 76 West Yanta Road, Xi'an, 710061, Shaanxi, China.
2. Department of Pathology, Xi'an People's Hospital (Xi'an Fourth Hospital), Affiliated Guangren Hospital, Xi'an Jiaotong University Health Science Center, 21 Jiefang Road, Xi'an, 710005, Shaanxi, China.
3. Cardiovascular Research Centre, School of Basic Medical Sciences, Xi'an Jiaotong University Health Science Center, 76 West Yanta Road, Xi'an, 710061, Shaanxi, China.
4. Baker Heart and Diabetes Institute, 75 Commercial Road, Melbourne, Victoria 3004, Australia.
5. Rutgers New Jersey Medical School, Department of Cell Biology and Molecular Medicine, New Jersey, United States of America.
Abstract
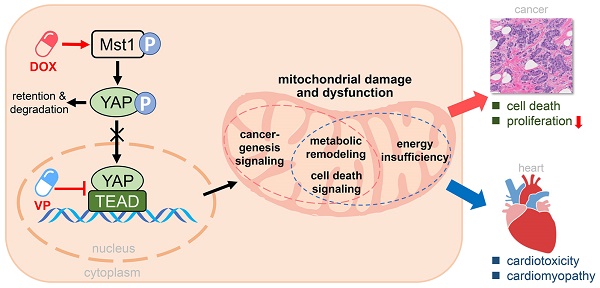
Rationale: Chemotherapy is a common clinical strategy for cancer treatment. However, the accompanied cardiomyopathy renders cancer patients under risk of another life-threatening condition. Whereas Hippo pathway is known to play key roles in both cancerogenesis and heart disease, it remains unclear whether Hippo pathway activation mediates chemotherapy-induced cardiomyopathy.
Methods and Results: In human breast cancer cells, doxorubicin (DOX) significantly induced upregulation of Hippo kinase Mst1, inhibitory phosphorylation of YAP, mitochondrial damage, reduced cell viability and increased apoptosis. Hippo pathway inactivation by Mst1-siRNA transfection effectively improved cell survival and mitigated mitochondrial damage and cell apoptosis. Another anti-cancer drug YAP inhibitor verteporfin also induced lower cancer cell viability, apoptosis and mitochondrial injury. Chronic treatment with DOX in vivo (4 mg/kg/week for 6 weeks) caused mitochondrial damage and dysfunction, oxidative stress and cardiac fibrosis, while acute DOX treatment (16 mg/kg single bolus) also induced myocardial oxidative stress and mitochondrial abnormalities. Chronic treatment with verteporfin (2 months) resulted in cardiomyopathy phenotypes comparable to that by chronic DOX regimen. In transgenic mice with cardiac overexpression of kinase-dead mutant Mst1 gene, these adverse cardiac effects of DOX were significantly attenuated relative to wild-type littermates.
Conclusions: Anti-cancer action of both DOX and verteporfin is associated with Hippo pathway activation. Such action on cardiac Hippo pathway mediates mitochondrial damage and cardiomyopathy.
Keywords: Hippo pathway, mitochondrial damage, anti-cancer chemotherapy, doxorubicin, verteporfin, cardiotoxicity, cardiomyopathy
 Global reach, higher impact
Global reach, higher impact