13.3
Impact Factor
Theranostics 2022; 12(15):6740-6761. doi:10.7150/thno.75899 This issue Cite
Review
Multiplexed strategies toward clinical translation of extracellular vesicles
1. State Key Laboratory of Cancer Biology, Department of Biochemistry and Molecular Biology, Fourth Military Medical University, Xi'an, 710032, People's Republic of China.
2. Department of Plastic and Reconstructive Surgery, Xijing Hospital, Fourth Military Medical University, Xi'an, 710032, People's Republic of China.
3. Cadet Team 6 of School of Basic Medicine, Fourth Military Medical University, Xi'an, People's Republic of China.
4. Department of Ultrasound Diagnostics, Tangdu Hospital, Fourth Military Medical University, Xi'an, 710038, People's Republic of China.
Received 2022-6-7; Accepted 2022-9-7; Published 2022-9-21
Abstract
Extracellular vesicles (EVs), of which exosomes are a representative subgroup, are naturally secreted nanoparticles with a variety of payloads. With the intrinsic merits of stability, biocompatibility, low immunogenicity, and large capacity, EVs are widely regarded as effective carriers of drug delivery. However, disadvantages, such as low yield, complicated isolation procedures, and low loading efficiency, hinder its clinical translation. In this review, we systematically summarize the advances in EV (especially exosomes) engineering for clinical application, focusing on strategies toward high yield, facile isolation, efficient cargo loading, improved delivery, and optimized manufacturing, which might unleash the infinite power of EVs in clinical translation.
Keywords: extracellular vesicles, clinical translation, yield, isolation, cargo loading, delivery, manufacturing
Introduction
Nanomedicine-based theranostics has significantly impacted conventional therapeutic modalities. Rapid developments in nanotechnology have allowed us to synthesize nanomaterials with designed properties, such as electromagnetism, targeting ability, and thermal responsiveness, enabling clinicians to treat diseases precisely [1]. Drug delivery is at the core of clinical therapeutics. Due to the large ratio of surface area to volume, excellent biocompatibility, and prolonged circulation time, artificially assembled nanoparticles are considered a fascinating carrier, bringing about revolutionary changes in treatment. Nevertheless, the blockade effect from physiological barriers and unwanted uptake by the reticuloendothelial system impede the application of exogenous nano-systems [2, 3]. Conventional viral vectors used in gene therapy also have limited application due to oncogenicity, immunogenicity, and intrinsic pathogenicity [4]. Scientists are, therefore, actively seeking a promising substitute with superior performance.
Extracellular vesicles (EVs), including microvesicles (MVs) and exosomes or exosome-like vesicles (ELVs), have been used as effective carriers in drug delivery with remarkable capability to extravasate through vessel fenestrations and move through extracellular matrix [5]. The focus of this review is on exosomes, a major subgroup of EVs. As organelles of ~30 to ~200 nm in diameter and 1.08 to 1.22 g/mL in density [6, 7], exosomes are budded from the endosome membrane and endowed with a variety of distinctive features, playing a significant role in multiple biological processes [8, 9]. Previous studies have unraveled that exosomes can cross the blood-brain barrier [10] and maternal-placental barrier [11]. The phospholipid bilayer provides excellent membrane permeability and protects the encapsulated agents from degradation [12]. Modes of administration for EVs range from nasal and oral administration to stereotactic and intravenous injection, rendering options for various diseases [13]. EVs have shown unprecedented potential in diagnostics and therapeutics [14-16].
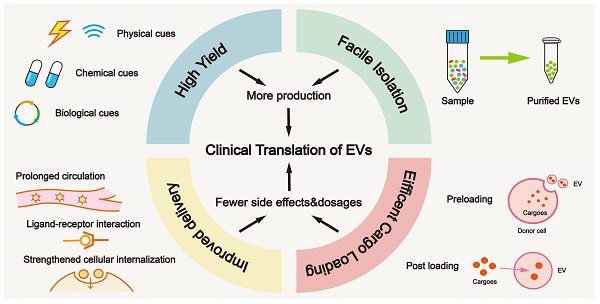
Building blocks for clinical translation of EVs. Successful application requires an integrated platform, incorporating methodologies for high yield, facile isolation, efficient cargo loading, and improved delivery. Based on diverse disease patterns, we could customize a concrete schedule for each patient to achieve personalized treatment. EV, extracellular vesicle; PEG, polyethylene glycol.
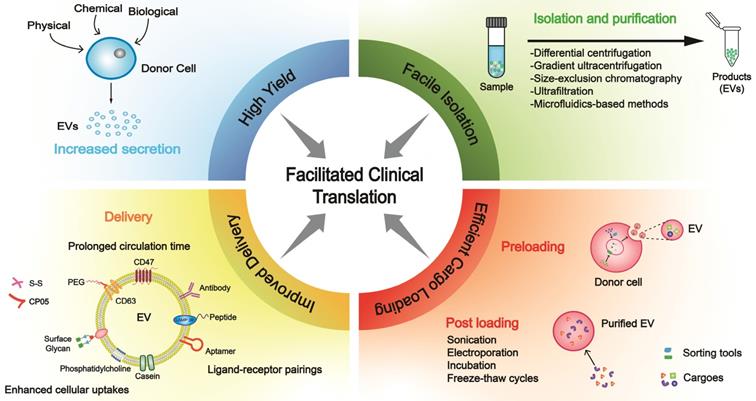
Clinical translation of EVs for drug delivery appears to be within reach. Preclinical data published by Codiak BioSciences Inc. demonstrated the potent anti-neoplastic activity of the candidate product exoASO-STAT6 [17]. Also, several pharmaceutical products have already entered human trials, such as Plexaris and Cevaris of ExoPharm and CAP-2003 of Capricor Therapeutics. Currently, many companies dedicated to cell therapy are diligently developing EV products for clinical applications [16, 18]. However, several bottlenecks of productivity, biopotency, and biosafety remain and must be resolved. Yuan et al. proposed the “STOP” criterion that refers to high Safety, good Targeting ability, rapid Obtainment, and high Purity [19]. Unlike laboratory experiments, there is still a huge gap between inadequate yield and sustainable medical demands. Additionally, the inefficiency of cargo encapsulation has restricted the progress of EV application [20]. Upgrading engineering strategies, isolation methods, and introducing well-designed modifications to EVs, will undoubtedly promote their clinical application.
In this review, we focused on strategies in areas of high yield, facile isolation, efficient cargo loading, improved delivery, and optimized manufacturing (Figure 1), which are closely interlinked with clinical translation.
Approaches to overcome low yield bottleneck
The history of exosomes dates back to 1983, when they were described to originate from sheep reticulocytes as released vesicles [21]. However, the vesicles were not given the name “exosome” until 1987 by scientists from McGill University [22], depicting its structure vividly as “though tiny, perfectly formed”. Bioactive substances, such as proteins, lipids, and nucleic acids are encapsulated and subsequently delivered to recipient cells, eventually leading to phenotypic changes. As secreted vesicles, the biogenesis of exosomes is a finely-tuned process mediated by modulatory machinery. With the maturation of early to late endosomes, the endosomal membrane invaginates inward and transforms into a multivesicular body (MVB) [16, 23] with the support of the endosomal sorting complex required for transport (ESCRT) of complexes 0, I, II, and III [24]. After the fusion between MVBs and the cellular membrane, intraluminal vesicles (ILVs) are successsfully released, which are widely acknowledged to be exosomes or ELVs [16]. There are other ESCRT-independent pathways for the formation of exosomes, either triggered by the cone-shaped ceramide to generate a spontaneous negative curvature [25, 26], or marked and controlled by RAB31 to prompt epidermal growth factor receptor (EGFR) entry into MVBs [27]. EVs are composed of diverse constituents, which are incorporated via sorting mechanisms such as AAUGC motif for miRNAs [28] and ubiquitin-like 3 (UBL3) modification for proteins [29]. From the opposite perspective, clearing MVBs by fusion with lysosomes or autophagosomes is also a critical stage in their lifecycle [30].
Bioinspired strategies for increasing EV yield. (A) Specific physical stimulations are feasible to raise the output of EVs. (B) The addition of drugs is a general form of chemical strategy in mass production by impacting different phases of EV biogenesis. (C) Altering the expression of specific genes leads to an increase in yield through genetic engineering. Exosome boosters are effective targets to be manipulated. (D) Several alternative means can be utilized to provide reliable resources. PSiNPs, porous silicon nanoparticles.
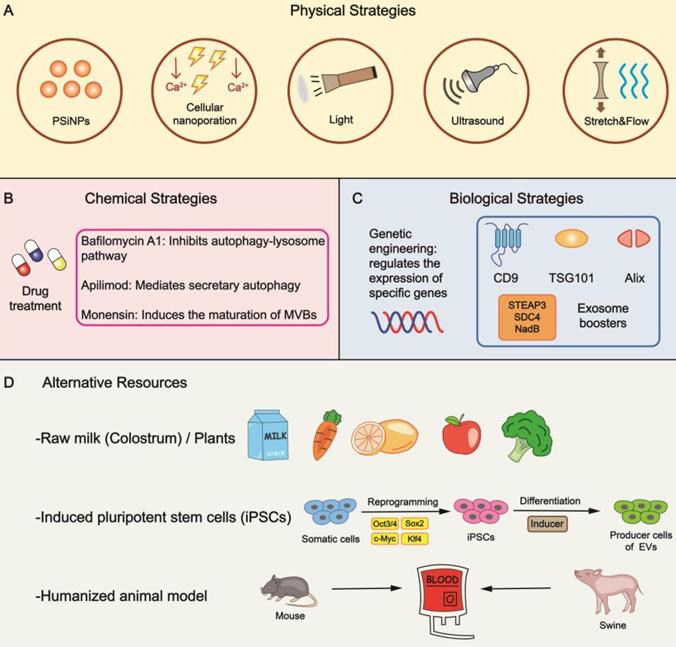
The quantity of naturally secreted EVs by mammalian cells is relatively low, inevitably hindering their clinical application. In this section, we will summarize promising methods to increase the biogenesis of EVs (mainly exosomes) (Figure 2).
Physical strategies
Physical stimuli in various forms are explored to enhance EV yield. For example, tumor cells can generate a nearly 34-fold output of exosomes when incubated with porous silicon nanoparticles (PSiNPs) with a minimal effect on exosome identity [31]. Mechanically, PSiNPs booster exosome biogenesis in an autophagy-dependent manner owing to their non-degradability in the lysosomal acidic microenvironment. Theoretically, the strategy should be applicable to other cells, including non-malignant cells. Yang et al. reported that by regulating the flow of calcium ions and generating electrical stimulus in a focal and transient pattern, cellular nanoporation (CNP) induced a 50-fold increase in exosome yield [32]. Also, 3D scaffolds could be viewed as an innovation of traditional 2D cell cultures. Mediated by yes-associated protein (YAP) mechanosensitivity, mechanical forces remarkably fostered EV release on 3D scaffolds in the form of flow and cyclic stretch [33]. Treatments such as light induction and ultrasound stimulation also led to a 13-fold and 4.14-fold increase in EV secretion respectively [34, 35]. These two methods are relatively convenient to operate, but more attention should be on addressing safety and stability concerns. Physical stimuli would inevitably affect the morphology of EVs to varying degrees; therefore, ascertaining appropriate parameters (e.g., intensity, frequency, duration) is imperative.
Besides promoting biogenesis, serial extrusion is a feasible and economical approach to producing exosome-mimetics (EMs) or ELVs. Cells could be extruded by filtration with filters of specific sizes, such as titanium meshes [36], polycarbonate filters [37] and nanoporous membranes [38]. The entire process averts the time-consuming course of ultracentrifugation and only requires a reasonable number of cells, avoiding time consumption and manpower for different purification procedures. This technique was upgraded by introducing iron oxide nanoparticles, and hypotonic treatment and homogenization were employed to harvest intracytoplasmic organelles as preliminary products for further processing [38]. Notably, the EMs or ELVs are artificial products that share morphology and conformation with native exosomes. Also, high yield and scalability are their superior features, along with improved manufacturing technology. Wan et al. showed that components are instantaneously loaded into EMs during extrusion while certain cytosolic cargoes undergo selective sorting procedures into EVs [39]. Measures to trace and identify the purity of collected EMs require further exploration as naturally secreted EVs, and endogenous components escaped from the cytosol during extrusion might be deemed as contaminants [40].
As for the clinical translation, production personnel should consider the expense and output rate balance. Proper modifications of cell culture interfaces provide a compatible environment for donor cells to grow and proliferate; hence, investments in developing advanced materials, such as a 3D production system, microporous frameworks, and multi-pronged bioreactors with a conflict-free combination of these principles might be a promising approach.
Chemical strategies
Numerous chemically active agents have been shown to be involved in regulating the behaviors of parental cells. Hence, we can artificially change the culture conditions by applying drugs. Monensin (MON), a Na+/H+ exchanger, triggers the maturation of dilated MVBs in a calcium-dependent manner. On the negative side, dimethyl amiloride (DMA) suppresses the release of exosomes by perturbing calcium signaling [41]. The autophagy-lysosome pathway (ALP) inhibitor bafilomycin A1(BafA1) induces cholesterol accumulation and the separation of MVBs from the plasma membrane [42], which can be considered as augmentation of exosomes by inhibiting autophagic degradation [43, 44]. Phosphoinositide kinase PIKfyve showed a suppression effect in EV yield that could be reversed by its inhibitor apilimod [45]. Chemical treatment is a simple method without complicated procedures; nevertheless, latent toxicity and appropriate dosages need to be assessed carefully.
Biological strategies
Altering the levels of particular genes might instigate a stronger release of EVs; thus, we can control the production rate through manual interventions. Fussenegger et al. reported that specific exosome production boosters, including STEAP3, syndecan-4 (SDC4), and a fragment of L-aspartate oxidase (NadB), impact diverse stages of exosome biogenesis to raise yield [46]. Upregulating CD9 promoted the secretion of exosomes, whereas Alix and TSG101 exhibited opposite effects [47]. Markedly, various molecules associated with lipid metabolism play a significant role in EV secretion. Lipid transporters, such as the ATP-binding cassette (ABC) transporter A3, mediate exosome secretion via impacting lipid clustering and the biogenesis of MVB [48]. Moreover, LPS/ATP stimulation resulted in increased release through inflammasome-induced cleavage of the trafficking adaptor protein RILP [28].
With advancements in transgenic technology, this method holds great promise in the clinic. Based on massive data, gene libraries, and visualization tools that can subsequently be verified under rigorous criteria, bioinformatics and multilevel omics have been extremely helpful in identifying biomarkers and molecular subtypes in various diseases, such as cancer and neurological disorders [49-51], which are applicable to the validation of targets with yield-boosting potency as well.
Accessibility is a critical factor in mass production. Studies have shown raw milk to be a scalable and cost-effective source for collecting large quantities of exosomes, while colostrum powder shows even greater potential [52]. Also, we could utilize edible plants such as carrot, lemon, apple, broccoli, etc. as these plants are abundantly available sources. Derived exosome-like nanoparticles have shown significant therapeutic effects under many circumstances [53-55]. A significant challenge of using these plants as donors is the underlying immunogenicity, which motivates the needs of in-depth investigations in vivo. Notably, the immunogenicity might be beneficial in the context of tumor vaccine and immunotherapy. In addition, oral delivery for certain diseases should be acceptable and tolerable.
Given the successful allogeneic blood transfusion within accordant blood types, human red blood cells, especially group O, seem to be ideal derivations with universal donors [56]. The adequate reserves in blood banks offer a constant supply, and the absence of nuclear and mitochondrial DNA simplifies the purification [57]. In some cases, we can replace autologous resources with allogeneic substitutes, which is appropriate for humanized animal models, including mice and swine, providing a prelude to scale-up production [58]. Priority should be given to establishing appropriate models with stable expression of humanized genes and minimal individual differences. Remarkably, somatic cells could be reprogrammed into induced pluripotent stem cells (iPSCs), which can undergo differentiation into numerous cell types, such as mesenchymal stem cells (MSCs) and cardiac progenitor cells, providing a stable source for EVs [59-62]. These approaches might be limited due to high research and development expenditure, but the cost might be acceptable if the strategy could be industrialized and commercialized. For example, once the iPSC lineages are established, it is relatively cheap to maintain the culture. The procedure has high similarity with antibody manufacturing, and investigators can also learn from the experience of the antibody industry and commercialization.
Cell derivations significantly determine the properties of EVs (Table 1). Although the immunogenic nature of EVs and biological/chemical impurities are vital, accumulating studies reveal that EVs from cell lines or other species should be tolerable. For personalized medicine, the patient-specific cell origin is preferred, whereas scalable cell lines are more appropriate for commercialization.
Physical/chemical/biological strategies have all contributed to increasing EV production (Figure 2). Rapid vesiculation enables us to deal with variable clinical environments and sustain long-term treatments, independent of just-in-time or off-the-shelf product modalities. However, from a negative perspective, it might impact the components of secreted EVs and lead to the decrease of active ingredients, either import of exogenous drugs or evasion of endogenous substances; hence, heterogeneity ought to be controlled accurately. The strategy of mass production in combination with excessive loading could ensure content level. Besides optimizing these methods, such as setting appropriate parameters and utilizing eliminable chemical reagents, screening programs are also beneficial for acquiring uniform products and reducing batch-to-batch variations. In the future, biomimetic methodologies could be employed for this purpose.
Refinement of isolation methods
The isolation of EVs has always been a topic of interest in academic and industrial communities. Cellular components with similar size and density properties as EVs are present in culture media and biological fluids. Therefore, there is always a risk of contamination from cell debris, proteins, and apoptotic bodies [100]. Moreover, different protocols of EV isolation are used by different investigators, impeding the development of a standard protocol. Facile isolation of EVs with high purity and cost efficiency is a prerequisite for clinical translation, encouraging us to design innovative procedures.
Current methods
Recently, several methods ranging from conventional to newly-developed techniques have emerged that are summarized in Table 2.
Differential centrifugation (DC) is acknowledged as one of the most popular methods in the realm of isolation procedures. By adjusting the RPM, ingredients with different densities are either separated or discarded, while EVs are harvested in the form of pellets. It is important to establish whether EVs are in the precipitate or supernatant. Density gradient ultracentrifugation (DG UC) is employed as a form of DC using a gradient medium of sucrose or iodixanol to improve the purity of EVs. Nonetheless, the phenomena of aggregation, and contamination including high-density lipoprotein (HDL), low-density lipoprotein (LDL), or other elements will affect the purity of isolated EVs [101, 102]. These two methods are commonly implemented for research purpose and account for a significant proportion (81%) of all applications for their relatively stable yield and standardized protocols [103]. However, the pharmaceutical production of EVs requires high-expense investment for instruments and manpower.
The methods of size-exclusion chromatography (SEC) and ultrafiltration are based on molecular size. The stationary phase of SEC uses cross-linked porous polymers with a bead-like structure, while ultrafiltration capitalizes on membranes with various apertures. Lobb et al. suggested that the efficiency of SEC is approximately equivalent to DG [104]. Notably, SEC requires extra procedures to harvest clinical-grade EVs, increasing the complexity; therefore, designing an automatic workflow may be desirable. Other methods based on precipitation and affinity appear to be promising. For example, Wang et al. capitalized on specific peptides (CLIKKPF) to capture exosomes attributed to its high affinity for phosphatidylserine on exosomal surfaces, and silica (SiO2) microspheres are designated sites to immobilize these peptides [105].
Derivations of EVs and their applications
| Derivation | Merits | Drawbacks | Application Scenarios | Ref. |
|---|---|---|---|---|
| Differentiated cells from induced pluripotent stem cells | -Artificial induction with selectivity -Stable sources of EVs (differentiate into various cell types) -Consecutive production of autologous EVs -Bypass ethical issues | -Underlying genetic defects -Tumorigenicity -Heterogeneity due to inconsistency of differentiation -Incomplete mechanisms | -Treatment of heart failure and myocardial infarction -Regenerative medicine -Drug tests and screening -Disease modeling -Applicable to precision and personalized medicine | [63, 64] |
| Cancer Cells | -Characteristic biomarkers and bioactive molecules -Separation from bio-fluids -Benefit from the development of detection technology | -Limited protein profiling for derived EVs -Incomplete understanding of the mechanisms -Safety concerns about inducing immunologic tolerance and negative immune regulation | -Biomarkers for diagnosis -Engagement in cancer development -Therapeutic function | [65, 66] |
| Established cell line | ||||
| HEK293T/AML12/RAW264.7 | -Guaranteed source -mass production capacity for industrialization -Standardized protocols (applicability and universality) | -May contain carcinogenic ingredients | -Therapeutic effect -Anti-tumor features | [67] |
| Immune cell | ||||
| Dendritic cell | -Alter the properties under feasible stimulus (antigens/cytokines), -Expression of multiple immune-associated molecules -Activator of immune responses | -Heterogeneity in different stages (deficiency of MHC-II and CD86 in immature stage) | -Chimeric antigen receptor T cell therapy -Induce the apoptosis of cancer cells -Activate natural killer cells -Treatment of Alzheimer's disease | [68-70] |
| Neutrophil | -Tumor-targeting ability -Intrinsic inflammation-tropism -Outstanding blood-brain barrier crossing capability | -Latent pathogenicity | -Suppress tumor growth -Therapy for glioma -Induce chronic obstructive pulmonary disease and bronchopulmonary dysplasia | [71-73] |
| Macrophages | -Intrinsic inflammatory chemotaxis -Expression of specific receptors -Involve multiple cytokines | -Heterogeneity in functions and phenotypes of different sources | -Alleviate rheumatoid arthritis -Management of atherosclerosis- -Remodel the tumor microenvironment -Enhance antitumor effect | [17, 74-76] |
| Blood | ||||
| Plasma | -Accessibility -High bio-safety -Wide distribution in vivo -Maneuverability | -Difficulty in precise separation | -Alleviate spontaneous hypertension -Biomarker in the diagnosis of multiple diseases | [77-79] |
| Red blood cells | -Biosafety (devoid of nuclear and mitochondrial DNA) -Abundant resources in the blood bank -Accessibility -Brain targeting ability | -Underlying side effect -Various factors affect half-life and shelf life | -Therapy for Parkinson's disease -Drug delivery | [56, 80, 81] |
| Platelet | -Tumor-homing property -Apheresis facilitates the collection procedure -Enrichment of various bioactive molecules -Nuclear-free (minimized teratogenicity) | -Heterogeneity among donors -Probable pathogen contamination | -Chemo-dynamic/Photothermal therapy for cancer -Regenerative medicine -Drug delivery | [82, 83] |
| Neural cells | ||||
| Microglia | -Rich in trophic factors | -Difficulty in cell acquisition | -Prevent ischemia-reperfusion injury -Enhance neurogenesis and neuroprotective effect -Cure traumatic brain injury | [84, 85] |
| Astrocytes | -Most abundant neuroglial cells | As above | -Mitigate neurotoxicity in Alzheimer's disease | [35] |
| Adipose tissue | ||||
| Brown adipose tissue | -Promote energy metabolism -Rich in mitochondria | -Limited supplement (primarily in infants or neonatal mammals) | -Anti-obesity -Alleviate metabolic syndrome | [86] |
| White (visceral) adipose tissue | -Ability to cross the placenta -Effective uptake by vascular endothelial cells | -Relatively finite sources | -Related to fetal cardiac dysfunction -Management of colitis | [87, 88] |
| Mesenchymal stem cell | ||||
| Classification as below | -Self-renewal capacity -Multiple differentiation potential -Strong reproductive activity in vitro -Immunoregulation ability | -Safety concerns between donor and recipient -Require expansion | Varying from cell types as below | [89, 90] |
| Bone marrow mesenchymal stem cells | -Various bio-signal molecules | -Discrepancy in clinical trials -Limited knowledge of working principles | -Tissue engineering -Treatment of osteoarthritis -Neuro-degenerative diseases | [15, 91, 92] |
| Human umbilical cord mesenchymal stem cells | -Phenotypes change under stimuli -Primitiveness -lower reject reaction | -Relatively limited source -Ethical issue | -Treatment of spinal cord injury -Accelerate chronic diabetic wound healing | [93, 94] |
| Adipose-derived stem cells | -Contain Various cytokines -Broad distribution in the human body | -Heterogeneity in methods of administration -Biosafety in vivo under evaluation | -Remodel inflammatory microenvironment -Tissue repair and reconstruction -Treatment of rheumatoid arthritis/intervertebral disc degeneration/tendon lacerations | [95-99] |
Summary of the isolation methods for EVs
| Isolation methods | Operating principles | Pros | Cons |
|---|---|---|---|
| Differential centrifugation | Adjust centrifugal force via altering RPM; suspension or sediment in line with density | Widely applied and relatively standardized protocols; volume production; low cost of extraction in the condition of existing instruments | High expense of stationary instruments; cost of time and manpower; protein aggregation |
| Gradient ultracentrifugation | Form density gradient through medium; constituents with various densities lie in different positions | Highly purified; handle easily with protocols | High cost of stationary equipment; time-consuming; low productivity; latent changes under mechanical force |
| Size-exclusion chromatography | Sieve pores through crosslinking of polymers; separation on the basis of sizes | High purity; maintenance of original structure; applicable to various derivations | Relatively high expense of devices; requirement of extra procedures |
| Ultrafiltration | Filter through membranes of different apertures; isolation in dependence on the sizes | Great portability without costly equipment; fast operation; capable of mass production | Moderate purity; membrane obstructions and trapping; potential destructive effects of shear stress |
| Extrusion | Utilize extruder and designed membranes; sized-based technique | Fast preparation; high yield | Additional operations of ultracentrifugation; output as exosome-mimetics |
| Precipitation | Harness water-excluding polymers to remodel the solubility | Scalability; simple and practicable | Coprecipitation with proteins and polymers harming purity; requisite procedures of clean-up; contaminants may interfere downstream analysis |
| Immunoaffinity | High affinity between immobilized antibodies (ligands) and exosomal receptors | Highly specificity; high purity; lessened contaminants | High cost of antibodies; narrow scope of application (cell-free samples with distinct markers); elution step may impair the original states |
| Microfluidics-based method | Construct microfluidic channel systems to filter EVs; established on different principles including size, density and immunoaffinity | Focus of research with tremendous room for upgradation; high recovery rate; achievable automatic operating system; portability; development of biochips boosts this technique | Low sample capacity; require standardization of operational procedures |
| Commercial kits | Multiple product types based on the above-mentioned principles | Portability without stationary apparatus; apt to commercialization; cost-effective in the absence of stationary instruments | Low purity; different efficiencies of disparate products; relatively expensive in specific circumstances |
The development of the microfluidic-based method is a milestone in EV purification. As an interdisciplinary technique across nanotechnology and bioengineering, it focuses on constructing a microchannel system with specificity and sensitivity by utilizing external actuators and has already been applied in extracting EVs from urine, whole blood, plasma and other samples [7, 106-109]. Tangential flow filtration (TFF) is an improved version of the microfluidic-based method, utilizing ultrafiltration membrane filters to collect exosomes from pre-treated supernatants after incubation and centrifugation [95]. Likewise, samples can be purified through the 'dead-end' membranes along with perpendicular flow [7]. An integrated system based on microfluidics has been enriched to a great extent, for which examples include acoustics and microfluidics [110], and immunomagnetic beads and microfluidics [111]. Significantly, this technique can collect freshly released EVs immediately to avoid endocytosis by parental cells. Currently, portable commercial kits at a reduced cost and avoiding large stationary instruments are being invented extensively. Typical examples include miRCURY Exosome Plasma/Serum Kit, Saliva Exosome Purification Kit, and ExoQuick-Ultra [16]. These products provide operators with preferable selections based on diverse working principles.
Low recovery rate and contamination induced by various methods adding to the impurity profile are major issues in EV isolation. Incorporating an interception unit into a microfluidic-based system might be beneficial in reducing the impurity of EV samples. Multifunctional instruments conducting isolation and detection synchronously are also a viable solution, enabling the EV recovery analysis in real-time. Also, purification based on yield boosting would significantly improve the proportion of purified EVs compared to contaminants.
Assisted separation
Introducing specific devices might be a viable solution. For instance, researchers anchored superparamagnetic nanoparticles (SPMNs) onto exosomes via Tf-TfR (transferrin and transferrin receptor) interaction, labeled as SMNC-EXO. Under the impact of adjustable magnetic fields (MFs), exosomes could be effectively separated from the solution, as has been shown with reticulocyte-derived exosomes [112]. This method has been optimized by attaching polyethylene glycol (PEG) spacers, shortening the time interval from extraction to acquisition to 3.5 h [19]. Since the binding and disaggregation between Tf and TfR are pH-responsive, adjusting relevant conditions increased the precision of isolation and better maintained the original shape of exosomes. Other factors, such as temperature and ion strength, should also be considered [78].
In another study, a stimuli-mediated platform was exploited for EV isolation from plasma based on a synthetic copolymer (poly (N-isopropylacrylamide-co-N-acryloxysuccinimide), PNN). Thermal-mediated hydrophilic-to-hydrophobic phase transition induced EV accumulation, while the painting and removal of PNN on EVs could be manipulated [113]. Recently, the chimeric nanocomposites of lactoferrin (LF)-conjugated 2,2-bis(methylol)propionic acid (MPA) dendrimer-coated magnetic nanoparticles (MNPs) (LF-bis-MPA-MNPs) achieved high purity and recovery rate of EVs. The nanocomposites utilized various interactions, including electrostatic attraction between electropositive LF and electronegative surface of EVs, physical adsorption, and biorecognition between LF and LF receptors [114]. Technically, Chen et al. established the ultrafast-isolation system (EXODUS) to purify exosomes automatically by undergoing negative pressure oscillation and membrane vibration driven by double coupled harmonic oscillator [115].
Notably, for EV-based diagnostics, which starts from tiny amounts of samples, specific lab-based isolation methods such as microfluidic-based techniques appear to be practical. In contrast, methods such as DC and DG/UC, which need a large quantity of samples, might not be smart choices.
Pipeline integrating biogenesis and isolation
Under specific circumstances, a rational and flexible combination of selected techniques is conducive to purification. For example, Watson et al. developed a strategy incorporating the bioreactor culture, TFF, and preparative-scale SEC and received desired effects, especially in lowering the contamination of ferritin [116]. By integrating multiple advantages of different methods, the constructed pipeline exhibits the greatest promise to become the mainstream approach. As for each newly designed workflow, setting conflict detection programs can prompt the transition from experimental units to industrialization.
The strategies of high yield and facile isolation contribute to meeting clinical demands. It is worth investigating systematically the relative merits and compatibility in follow-up studies. Furthermore, cell culture, EV sensing, and quality control innovations would certainly be beneficial for clinical applications. The ability to automatically monitor the production, precisely booster biogenesis, and real-time isolation are urgently needed.
Strategies to improve cargo loading
Key breakthrough in interpreting the role of EVs was made in 2007 when Valadi et al. discovered that exosomal miRNAs and mRNAs shuttled between cells [117]. Since then, encapsulation of cargoes into this cell-free system has been in the spotlight. Enhancing the stability of the contents in the lumen of EVs is of prime importance, either in circulation or preservation, requiring innovations in loading methodologies. Each method has pros and cons in terms of endogenous or exogenous substances, correlating with molecular weight, functional groups, and lipophilicity. For preloading, cargoes are sorted during the biogenesis of EVs, and modulating the status of parental cells would impact the loading efficiency, while for post-loading, cargoes are directly packaged into EVs through manual interventions.
Loading during biogenesis
Preloading begins before the formation of EVs with sorting mechanisms specific for different components (Figure 3A). Intracellular nucleic acids constitute a crucial branch of cargoes, and the RNA-binding protein (RBP), a constituent of the ribonucleoprotein (RNP) complex, is considered a pivotal tool to load RNA. It has been reported that fragile X mental retardation 1 (FMR1, an RBP) participated in the selective packaging of exosomal miRNA under inflammatory circumstances by recognizing specific motifs of miRNA [28]. There are various reported sorting tools with nucleic acid loading ability, such as Y-box protein 1 (YBX1) [118], sumoylated heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) [119], and VAP-A along with ceramide transfer protein (CERT) [120].
Our group successfully constructed a DNA aptamer to load mRNA consisting of two segments. The single strand segment could interact with the AUG region of mRNA, suppressing mRNA translation and ribosome assembly, whereas the double strand segment could encapsulate mRNA into EVs when recognized by CD9-ZF (zinc finger) motifs [121]. In another study, we fused the exosomal membrane protein CD9 with human antigen R (HuR, an RBP), facilitating miR-155 loading into exosomes specifically [122]. With the emerging information on the sorting machinery of miRNA, especially the sEV-export sequences (EXOmotifs) and cellular-retention sequences (CELLmotifs) [123], it is feasible to modulate the recognition network and generate RNA-based loading by tagging them with particular motifs.
Strategies for cargo loading into EVs. Mechanisms and techniques for cargo loading into EVs are diverse. Preloading and post-loading are two main patterns. (A) Preloading is related to the sorting mechanism during biogenesis. Transfection is a frequently-used technology to increase the content level. Post-loading relies on the development of methods including (B) electroporation, (C) sonication, (D) incubation, (E) click chemistry, (F) membrane fusion. Different methods exhibit advantages towards different cargoes, providing cues for patient-centered clinical application. hnRNPA2B1, heterogeneous nuclear ribonucleoprotein A2B1; MVB, multivesicular body; FMR1, fragile X mental retardation 1; YBX1, Y-box protein 1; EXPLOR, exosomes for protein loading via optically reversible protein-protein interaction; CRY2, cryptochrome 2; CIBN, a truncated version of CIB1; GFP, green fluorescent protein; PTX, paclitaxel; Er, Erastin; RB, Rose Bengal; EV, extracellular vesicle.
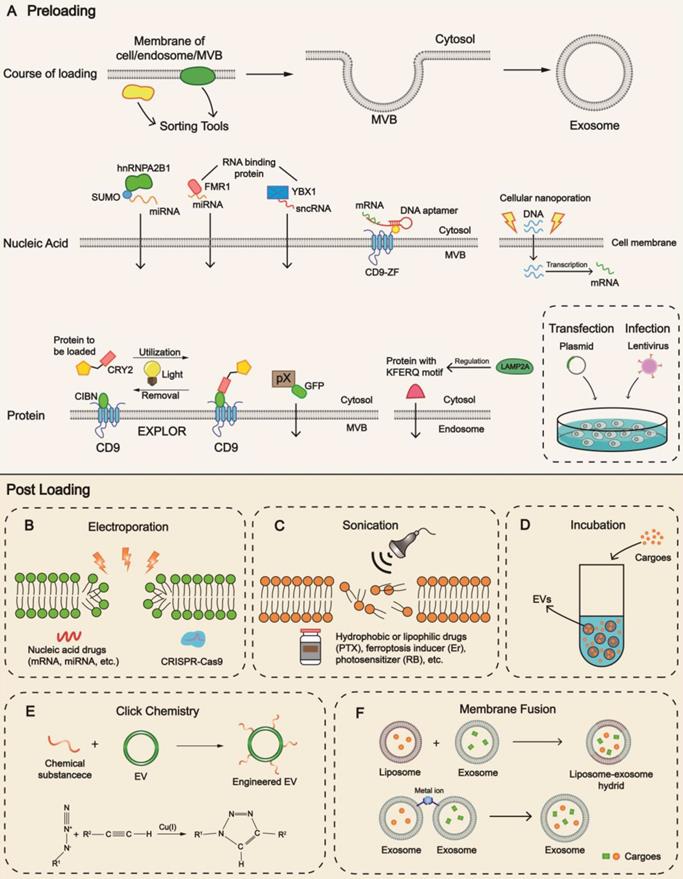
The sorting mechanism of proteins has been clarified in recent years. Choi et al. reported an excellent pattern of 'exosomes for protein loading via optically reversible protein-protein interactions' (EXPLORs) [124], allowing operators to have better control of the loading. The status of the key connector cryptochrome 2 (CRY2) is regulated via altering light conditions, changing between quiescent and photo-activated phases through phosphorylation and dephosphorylation [125]. Some proteins also contain specific motifs which facilitate their loading into EVs. For example, LAMP2A mediates the sorting of proteins (e.g., hypoxia-inducible transcription factor 1, HIF1A) through the KFERQ motif pentapeptide [126]. Researchers from AstraZeneca recently reported that TSPAN14, CD63, CD63TM, and CD81TM were highly efficient EV sorting proteins for loading green fluorescent protein (GFP) [127]. From the engineering perspective, Kim and his colleagues constructed a truncated CD9 scaffold to load angiotensin-converting enzyme 2 (ACE2) to small extracellular vesicles (sEVs) through biogenesis, offering a binding site specific to the viral spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [128]. It has also been reported that the structural protein pX of hepatitis A virus (HAV) facilitated eGFP (enhanced GFP) entry into MVB by interacting with the region of apoptosis-linked gene 2-interacting protein X (ALIX) [129]. Future directions should concentrate on identifying more concrete domains with loading capacity.
Transfection/infection is a common technique of packaging exogenous substances into donor cells, increasing the content level and subsequently affecting the released EVs. Firefly luciferase (Fluc) and mCherry applied for EV tracking are loaded using this method [130]. Technically, CNP increased the yield and encapsulated large quantities of DNA into exosomes non-genetically via creating nanochannels. Generated by electrical pulses, parental cells release plentiful exosomes with significant elevation in mRNA transcripts [32].
Loading after biogenesis
Even after EV isolation, cargo loading is possible. In the context of loading strategies of liposomes, electroporation has been a mainstream method in encapsulating nucleic acid drugs (Figure 3B). Usman et al. confirmed that the tool for gene manipulation, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system, could be packaged into EVs through electroporation [56]. This technique omits the inconvenience of designing and constructing miscellaneous vectors, inserting the cargoes into EVs via nanoscale channels directly. However, electroporation may cause the loss of endogenous components and subvert the original traits of EVs. It exhibits great potential in loading small RNAs, such as siRNA and miRNA but remains technically challenging in loading mRNA, lncRNA, or other large nucleic acid drugs. In-depth research should focus on identifying the most appropriate system and the optimal technical parameters, such as voltage and quantity of electric charge, to minimize harmful effects. Also, reducing the expense of instruments for industrial manufacturing is essential.
Sonication is another promising technique utilized in drug encapsulation of EVs by perturbing the morphology and topology of the membranes, which is better suited to hydrophobic and lipophilic cargoes (Figure 3C). Anticancer drugs such as paclitaxel (PTX), ferroptosis inducer (Erastin), and photosensitizer (Rose Bengal) can be packaged with this method [76, 131]. However, without proper understanding (e.g., unfit intensity and frequency of sonic waves), an obscure alteration in physicochemical properties will result in biological dysfunction, posing a risk to safety and reproducibility.
Incubation is a spontaneous process with low efficiency [83] and is a simple method widely adopted in cargo loading of EVs, where the basic principle is passive diffusion (Figure 3D). Cargoes of interest and isolated EVs are mixed together while the duration and temperature of incubation are determined by cargo types. The membrane lipid layer of EVs accelerates the diffusion of lipophilic cargos such as fluorescent probes DiR, and DiD [130]. In addition, therapeutic nucleic acids can be packaged into the liposomes to acquire lipophilicity [132]. As a biosurfactant and permeabilizer, saponin can induce pore formation on the membranes by acting on cholesterol and phospholipids [133, 134]. EVs incubated with saponin have been reported to exhibit a higher encapsulation percentage of hollow gold nanoparticles (HGNs) than electroporation [135].
Besides effectively producing EV mimetics, serial extrusion also has a great potential in cargo packaging, such as synthetic materials like gold nanoparticles (AuNPs) [136] and chemotherapeutic drugs like PTX [137]. Extruders and filters with different diminishing pore sizes are the building blocks of this method. With repeated execution of the procedure, the dimensions of the particles can be manipulated. However, mechanical force may pose a threat to the quality and stability of EVs in subtle ways. Another technique in cargo loading of EVs is freeze-thaw cycles. For example, Haney et al. successfully loaded catalase into exosomes using this technique for treating Parkinson's disease [138]. Other methods like thermal shock for HGNs, hypotonic dialysis for porphyrins, and the pH gradient method for nucleic acids have also been explored [135, 139-141].
The introduction of lipids benefits the loading of EVs. Lipids, such as cholesterol, have membrane-anchoring properties through hydrophobic interactions with the phospholipid layer. Guo et al. attached arrow-shaped RNA to EVs and altered its function by switching cholesterol positions, either in the arrowhead or arrow tail [142].
After it was first introduced by Sharpless in 2001 [143], click chemistry has been broadly adopted in biomedical materials with high atom economy and was utilized for EV engineering (Figure 3E). Substrates, such as functional groups of primary metabolites, can be synthesized and conjugated through versatile crosslinkers into many products with anticipated characteristics [144]. For instance, ultrasmall Prussian blue nanoparticles (uPB) for mitigating oxidative stress [145] can be decorated onto exosomes through copper-free click chemistry.
Membrane fusion is a viable strategy in fabricating engineered EVs, which could be generated between liposomes and EVs or with two individual EVs. The evolution of liposomes has exceeded half a century since Bangham first proposed it in 1965 [146]. Because of their distinctive conformation and optimized pharmacodynamics, liposomes have been extensively applied and commercialized in drug delivery [3]. The selective packaging of cargoes into exosomes can be achieved through the fabrication of exosome-liposome hybrids (Figure 3F). For example, Li and his coworkers used biohybrids encapsulated with triptolide (TP) and miR497 for the treatment of ovarian cancer [147]. Multiple methods can be implemented to construct exosome-liposome hybrids, including freeze‑thawing, co‑extruding, and incubation [40]. Native exosomes may lack specific therapeutic agents or sufficient content levels. On the contrary, biohybrids (semi-artificial exosomes) can be modified with desired physicochemical features for which the technology is relatively well-established, offering better chances to carry a variety of payloads. However, before its application in clinical settings, the identity of the exosome-liposome hybrid should be established. It is important to determine whether the hybrid is more like an exosome or a liposome, whether the stability, biocompatibility, and homing property of exosomes are sacrificed during their formation, and how to achieve balanced effects with improved loading and acceptable biocompatibility/kinetics. Remarkably, the fusion between exosomes can be controlled and mediated by supramolecular complexation. Scientists assembled multiple enzymes within different engineered exosomes into a minimal electron transport chain (an unprecedented cargo type) and innovatively formed biocatalytic cascades to produce ATP with the chemical bridging method [148].
With profound technological advances, numerous methods have emerged to enhance loading capacity and the categories of cargo have been broadened. Efficient cargo loading elevates the level of agents within a single EV, lowering the amount of EVs needed, promoting therapeutic efficiency, and significantly benefiting clinical translation. In addition, processed EVs comprise a heterogenous population, and it is imperative to eliminate the unloaded fraction to raise the safety and quality level.
Multifaceted routes towards improved delivery
Biosafety is an uncompromising benchmark in clinical practice as chemical reagents act on the human body. Furthermore, improved EV delivery should start from reducing non-specific cellular uptakes, promoting interactions with recipient cells and retention in targeted organs, and enhancing internalization efficiency (Figure 1).
Strategies to prolong half-life period in vivo
Reducing the off-target cellular uptake is critical to prolonging circulation time, minimizing the needed doses, and facilitating delivery in target cells. Although the nanoscale framework prevents EVs from phagocytosis by the mononuclear phagocyte system (MPS) in the spleen and liver to a certain degree [5], they are distributed and degraded primarily in these organs [149]. EVs need to be camouflaged to avoid uptake by untargeted tissues. CD47 is a well-known target in tumor immunology, which binds with the signal regulatory protein alpha (SIRPα, also known as CD172a) on macrophages, sending the signal of 'don't eat me'. CD47-SIRPα pathway is a classical immune checkpoint, permitting cancer cells to escape from immune system surveillance in the first place. This approach has been exploited to assist EVs in evading MPS by constructing corresponding plasmids to transfect the donor cells [131, 150]. Moreover, knockdown of specific endocytosis-associated genes in macrophages and injection of blocking agents such as siClathrin efficiently reduced endocytosis [151]. This methodology is conceptually feasible; nevertheless, its clinical prospect remains unproven. The technology of gene knockdown in humans is still challenging as the accuracy and latent mutations are difficult to control. MPS is a vital defense mechanism in pathological states, and its blockade may lead to immune response disorders. Also, Clayton et al. discovered that complement regulators CD55 and CD59 anchored on exosomal glycosylphosphatidylinositol (GPI) protected the exosomes from complement-mediated lysis, effectively extending their half-life [152]. Genetic engineering would enable us to apply similar modalities to the EVs of various origins.
Surface remodeling is believed to enhance the stability of EVs. PEG is a protective hydrophilic polymer and could be decorated onto EVs through specific peptides. Its high sensitivity to reactive oxygen species (ROS) results in the breakage of thioketal bonds between the peptide and PEG, leading to the removal of the outer coating of PEG, exposing the core unit (the entity of EV). The PEG corona (PEGylation) for EVs is conducive to avoiding aggregation, opsonization, and phagocytosis [153]. Exosome polymer hybrids based on photo-mediated atom transfer radical polymerization have also been proven feasible to increase blood cycling time as the grafted monomers included oligo (ethylene oxide) methacrylate (OEOMA), carboxybetaine methacrylate (CBMA) and poly(2-(methylsulfinyl) ethyl acrylate) (pMSEA) [154]. Strategies to maintain biological features of intrinsic exosomal surface molecules after manufacturing would be the focus of further research. Simplifying procedures and shortening reaction time (e.g., application of catalyst) might be workable solutions.
Overall, these methods significantly improve the pharmacokinetics of EVs and enhance their retention in circulation, while pre-sealed strategy of PEGylation holds great potential along with the advances in materials science and “grafting” technologies [154]. Nonetheless, its clinical prospect has yet to be determined as excessive usages of PEG will give rise to the generation of anti-PEG antibodies and even severe hypersensitivity reactions. The non-biodegradability of PEG should not be neglected as well, urgently encouraging the development of alternatives with superior biocompatibility [155-157].
Enhanced target-specific delivery and recognition by recipient cells
Scientists have discovered and utilized a wide range of pairing mechanisms based on the interaction between ligands and receptors (listed in Table 3). Several cell types possess intrinsic specificities, such as the inflammation-tropism capacity of M2 macrophages [158] and the tumor-homing property of neutrophils [71, 145], and the derived EVs could inherit these properties. Jang et al. adopted lymphocyte function-associated antigen (LFA-1) to match with overexpressed ICAM1 (a type of cell adhesion molecule) of endothelial cells in abnormal angiogenesis [37]. Among many similar examples are TNF superfamily ligands on dendritic cell-derived exosome target TNF receptors of natural killer cells [69]. The aggregation of human breast cancer cell (MDA-MB-231)-derived exosomes in the lung was due to the interaction between overexpressed exosomal integrin β4 and surfactant protein C of non-small cell lung cancer cells [159, 160].
One common trait in the examples mentioned above is that EVs are not modified. Although EVs have intrinsic targeting capacity based on natural recognition, they cannot accommodate incomputable disease patterns. Conferring an accurate orientation ability to EVs through appending suitable ligands represents an engineering modality that can endow EVs with desirable properties (Figure 4).
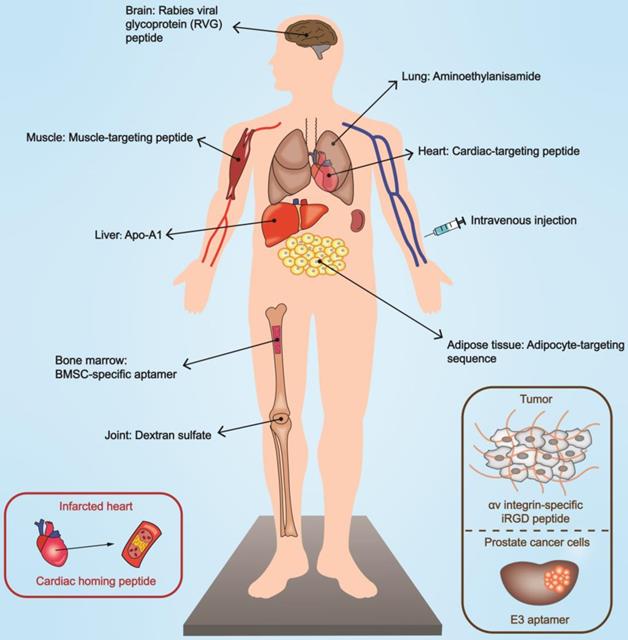
Genetic engineering is a critical approach to realizing the reprogramming of EVs [161]. By constructing plasmids or lentiviral vectors encoding the targeting moiety to transfect/infect producer cells, it is possible to harvest functionalized EVs. Functional peptides with targeting ability can be fused to EV membrane proteins to mediate biological navigation. In this context, lamp2 (lysosomal-associated membrane protein 2), a well-characterized and abundantly expressed exosomal membrane protein, is a perfect site for modifications. RVG (rabies viral glycoprotein) peptides anchored onto lamp2b are capable of recognizing acetylcholine receptors on organs such as the brain [70] and kidneys [162]. Other examples include αv integrin-specific iRGD peptide [12], cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide [163], and the muscle-specific peptide ligand [164] to hitch to tumor tissues, ischemic brain, and muscle respectively. The strategy of engineered glycosylation can contribute to maintaining the bioactivity of these well-designed peptides [165], and the phage display technology enables researchers to examine feasible peptides that can be attached to EVs [166].
Affibody is a scaffold protein consisting of 58 amino acids and is considered a targeting vector with excellent affinity [167]. Liang et al. fused HER2-binding affibody to the extra-exosomal N terminus of human lamp2 to target colon cancer cells with high expression of HER2 [67]. Similarly, cytokines, such as interleukin 3 (IL-3), could be coated onto exosomes as a mediator to prime the IL3 receptor of chronic myelogenous leukemia blasts [168]. As more potential loci are being elucidated, the binding sites are not restricted to lamp2b; CD63 (lamp3) [169] and platelet-derived growth factor receptor (PDGFR) [170] are also appropriate options.
The aptamer is a desirable ligand with a specific sequence that can decorate the surface of EVs and function in a dose-dependent manner within a specific range. It is a length of oligonucleotide sequence (DNA or RNA) in chemical nature, generated by multiple rounds of selection from a sequence library using the Cell-Internalization Systematic Evolution of Ligands by Exponential enrichment (SELEX) technique [171]. The stabilized Schiff base formation is established by the reaction between aldehyde group modification on the 5'-end of the aptamer and the amino group on the membrane proteins of the exosome [172]. For example, Sullenger and colleagues employed a designed aptamer (denoted as E3 aptamer) specific for prostate cancer cells [173]. In another study, investigators employed solid-phase phosphoramidite chemistry to conjugate designed aptamers with diacyllipid tails, serving as a linker to facilitate exosome binding [174].
Engineered EVs are cast as the intermediaries to narrow the distance between two cell types, representing bidirectional targeting. Inspired by the chimeric antigen receptor T (CAR T) cell therapy concept, Li et al. adopted tumor antigen to stimulate dendritic cells, which prompted exosomes to present CD86 co-stimulating molecules and histocompatibility (MHC)-antigen complexes, serving as CAR. Besides, anti-CD3 was used to target CD3 on T cells and anti-epidermal growth factor receptor (EGFR) to target EGFR on cancer cells [68]. This platform was denoted as synthetic multivalent antibodies retargeted exosomes (SMART-Exos) [170].
The interactions between chemical substances and receptors on specific membranes raise the possibility of targeted delivery of functionalized EVs by systemic administration. For example, linear 1,6-linked glucopyranoses with sulfate groups of dextran sulfate (DS) could be coupled with scavenger receptor class A (SR-A) of macrophages in treating rheumatoid arthritis (RA) [95]. In addition, engineering modifications (e.g., cross-linking reaction) to specific lipids such as dioleoylphosphatidylethanolamine (DOPE) or distearoylphosphatidylethanolamine (DSPE) enable them to serve as a linker between the exosomal spheroid and targeting moiety. A DOPE-NHS (dioleoylphosphatidylethanolamine N-hydroxysuccinimide) linker was adopted in connecting cardiac homing peptide (CSTSMLKAC) and the exosomes to target infarcted heart specifically [175]. Aminoethylanisamide (AA)-functionalized exosomes could be ligated to the sigma receptors on the lung cancer cells through the incorporation of DSPE-PEG-AA vector, as the terminal groups of PEGs are ideal sites for targeting moieties to link with [176].
Targeted delivery of EVs mediated by targeting moiety engineering. Engineered EVs possess the ability to target specific organs and lesion sites, contributing to bioeffect optimization. The modes of functionalization include peptide, antibody, aptamer, and chemical substances. Specific modifications can selectively act on damaged or diseased tissues. BMSC, bone marrow mesenchymal stem cell.
Mechanisms of targeting in EVs
| Ligand | Mechanisms | Derivation of EVs | Receptor | Recipient cell/Organ | Ref. |
|---|---|---|---|---|---|
| Peptide | |||||
| Muscle-targeting peptide | Conjugated with CD63 through CP05 | Murine myotubes | NA | Muscle | [166] |
| Neuron-specific rabies viral glycoprotein peptide (YTIWMPENPRPGTPCDIFTNSRGKRASNG) | Fused to Lamp2b | Dendritic cells | Acetylcholine receptor | Brain | [70] |
| αv integrin-specific iRGD peptide (CRGDKGPDC) | Fused to Lamp2b | Immature dendritic cells | αv integrin | Tumor tissues (MDA-MB-231 tumor) | [12] |
| Ischemic myocardium-targeting peptide (CSTSMLKAC) | Fused to Lamp2b | Mesenchymal stem cell | NA | Ischemic myocardium | [177] |
| Cardiac-targeting peptide (APWHLSSQYSRT) | Fused to Lamp2b | HEK 293 cells | NA | Heart | [149] |
| Cardiac homing peptide (CSTSMLKAC) | Conjugated with exosomes through a DOPE-NHS linker | Cardiosphere-derived stem cells | NA | Infarcted heart | [175] |
| Cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide | Click chemistry | Bone marrow-derived mesenchymal stromal cell | Integrin αvβ3 | Reactive cerebral vascular endothelial cells of ischemic brain | [163] |
| Adipocyte-targeting sequence (CKGGRAKDC) | Fused to Lamp2b | HEK293T | Prohibitin | Adipocyte | [121] |
| Antibody | |||||
| A33 antibody | Antigen-antibody reaction | LIM1215 cells | A33 antigen | Colon cancer cells | [178] |
| Anti-CD3 | Connected with exosome through DSPE-PEG-NHS linker | Tumor antigen-stimulated dendritic cells | CD3 | T cells | [68] |
| Anti-EGFR | As above | As above | EGFR | B16-OVA cancer cells | As above |
| Antibody | |||||
| Her2 binding affibody (VDNKFNKEMRNAYWEIALLPNLNNQQKRAFIRSLYDDPSQSANLLAEAKKLNDAQAPK) | Fused to the extra-exosomal N terminus of human Lamp2 | HEK293T cells | Her2 | 5-FU-resistant HCT-116 colon cancer cells | [67] |
| Aptamer | |||||
| Bone marrow mesenchymal stem cell (BMSC)-specific aptamer (5′-ACGACGGTGATATGTCAAGGTCGTATGCACGAGTCAGAGG-3′) | Schiff base reaction | Bone marrow stromal cell | NA | BMSC | [172] |
| E3 aptamer | Thiol-maleimide cross-linking reaction | HEK293T cells | NA | Prostate cancer cells | [179] |
| Cytokines | |||||
| IL-3 | Fused to Lamb2b | HEK293T cells | IL3 receptor | Chronic myelogenous leukemia cells | [168] |
| Chemokine receptors | |||||
| CD44 | Intrinsic inflammation-tropism capability | M2 macrophage | E-selectin | Inflammatory endothelial cells | [158] |
| VLA4 | As above | As above | VCAM1 | As above | As above |
| LFA1 | As above | As above | ICAM1 | As above | As above |
| Others | |||||
| Aminoethylanisamide | Incorporation of AA-PEG vector moiety | Primary bone-marrow derived macrophages | Sigma receptor | Lung cancer cells | [176] |
| Apo-A1 | Fused to CD63 | HEK293T cells | SR-B1 | HepG2 cells | [169] |
| Dextran sulfate | Click chemistry | Adipose-derived stem cells | SR-A | Activated macrophages in inflamed joints | [95] |
| Integrin β4 | Overexpression of integrin β4 | Breast cancer cells (MDA-MB-231) | Surfactant protein C | Non-small cell lung cancer cells | [160] |
| Folic acid | Attached folic acid covalently | Colostrum powder | Folate receptor α | Lung cancer cell line A549 | [52] |
CD, cluster of differentiation; Lamp2b, Lysosomal-associated membrane protein 2b; DOPE-NHS, dioleoylphosphatidylethanolamine N-hydroxysuccinimide; DSPE-PEG-NHS, phospholipid-polyethylene glycol-succinimide; EGFR, epidermal growth factor receptor; Her2, human epidermal growth factor receptor 2; BMSC, bone marrow mesenchymal stem cell; IL-3, interleukin 3; VLA4, very late antigen 4; VCAM1, vascular cell adhesion molecule 1; LFA1, lymphocyte function-associated antigen 1; ICAM1, endothelial intercellular adhesion molecule 1; AA-PEG, aminoethylanisamide-polyethylene glycol; SR-B1, scavenger receptor class B type 1; SR-A, scavenger receptor class A; NA, not available.
Notably, the local delivery of EVs can increase the distribution in targeted organs with multiple auxiliary techniques. Ultrasound acts as a safety switch in controlling the activities of EVs. Microbubbles guided by ultrasound can interfere with the stability of the cell membrane and enhance penetrability of cavitation nuclei [180]. We have previously elaborated the role of ultrasound-targeted microbubble destruction (UTMD) in the delivery of exosomes in refractory tissues [181]. Sonication-sensitive materials can be adopted in the cascade reactions to realize local delivery. Sonosensitizer chlorin e6 (Ce6) can produce ROS under the effect of ultrasound irradiation, inducing the deshielding of PEG for further procedures [153]. Physical connections have also found an increased application. Superparamagnetic iron oxide nanoparticles regulated by an external magnetic field adhered to exosomes through the Tf-TfR interaction with excellent targeting capability [71].
External carriers can also be adapted to facilitate the local delivery of EVs. Hydrogel, structured with a three-dimensional network, is an ideal candidate for EV-based delivery due to its excellent cellular biocompatibility, inherent antibacterial activity, and sustained release capability. The immobilization of exosomes can be achieved through the interaction between the accessional adhesive peptide PPFLMLLKGSTR of hydrogel and integrins on exosomal membranes [93]. Bioactive scaffolds with high plasticity are desirable carriers as they display excellent competency [98].
Promoting endocytosis and membrane fusion
Effective cellular uptake of EVs guarantees their biological functions. The primary form of endocytosis of EVs is pinocytosis, which can be subdivided into clathrin-dependent endocytosis (CDE), clathrin-independent endocytosis (CIE), and macropinocytosis (MP), among which CIE and MP are the commonest modalities [182-184]. Similar conformation of lipids and shared molecular features increase the chances of membrane fusion. Engineered modifications to the membrane composition are also helpful in this context. In another example, the greatly enriched milk protein casein can self-assemble into micelles, and EVs mixed with casein showed enhanced cellular uptake [185]. Similarly, Kang et al. incorporated amphiphilic phosphatidylcholine (PC) molecules into the phospholipid layer of reticulocyte-derived exosomes by incubation, vastly promoting their cellular internalization efficiency [186]. Also, glycoengineering could selectively adjust the surface glycan constitution to alter the intensity of interactions between EVs and recipient cells [187]. Noteworthy, we need to ensure that the newly introduced materials do not affect the original functions of EVs.
In the context of clinical translation, improved delivery minimizes the dosages of drugs and reduces side effects due to the toxicity to healthy cells. Vaccination is another important application of EVs, as unique nebulization treatments and inhalable modalities provide multi-protection compared with intramuscular injection [188]. The immunogenicity and pathogenicity of EV vaccines should be precisely determined to achieve clinical standards. A multidimensional editing strategy without sacrificing bioactivity lays the foundation for improved delivery. EVs of autogenous origin tend to be the first choice with utmost adaptability and delivery efficiency for individual patients.
Optimized manufacturing: formulation and characterization
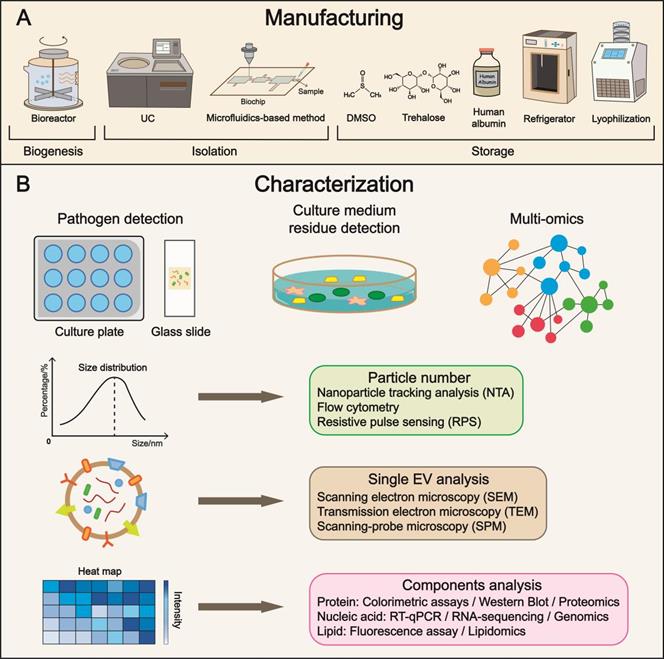
With the recent progress in yield, isolation, cargo loading, and delivery, manufacturing issues have become increasingly important. In addition, quality control is a central issue that deserves further investigation (Figure 5) [189].
Bioreactor suspension cultures, in which the cells are dispersed, oscillated, and rotated in the medium, are widely applied in cell-based manufacturing. For the industrialization of EVs, the bioreactor-based culture system is preferred. Also, it is important to avoid using biological molecules from other species. For example, humanized materials, such as human growth factors and human serum albumin instead of bovine serum albumin, should be employed. More significantly, a system should be established to detect and prevent contamination during cultivation.
Suitable parameters for stable storage ensure the preservation of biosafety. Temperature is a determinant in EV storage. At the 4 °C-storage temperature, a reduction in the quantity and antibacterial capacity of EVs was observed within a short period, probably associated with aggregation, fusion, adsorption onto tube walls, and decomposition [190]. Storage at -20 °C affects the EV size, whereas the total quantity is comparably stable [191]. The temperature of -80 °C appears to be the most appropriate for long-term storage. Although multiple studies have reported controversial outcomes, for example, Brasier et al. denoted that -80 °C caused a 25% increase in diameter and an apparent decline in the zeta potential of EVs [192], the cold chain logistics industry brings new opportunities to -80 °C setting. The development of temperature control relies on the mechanical engineering industry with collective efforts from multiple fields.
Phosphate-buffered saline (PBS) is used for stabilizing EVs, protecting them from denaturation or degradation, and maintaining pH and electrolytes at normal levels. Trehalose has been broadly utilized as a protein stabilizer and cryoprotectant, and its nontoxicity and low moisture absorption are suitable for EV preservation. Bosch et al. revealed that particle size distribution narrowed and increased the concentration of EVs per milligram of protein after trehalose treatment [193]. The recovery rate of EVs could be improved in PBS supplemented with human albumin and trehalose at -80 °C [194]. Another practical method includes dimethyl sulfoxide (DMSO) cryopreservation [195]. To sum up, flexible adjustments of additives may generate surprisingly positive outcomes. Lyophilization is an epoch-making technology in the pharmaceutical industry that greatly benefits transportation. The stability of colloidal EVs (lyophilized EV formulations) is significantly enhanced within sucrose or potassium phosphate buffer, while the import of traces of poloxamer 188 would augment this effect [196]. Chemical substances with amplification effect in storage capacity need to be discovered, ranging from native to synthesized compounds.
Multidimensional characterization is the major form of quality control with precision through cross-validation. The entities include producer cells (e.g., phenotypes, vesiculation capacity), isolated EVs (e.g., single-EV analysis, batch heterogeneity), and potential contaminations (e.g., bacteria, viruses, residua of the mediums, reagents) [189]. Testing tools, such as versatile microfluidic chips and advanced nano-flow cytometry for single-EV analysis, are being constantly developed [108, 197]. Lu et al. reported a microarray platform constituted by a series of membrane protein-specific antibodies and microscopic hyperspectral imaging to distinguish EVs. Moreover, by constructing molecular fingerprints, the range of characterization is not limited to EVs, which also incorporates the donor cells [198]. The aspects of quality control include bioactivity, physiological status, purity, and homogeneity. Herein, detection approaches are summarized based on the published literature. A systematic and standardized evaluation system remains to be established, forming consensus in the EV field.
Manufacturing, characterization, and subsequent clinical translation. (A) Refinement in manufacturing is the focus of future research, covering a broad range of aspects from cell culture to storage. Multiple parameters such as temperature and additives have been identified to maintain the biological features of EVs. (B) Characterization enables precise evaluation of EVs and substantially expedites the progress of clinical translation. Pathogen and residue detections are approaches to examining purity and biosafety. The quality control of EV includes particle number, single EV, and components analysis using advanced techniques. UC, ultracentrifugation; DMSO, dimethyl sulfoxide.
Representative clinical trials of EVs
| Trial identifier | Source (product name) | Modifications | Study aim | Recruitment status |
|---|---|---|---|---|
| NCT04652531 | Autologous Serum | -Unmodified | Treatment for venous trophic lesions | Recruiting |
| NCT05499156 | Human placenta MSC | -Unmodified | Safety evaluation of EVs for treating perianal fistula in patients with Crohn's disease | Active, not recruiting |
| NCT04276987 | Allogenic adipose MSC | -Unmodified | Treatment for severe novel coronavirus pneumonia | Completed |
| NCT04202770 | Amniotic fluid | -Delivery aided by focused ultrasound | Therapy of refractory depression, anxiety, and neurodegenerative dementias | Suspended |
| NCT05043181 | Bone marrow MSC | -Loaded with LDL receptor mRNA | Treatment of homozygous familial hypercholesterolemia | Not yet recruiting |
| NCT03608631 | Mesenchymal stromal cells | -Loaded with small interference RNA against KrasG12D | Treating metastatic pancreas cancer with KrasG12D mutation | Recruiting |
| NCT01294072 | Plant | -Loaded with curcumin | Delivering curcumin to normal and colon cancer tissue | Recruiting |
| NCT05375604 | HEK 293 cells (exoASO-STAT6) | -Loaded with STAT6-targeting ASOs | Verifying the antitumor effect of exoASO-STAT6 | Recruiting |
| NCT04592484 | HEK 293 cells (ExoSTING) | -Loaded with STING agonist | Treatment for patients with certain solid tumors | Active, not recruiting |
| NCT01159288 | Dendritic cell | -Loaded with tumor antigen | Vaccination for unresectable NSCLC patients | Completed |
MSC, mesenchymal stem cells; LDL, low-density lipoprotein; STAT6, signal transducer and activator of transcription 6; ASO, antisense oligonucleotide; STING, STimulator of InterferoN Genes; NSCLC, non-small-cell lung cancer.
Application of the EV manipulating strategies in clinical trials
Currently, a multitude of clinical trials have been designed and conducted. Importantly, native EVs (without extra modifications) of diverse origins have found utility in various diseases. To overcome the limitation of the low yield of EVs from autologous or allogenic cells, EVs from HEK293 cells are explored in clinical trial settings (NCT05375604 and NCT04592484). EVs from plant have been also tested in cancer therapy (NCT01294072). Beyond the donor cell source optimization, the strategies of loading have also been successfully adopted in EV functionalization. A sequence of trials sponsored by Codiak BioSciences Inc. have been attempting to confirm that cargoes including STimulator of InterferoN Genes (STING) agonists (NCT04592484) and STAT6-targeting antisense oligonucleotide (ASOs) (NCT05375604) could be loaded into EVs to exert antitumor effects. Our group has designed the first-in-human study (NCT05043181), in which bone marrow-derived MSCs with LDL receptor (LDLR) overexpressed served as parental cells to ensure efficient mRNA loading. Further, with the goal of bolstering delivery efficacy, in an open-label study, researchers would utilize focused ultrasound to facilitate the delivery of EVs derived from amniotic fluid to the subgenual target for patients with refractory depression (NCT04202770). In summary, the strategies toward high yield, facile isolation, improved delivery and efficient loading are just being recognized in the clinical trials (Table 4).
As our knowledge of EVs deepens, we are faithful that a rational design of clinical trials and a throughout analysis of the achieved results will collectively contribute to the clinical translation of EVs. Going forward, we suggest the researchers to give more consideration to the strategy of improved delivery (e.g., decoration of ligands for targeting) when designing a clinical trial.
Conclusion and prospective
EV-based therapy has a bright prospect attributed to the unique structure and composition of EVs. However, the insufficient yield of EVs has restricted their clinical translation. Researchers have made enormous progress with significant advances in mass production, purification, cargo loading, and EV delivery (Figure 1). We believe a rational combination of these strategies would promote the clinical translation for specific diseases. A systematic comparison of the parallel methods for a specific purpose is a prerequisite to choosing the best one. Then, as the strategies might conflict, a disease/patient-specific combination strategy should be explored by small-scale experiments before manufacturing. Finally, the most optimized strategy should be chosen for manufacturing.
Standardization of protocols is crucial for manufacturing [199]. Furthermore, attention must be focused on quality control and the long-term storage of EVs. With technical support from electrical automation and machine learning, an artificially intellectualized workstation is expected, holding tremendous potential to be mined.
Strategies in EV manufacturing and quality control aspects should be performed in a coordinated manner, contributing to the ultimate goal of EV-based therapy. A sequential pattern of “Secretion-Isolation-Purification-Encapsulation/Modulation-Characterization/Quality control-Delivery-Evaluation” could be a conceptual roadmap for clinical translation of EV-based therapy. Manufacturing dominates the steps before “Delivery” while the rest of the flow is assigned to medical workers. Future direction lies in appending branches to this patient-centered route based on feedback response, turning it into a multi-threaded network. Delightedly, a vast number of scientists are already engaged in.
For commercialization and clinical translation of EVs, advanced animal experiments and more clinical trials must be conducted to detect probable adverse effects. Scientists should also execute a comprehensive preclinical examination of pharmacokinetics for EV-based products. On the strength of these strategies, we firmly believe that the pace of clinical translation for EVs can be accelerated markedly.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 81970737, No. 82072182). The study is also funded by Provincial Scientific Foundation of Shaan'Xi (2020TD-038), and Clinical Trial Fund of Tangdu Hospital (2021LCYJ006).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hu H, Feng W, Qian X, Yu L, Chen Y, Li Y. Emerging Nanomedicine-Enabled/Enhanced Nanodynamic Therapies beyond Traditional Photodynamics. Adv Mater. 2021;33:e2005062
2. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941-51
3. Zahednezhad F, Saadat M, Valizadeh H, Zakeri-Milani P, Baradaran B. Liposome and immune system interplay: Challenges and potentials. J Control Release. 2019;305:194-209
4. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541-55
5. van den Boorn JG, Schlee M, Coch C, Hartmann G. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29:325-6
6. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487-514
7. Shirejini SZ, Inci F. The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits. Biotechnol Adv. 2022;54:107814
8. Li YY, Tao YW, Gao S, Li P, Zheng JM, Zhang SE. et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209-20
9. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594-600
10. Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P. et al. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172-7
11. Shi R, Zhao L, Cai W, Wei M, Zhou X, Yang G. et al. Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochem Biophys Res Commun. 2017;483:602-8
12. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383-90
13. Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y. et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926-44
14. Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q. et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546
15. Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lasser C, Segaliny AI. et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano. 2019;13:6670-88
16. Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21:379-99
17. Kamerkar S, Leng C, Burenkova O, Jang SC, McCoy C, Zhang K. et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci Adv. 2022;8:eabj7002
18. Zipkin M. Exosome redux. Nat Biotechnol. 2019;37:1395-400
19. Qi H, Yang L, Li X, Zhan Q, Han D, Zhao J. et al. Exosomes separated based on the “STOP” criteria for tumor-targeted drug delivery. J Mater Chem B. 2018;6:2758-68
20. Kooijmans SAA, Vader P, Schiffelers RM. Tumour-bound RNA-laden exosomes. Nat Biomed Eng. 2017;1:634-6
21. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-78
22. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-20
23. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977
24. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864-9
25. Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244-7
26. Verderio C, Gabrielli M, Giussani P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J Lipid Res. 2018;59:1325-40
27. Wei D, Zhan W, Gao Y, Huang L, Gong R, Wang W. et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31:157-77
28. Wozniak AL, Adams A, King KE, Dunn W, Christenson LK, Hung WT. et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol. 2020;219:e201912074
29. Ageta H, Ageta-Ishihara N, Hitachi K, Karayel O, Onouchi T, Yamaguchi H. et al. UBL3 modification influences protein sorting to small extracellular vesicles. Nat Commun. 2018;9:3936
30. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
31. Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F. et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10:3838
32. Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T. et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4:69-83
33. Guo S, Debbi L, Zohar B, Samuel R, Arzi RS, Fried AI. et al. Stimulating Extracellular Vesicles Production from Engineered Tissues by Mechanical Forces. Nano Lett. 2021;21:2497-504
34. Ruan S, Erwin N, He M. Light-induced high-efficient cellular production of immune functional extracellular vesicles. J Extracell Vesicles. 2022;11:e12194
35. Deng Z, Wang J, Xiao Y, Li F, Niu L, Liu X. et al. Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-beta-induced neurotoxicity. Theranostics. 2021;11:4351-62
36. Zha Y, Lin T, Li Y, Zhang X, Wang Z, Li Z. et al. Exosome-mimetics as an engineered gene-activated matrix induces in-situ vascularized osteogenesis. Biomaterials. 2020;247:119985
37. Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J. et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698-710
38. Guo P, Busatto S, Huang J, Morad G, Moses MA. A facile magnetic extrusion method for preparing endosome-derived vesicles for cancer drug delivery. Adv Funct Mater. 2021;31:2008326
39. Wen Y, Fu Q, Soliwoda A, Zhang S, Zheng M, Mao W. et al. Cell-derived nanovesicles prepared by membrane extrusion are good substitutes for natural extracellular vesicles. Extracellular Vesicle. 2022;1:100004
40. Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S. et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. 2021;19:242
41. Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083-90
42. Edgar JR, Manna PT, Nishimura S, Banting G, Robinson MS. Tetherin is an exosomal tether. Elife. 2016;5:e17180
43. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
44. Minakaki G, Menges S, Kittel A, Emmanouilidou E, Schaeffner I, Barkovits K. et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy. 2018;14:98-119
45. Hessvik NP, Overbye A, Brech A, Torgersen ML, Jakobsen IS, Sandvig K. et al. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell Mol Life Sci. 2016;73:4717-37
46. Kojima R, Bojar D, Rizzi G, Hamri GC, El-Baba MD, Saxena P. et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment. Nat Commun. 2018;9:1305
47. Boker KO, Lemus-Diaz N, Rinaldi Ferreira R, Schiller L, Schneider S, Gruber J. The Impact of the CD9 Tetraspanin on Lentivirus Infectivity and Exosome Secretion. Mol Ther. 2018;26:634-47
48. Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M. et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A. 2011;108:15336-41
49. Reel PS, Reel S, Pearson E, Trucco E, Jefferson E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol Adv. 2021;49:107739
50. Montaner J, Ramiro L, Simats A, Tiedt S, Makris K, Jickling GC. et al. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat Rev Neurol. 2020;16:247-64
51. Lindskrog SV, Prip F, Lamy P, Taber A, Groeneveld CS, Birkenkamp-Demtroder K. et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun. 2021;12:2301
52. Kandimalla R, Aqil F, Alhakeem SS, Jeyabalan J, Tyagi N, Agrawal A. et al. Targeted Oral Delivery of Paclitaxel Using Colostrum-Derived Exosomes. Cancers (Basel). 2021;13:3700
53. Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17:53-69
54. Komori H, Fujita D, Shirasaki Y, Zhu Q, Iwamoto Y, Nakanishi T. et al. MicroRNAs in Apple-Derived Nanoparticles Modulate Intestinal Expression of Organic Anion-Transporting Peptide 2B1/SLCO2B1 in Caco-2 Cells. Drug Metab Dispos. 2021;49:803-9
55. Deng Z, Rong Y, Teng Y, Mu J, Zhuang X, Tseng M. et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol Ther. 2017;25:1641-54
56. Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B. et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9:2359
57. Shi J, Kundrat L, Pishesha N, Bilate A, Theile C, Maruyama T. et al. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc Natl Acad Sci U S A. 2014;111:10131-6
58. Wang X, Xiang Z, Liu Y, Huang C, Pei Y, Wang X. et al. Exosomes derived from Vdelta2-T cells control Epstein-Barr virus-associated tumors and induce T cell antitumor immunity. Sci Transl Med. 2020;12:eaaz3426
59. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-76
60. Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771-5
61. Tang Q, Lu B, He J, Chen X, Fu Q, Han H. et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. 2022;280:121320
62. Zhu D, Li Z, Huang K, Caranasos TG, Rossi JS, Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat Commun. 2021;12:1412
63. Yang PC. Induced Pluripotent Stem Cell (iPSC)-Derived Exosomes for Precision Medicine in Heart Failure. Circ Res. 2018;122:661-3
64. Gao L, Wang L, Wei Y, Krishnamurthy P, Walcott GP, Menasche P. et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med. 2020;12:eaay1318
65. Xiong H, Huang Z, Yang Z, Lin Q, Yang B, Fang X. et al. Recent Progress in Detection and Profiling of Cancer Cell-Derived Exosomes. Small. 2021;17:e2007971
66. Hosseini R, Asef-Kabiri L, Yousefi H, Sarvnaz H, Salehi M, Akbari ME. et al. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol Cancer. 2021;20:83
67. Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K. et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18:10
68. Fan M, Liu H, Yan H, Che R, Jin Y, Yang X. et al. A CAR T-inspiring platform based on antibody-engineered exosomes from antigen-feeding dendritic cells for precise solid tumor therapy. Biomaterials. 2022;282:121424
69. Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1:1074-83
70. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-5
71. Zhang J, Ji C, Zhang H, Shi H, Mao F, Qian H. et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022;8:eabj8207
72. Wang J, Tang W, Yang M, Yin Y, Li H, Hu F. et al. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials. 2021;273:120784
73. Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD. et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell. 2019;176:113-26 e15
74. Li H, Feng Y, Zheng X, Jia M, Mei Z, Wang Y. et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16-30
75. Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L. et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901-19
76. Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H. et al. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics. 2019;9:1714-27
77. Wang C, Xing C, Li Z, Liu Y, Li Q, Wang Y. et al. Bioinspired therapeutic platform based on extracellular vesicles for prevention of arterial wall remodeling in hypertension. Bioact Mater. 2022;8:494-504
78. Yang L, Han D, Zhan Q, Li X, Shan P, Hu Y. et al. Blood TfR+ exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy. Theranostics. 2019;9:7680-96
79. Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL. et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90
80. Qu M, Lin Q, Huang L, Fu Y, Wang L, He S. et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease. J Control Release. 2018;287:156-66
81. Chiangjong W, Netsirisawan P, Hongeng S, Chutipongtanate S. Red Blood Cell Extracellular Vesicle-Based Drug Delivery: Challenges and Opportunities. Front Med (Lausanne). 2021;8:761362
82. Ma Y, Zhang Y, Han R, Li Y, Zhai Y, Qian Z. et al. A cascade synergetic strategy induced by photothermal effect based on platelet exosome nanoparticles for tumor therapy. Biomaterials. 2022;282:121384
83. Johnson J, Wu YW, Blyth C, Lichtfuss G, Goubran H, Burnouf T. Prospective Therapeutic Applications of Platelet Extracellular Vesicles. Trends Biotechnol. 2021;39:598-612
84. Song Y, Li Z, He T, Qu M, Jiang L, Li W. et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. 2019;9:2910-23
85. Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol Ther Nucleic Acids. 2017;7:278-87
86. Zhou X, Li Z, Qi M, Zhao P, Duan Y, Yang G. et al. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10:8197-210
87. Liu Y, Wang Y, Wang C, Shi R, Zhou X, Li Z. et al. Maternal obesity increases the risk of fetal cardiac dysfunction via visceral adipose tissue derived exosomes. Placenta. 2021;105:85-93
88. Wei M, Gao X, Liu L, Li Z, Wan Z, Dong Y. et al. Visceral Adipose Tissue Derived Exosomes Exacerbate Colitis Severity via Pro-inflammatory MiRNAs in High Fat Diet Fed Mice. ACS Nano. 2020;14:5099-110
89. Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63
90. Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111:3100-10
91. Wang N, Liu X, Tang Z, Wei X, Dong H, Liu Y. et al. Increased BMSC exosomal miR-140-3p alleviates bone degradation and promotes bone restoration by targeting Plxnb1 in diabetic rats. J Nanobiotechnology. 2022;20:97
92. Zhang FX, Liu P, Ding W, Meng QB, Su DH, Zhang QC. et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278:121169
93. Mu J, Li L, Wu J, Huang T, Zhang Y, Cao J. et al. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater Sci. 2022;10:1803-11
94. Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W. et al. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019;9:65-76
95. You DG, Lim GT, Kwon S, Um W, Oh BH, Song SH. et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7:eabe0083
96. Xing H, Zhang Z, Mao Q, Wang C, Zhou Y, Zhou X. et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J Nanobiotechnology. 2021;19:264
97. Zhang X, Cai Z, Wu M, Huangfu X, Li J, Liu X. Adipose Stem Cell-Derived Exosomes Recover Impaired Matrix Metabolism of Torn Human Rotator Cuff Tendons by Maintaining Tissue Homeostasis. Am J Sports Med. 2021;49:899-908
98. Li W, Liu Y, Zhang P, Tang Y, Zhou M, Jiang W. et al. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl Mater Interfaces. 2018;10:5240-54
99. Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y. et al. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front Cell Dev Biol. 2020;8:574223
100. Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE. et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319
101. Yuana Y, Levels J, Grootemaat A, Sturk A, Nieuwland R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J Extracell Vesicles. 2014;3:23262
102. Sodar BW, Kittel A, Paloczi K, Vukman KV, Osteikoetxea X, Szabo-Taylor K. et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep. 2016;6:24316
103. Yang B, Chen Y, Shi J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv Mater. 2019;31:e1802896
104. Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A. et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031
105. Yang K, Jia M, Cheddah S, Zhang Z, Wang W, Li X. et al. Peptide ligand-SiO2 microspheres with specific affinity for phosphatidylserine as a new strategy to isolate exosomes and application in proteomics to differentiate hepatic cancer. Bioact Mater. 2022;15:343-54
106. Yang Q, Cheng L, Hu L, Lou D, Zhang T, Li J. et al. An integrative microfluidic device for isolation and ultrasensitive detection of lung cancer-specific exosomes from patient urine. Biosens Bioelectron. 2020;163:112290
107. Sancho-Albero M, Sebastian V, Sese J, Pazo-Cid R, Mendoza G, Arruebo M. et al. Isolation of exosomes from whole blood by a new microfluidic device: proof of concept application in the diagnosis and monitoring of pancreatic cancer. J Nanobiotechnology. 2020;18:150
108. Lu Y, Ye L, Jian X, Yang D, Zhang H, Tong Z. et al. Integrated microfluidic system for isolating exosome and analyzing protein marker PD-L1. Biosens Bioelectron. 2022;204:113879
109. Ding L, Yang X, Gao Z, Effah CY, Zhang X, Wu Y. et al. A Holistic Review of the State-of-the-Art Microfluidics for Exosome Separation: An Overview of the Current Status, Existing Obstacles, and Future Outlook. Small. 2021;17:e2007174
110. Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C. et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114:10584-9
111. Niu F, Chen X, Niu X, Cai Y, Zhang Q, Chen T. et al. Integrated Immunomagnetic Bead-Based Microfluidic Chip for Exosomes Isolation. Micromachines (Basel). 2020;11:503
112. Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X. et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano. 2016;10:3323-33
113. Liu X, Zong Z, Liu X, Li Q, Li A, Xu C. et al. Stimuli-Mediated Specific Isolation of Exosomes from Blood Plasma for High-Throughput Profiling of Cancer Biomarkers. Small Methods. 2022;6:e2101234
114. Dao TNT, Kim MG, Koo B, Liu H, Jang YO, Lee HJ. et al. Chimeric nanocomposites for the rapid and simple isolation of urinary extracellular vesicles. J Extracell Vesicles. 2022;11:e12195
115. Chen Y, Zhu Q, Cheng L, Wang Y, Li M, Yang Q. et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods. 2021;18:212-8
116. Watson DC, Yung BC, Bergamaschi C, Chowdhury B, Bear J, Stellas D. et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles. 2018;7:1442088
117. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-9
118. Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R. et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci U S A. 2017;114:E8987-E95
119. Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980
120. Barman B, Sung BH, Krystofiak E, Ping J, Ramirez M, Millis B. et al. VAP-A and its binding partner CERT drive biogenesis of RNA-containing extracellular vesicles at ER membrane contact sites. Dev Cell. 2022;57:974-94 e8
121. Zhang S, Dong Y, Wang Y, Sun W, Wei M, Yuan L. et al. Selective Encapsulation of Therapeutic mRNA in Engineered Extracellular Vesicles by DNA Aptamer. Nano Lett. 2021;21:8563-70
122. Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R. et al. In vitro and in vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19-28
123. Garcia-Martin R, Wang G, Brandao BB, Zanotto TM, Shah S, Kumar Patel S. et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446-51
124. Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H. et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277
125. Kihm AJ, Kong Y, Hong W, Russell JE, Rouda S, Adachi K. et al. An abundant erythroid protein that stabilizes free alpha-haemoglobin. Nature. 2002;417:758-63
126. Ferreira JV, da Rosa Soares A, Ramalho J, Maximo Carvalho C, Cardoso MH, Pintado P. et al. LAMP2A regulates the loading of proteins into exosomes. Sci Adv. 2022;8:eabm1140
127. Silva AM, Lazaro-Ibanez E, Gunnarsson A, Dhande A, Daaboul G, Peacock B. et al. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J Extracell Vesicles. 2021;10:e12130
128. Kim HK, Cho J, Kim E, Kim J, Yang JS, Kim KC. et al. Engineered small extracellular vesicles displaying ACE2 variants on the surface protect against SARS-CoV-2 infection. J Extracell Vesicles. 2022;11:e12179
129. Jiang W, Ma P, Deng L, Liu Z, Wang X, Liu X. et al. Hepatitis A virus structural protein pX interacts with ALIX and promotes the secretion of virions and foreign proteins through exosome-like vesicles. J Extracell Vesicles. 2020;9:1716513
130. Lazaro-Ibanez E, Faruqu FN, Saleh AF, Silva AM, Tzu-Wen Wang J, Rak J. et al. Selection of Fluorescent, Bioluminescent, and Radioactive Tracers to Accurately Reflect Extracellular Vesicle Biodistribution in vivo. ACS Nano. 2021;15:3212-27
131. Du J, Wan Z, Wang C, Lu F, Wei M, Wang D. et al. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. 2021;11:8185-96
132. Pattni BS, Chupin VV, Torchilin VP. New Developments in Liposomal Drug Delivery. Chem Rev. 2015;115:10938-66
133. Mazzucchelli GD, Cellier NA, Mshviladzade V, Elias R, Shim YH, Touboul D. et al. Pores formation on cell membranes by hederacolchiside A1 leads to a rapid release of proteins for cytosolic subproteome analysis. J Proteome Res. 2008;7:1683-92
134. Orczyk M, Wojciechowski K. Comparison of the effect of two Quillaja bark saponin extracts on DPPC and DPPC/cholesterol Langmuir monolayers. Colloids Surf B Biointerfaces. 2015;136:291-9
135. Sancho-Albero M, Encabo-Berzosa MDM, Beltran-Visiedo M, Fernandez-Messina L, Sebastian V, Sanchez-Madrid F. et al. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11:18825-36
136. Khongkow M, Yata T, Boonrungsiman S, Ruktanonchai UR, Graham D, Namdee K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci Rep. 2019;9:8278
137. Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW. et al. A New Approach for Loading Anticancer Drugs Into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front Pharmacol. 2018;9:1116
138. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z. et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18-30
139. Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35-44
140. Jeyaram A, Lamichhane TN, Wang S, Zou L, Dahal E, Kronstadt SM. et al. Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles. Mol Ther. 2020;28:975-85
141. Xi XM, Xia SJ, Lu R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021;76:61-7
142. Pi F, Binzel DW, Lee TJ, Li Z, Sun M, Rychahou P. et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol. 2018;13:82-9
143. Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001;40:2004-21
144. Kumar GS, Lin Q. Light-Triggered Click Chemistry. Chem Rev. 2021;121:6991-7031
145. Zhang L, Qin Z, Sun H, Chen X, Dong J, Shen S. et al. Nanoenzyme engineered neutrophil-derived exosomes attenuate joint injury in advanced rheumatoid arthritis via regulating inflammatory environment. Bioact Mater. 2022;18:1-14
146. Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238-52
147. Li L, He D, Guo Q, Zhang Z, Ru D, Wang L. et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnology. 2022;20:50
148. Kumar S, Karmacharya M, Michael IJ, Choi Y, Kim J, Kim I. et al. Programmed exosome fusion for energy generation in living cells. Nat Catal. 2021;4:763-74
149. Kim H, Yun N, Mun D, Kang JY, Lee SH, Park H. et al. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem Biophys Res Commun. 2018;499:803-8
150. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503
151. Wan Z, Zhao L, Lu F, Gao X, Dong Y, Zhao Y. et al. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics. 2020;10:218-30
152. Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33:522-31
153. Guo Y, Wan Z, Zhao P, Wei M, Liu Y, Bu T. et al. Ultrasound triggered topical delivery of Bmp7 mRNA for white fat browning induction via engineered smart exosomes. J Nanobiotechnology. 2021;19:402
154. Lathwal S, Yerneni SS, Boye S, Muza UL, Takahashi S, Sugimoto N. et al. Engineering exosome polymer hybrids by atom transfer radical polymerization. Proc Natl Acad Sci U S A. 2021;118:e2020241118
155. Kozma GT, Shimizu T, Ishida T, Szebeni J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154-155:163-75
156. Kozma GT, Meszaros T, Vashegyi I, Fulop T, Orfi E, Dezsi L. et al. Pseudo-anaphylaxis to Polyethylene Glycol (PEG)-Coated Liposomes: Roles of Anti-PEG IgM and Complement Activation in a Porcine Model of Human Infusion Reactions. ACS Nano. 2019;13:9315-24
157. Roth GA, Picece V, Ou BS, Luo W, Pulendran B, Appel EA. Designing spatial and temporal control of vaccine responses. Nat Rev Mater. 2022;7:174-95
158. Wu G, Zhang J, Zhao Q, Zhuang W, Ding J, Zhang C. et al. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew Chem Int Ed Engl. 2020;59:4068-74
159. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-35
160. Nie H, Xie X, Zhang D, Zhou Y, Li B, Li F. et al. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale. 2020;12:877-87
161. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-95
162. Wang H, Wang B, Zhang A, Hassounah F, Seow Y, Wood M. et al. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol Ther. 2019;27:571-83
163. Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C. et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137-49
164. Flint PW, Li ZB, Lehar M, Saito K, Pai SI. Laryngeal muscle surface receptors identified using random phage library. Laryngoscope. 2005;115:1930-7
165. Hung ME, Leonard JN. Stabilization of exosome-targeting peptides via engineered glycosylation. J Biol Chem. 2015;290:8166-72
166. Gao X, Ran N, Dong X, Zuo B, Yang R, Zhou Q. et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018;10:eaat0195
167. Tolmachev V, Orlova A. Affibody Molecules as Targeting Vectors for PET Imaging. Cancers (Basel). 2020;12:651
168. Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A. et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017;7:1333-45
169. Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomedicine. 2018;13:585-99
170. Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, Zhang Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J Am Chem Soc. 2018;140:16413-7
171. Osborne SE, Ellington AD. Nucleic Acid Selection and the Challenge of Combinatorial Chemistry. Chem Rev. 1997;97:349-70
172. Luo ZW, Li FX, Liu YW, Rao SS, Yin H, Huang J. et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11:20884-92
173. Powell Gray B, Kelly L, Ahrens DP, Barry AP, Kratschmer C, Levy M. et al. Tunable cytotoxic aptamer-drug conjugates for the treatment of prostate cancer. Proc Natl Acad Sci U S A. 2018;115:4761-6
174. Zou J, Shi M, Liu X, Jin C, Xing X, Qiu L. et al. Aptamer-Functionalized Exosomes: Elucidating the Cellular Uptake Mechanism and the Potential for Cancer-Targeted Chemotherapy. Anal Chem. 2019;91:2425-30
175. Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG. et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8:1869-78
176. Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL. et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14:195-204
177. Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J. et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J Am Heart Assoc. 2018;7:e008737
178. Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F. et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018;14:1973-85
179. Han Q, Xie QR, Li F, Cheng Y, Wu T, Zhang Y. et al. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics. 2021;11:6526-41
180. Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1153-66
181. Sun W, Li Z, Zhou X, Yang G, Yuan L. Efficient exosome delivery in refractory tissues assisted by ultrasound-targeted microbubble destruction. Drug Deliv. 2019;26:45-50
182. Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol. 2001;11:406-12
183. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857-902
184. Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100-8
185. Aminzadeh MA, Fournier M, Akhmerov A, Jones-Ungerleider KC, Valle JB, Marban E. Casein-enhanced uptake and disease-modifying bioactivity of ingested extracellular vesicles. J Extracell Vesicles. 2021;10:e12045
186. Zhan Q, Yi K, Li X, Cui X, Yang E, Chen N. et al. Phosphatidylcholine-Engineered Exosomes for Enhanced Tumor Cell Uptake and Intracellular Antitumor Drug Delivery. Macromol Biosci. 2021;21:e2100042
187. Shimoda A, Miura R, Tateno H, Seo N, Shiku H, Sawada SI. et al. Assessment of Surface Glycan Diversity on Extracellular Vesicles by Lectin Microarray and Glycoengineering Strategies for Drug Delivery Applications. Small Methods. 2022;6:e2100785
188. Wang Z, Popowski KD, Zhu D, de Juan Abad BL, Wang X, Liu M. et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat Biomed Eng. 2022;6:791-805
189. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748-59
190. Evtushenko EG, Bagrov DV, Lazarev VN, Livshits MA, Khomyakova E. Adsorption of extracellular vesicles onto the tube walls during storage in solution. PLoS One. 2020;15:e0243738
191. Lorincz AM, Timar CI, Marosvari KA, Veres DS, Otrokocsi L, Kittel A. et al. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J Extracell Vesicles. 2014;3:25465
192. Maroto R, Zhao Y, Jamaluddin M, Popov VL, Wang H, Kalubowilage M. et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J Extracell Vesicles. 2017;6:1359478
193. Bosch S, de Beaurepaire L, Allard M, Mosser M, Heichette C, Chretien D. et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. 2016;6:36162
194. Gorgens A, Corso G, Hagey DW, Jawad Wiklander R, Gustafsson MO, Felldin U. et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J Extracell Vesicles. 2022;11:e12238
195. Tegegn TZ, De Paoli SH, Orecna M, Elhelu OK, Woodle SA, Tarandovskiy ID. et al. Characterization of procoagulant extracellular vesicles and platelet membrane disintegration in DMSO-cryopreserved platelets. J Extracell Vesicles. 2016;5:30422
196. Trenkenschuh E, Richter M, Heinrich E, Koch M, Fuhrmann G, Friess W. Enhancing the Stabilization Potential of Lyophilization for Extracellular Vesicles. Adv Healthc Mater. 2022;11:e2100538
197. Liu H, Tian Y, Xue C, Niu Q, Chen C, Yan X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J Extracell Vesicles. 2022;11:e12206
198. Wang Y, Zhang Q, Yuan W, Wang Y, Loghry HJ, Zhao Z. et al. Hyperspectral imaging-based exosome microarray for rapid molecular profiling of extracellular vesicles. Lab Chip. 2021;21:196-204
199. Piffoux M, Volatron J, Cherukula K, Aubertin K, Wilhelm C, Silva AKA. et al. Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability. Adv Drug Deliv Rev. 2021;178:113972
Author contact
![]() Corresponding authors: E-mail: yanggdedu.cn (Guodong Yang); yuanljedu.cn (Lijun Yuan); songbq2012com (Baoqiang Song).
Corresponding authors: E-mail: yanggdedu.cn (Guodong Yang); yuanljedu.cn (Lijun Yuan); songbq2012com (Baoqiang Song).
 Global reach, higher impact
Global reach, higher impact