13.3
Impact Factor
Theranostics 2022; 12(18):7821-7852. doi:10.7150/thno.78572 This issue Cite
Review
Development of functional nanomedicines for tumor associated macrophages-focused cancer immunotherapy
1. School of Preclinical Medicine, Chengdu University, Chengdu 610106, P. R. China.
2. Section of Molecular Dermatology, Medical Faculty Mannheim of Heidelberg University, Mannheim, Germany.
3. Department of Thoracic and Cardiac Surgery, Affiliated Hospital of Chengdu University, Chengdu 610081, P. R. China.
4. Evidence-Based Medicine Center, West China Hospital, Sichuan University, Chengdu, 610041, P. R. China.
Abstract
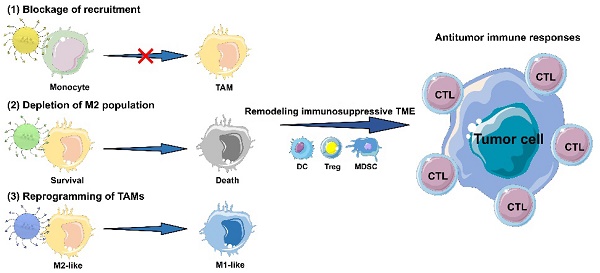
Clinical cancer immunotherapies are usually impeded by tumor immunosuppression driven by tumor associated macrophages (TAMs). Thus, TAMs can be considered as a promising therapeutic target for improved immunotherapy, and TAMs-focused molecular targeting agents have made ideal progress in clinical practice. Even so, most TAMs-targeting agents still cannot cover up their own shortcomings as free drugs. The emergence of multifunctional nanomaterials can expectedly endow these therapeutic cargoes with high solubility, favorable pharmacokinetic distribution, cell-specific delivery, and controlled release. Here, the underlying mechanisms of tumor immunosuppression caused by TAMs are first emphatically elucidated, and then the basic design of TAMs-focused immune-nanomedicines are discussed, mainly including diverse categories of nanomaterials, targeted and stimulus-responsive modifications, and TAM imaging in nanomedicines. A summary of current TAMs-targeting immunotherapeutic mechanisms based on functional nanomedicines for TAMs elimination and/or repolarization is further presented. Lastly, some severe challenges related to functional nanomedicines for TAMs-focused cancer immunotherapy are proposed, and some feasible perspectives on clinical translation of TAMs-associated anticancer immunonanomedicines are provided. It is hoped that, with rapid development of nanomedicine in cancer immunotherapy, TAMs-focused therapeutic strategies may be anticipated to become an emerging immunotherapeutic modality for future clinical cancer treatment.
Keywords: tumor associated macrophages, tumor immunosuppression, functional nanomedicines, cancer immunotherapy
 Global reach, higher impact
Global reach, higher impact