13.3
Impact Factor
Theranostics 2022; 12(15):6665-6681. doi:10.7150/thno.75444 This issue Cite
Research Paper
Design of stapled peptide-based PROTACs for MDM2/MDMX atypical degradation and tumor suppression
1. School of Medicine or Institute of Translational Medicine, Shanghai University, Shanghai 200444, China.
2. School of Pharmacy, Second Military Medical University, Shanghai 200433, China.
3. Key Laboratory of Medical Molecular Virology (MOE/NHC/CAMS), School of Basic Medical Sciences, Fudan University, Shanghai 200030, China.
4. The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, 710049, China.
5. Institute of Bioengineering, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang 310027, China.
#These authors contributed equally to this work.
Abstract
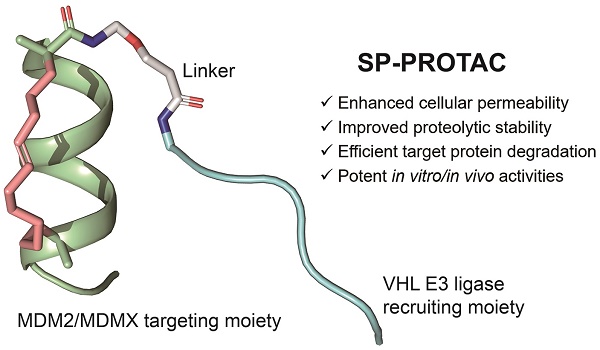
Rationale: Although stapled peptides offer a powerful solution to overcome the susceptibility of linear peptides to proteolytic degradation and improve their ability to cross membranes, an efficient and durable disease treatment strategy has not yet been developed due to the inevitable elimination of peptide inhibitors and rapid accumulation of target proteins.
Methods: Herein we developed stapled peptide-based proteolysis-targeting chimeras (SP-PROTACs), that simultaneously exhibited improved cellular uptake and proteolytic stability attributed to the stapled peptides, and efficient target protein degradation promoted by the PROTACs. Based on the PMI peptide with dual specificity for both MDM2 and MDMX, a series of SP-PROTACs were designed.
Results: Among them, the optimized SPMI-HIF2-1 exhibited similar binding affinity with MDM2 and MDMX but obviously higher helical contents, improved proteolytic stability, better cellular permeability, and a better pharmacokinetic profile compared with its linear counterpart. Importantly, SPMI-HIF2-1 could effectively kill cancer cells and inhibit tumor progression in subcutaneous and orthotopic colorectal cancer xenograft models through simultaneously promoting the atypical degradation of both MDM2 and MDMX and durable p53 activation. An FP-based binding assay and structural modeling analysis of the ternary complex suggested that SPMI-HIF2-1 simultaneously bound with the target protein and E3 ligase.
Conclusion: Our findings not only provide a new class of anticancer drug candidates, but also bridge the gap and reduce the physical distance between peptides and PROTACs.
Keywords: MDM2/MDMX, p53, stapled peptide, PROTAC, anticancer
 Global reach, higher impact
Global reach, higher impact