13.3
Impact Factor
Theranostics 2022; 12(15):6646-6664. doi:10.7150/thno.76574 This issue Cite
Research Paper
Dual-targeted magnetic mesoporous silica nanoparticles reduce brain amyloid-β burden via depolymerization and intestinal metabolism
1. Britton Chance Center for Biomedical Photonics, Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan, China.
2. MoE Key Laboratory for Biomedical Photonics, School of Engineering Sciences, Huazhong University of Science and Technology, Wuhan, China.
3. School of Biomedical Engineering, Hainan University, Haikou, Hainan 570228, China.
Abstract
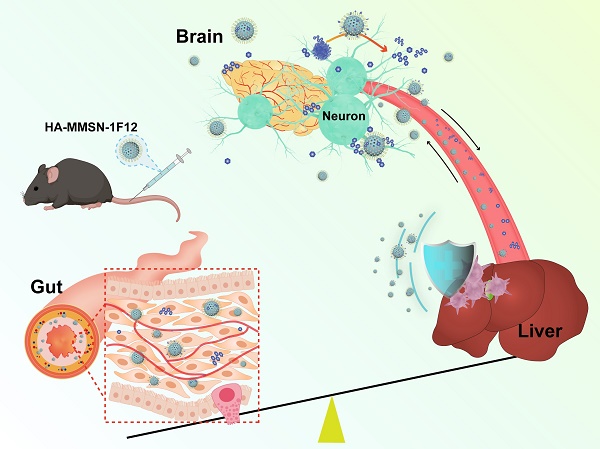
Rationale: Active removal of excess peripheral amyloid-β (Aβ) can potentially treat Alzheimer's disease (AD). However, the peripheral clearance of Aβ using an anti-Aβ monoclonal antibody (mAb) cannot remove PET-detectable Aβ within the brain. This may be due to the inability of mAb to cross the blood-brain barrier (BBB) to degrade insoluble brain Aβ plaques and block liver dysfunction.
Methods: We developed a dual-targeted magnetic mesoporous silica nanoparticle (HA-MMSN-1F12) through surface-coupled Aβ42-targeting antibody 1F12 and CD44-targeting ligand hyaluronic acid (HA).
Results: HA-MMSN-1F12 had a high binding affinity toward Aβ42 oligomers (Kd = 1.27 ± 0.34 nM) and revealed robust degradation of Aβ42 aggregates. After intravenous administration of HA-MMSN-1F12 into ten-month-old APP/PS1 mice for three weeks (4 mg/kg/week), HA-MMSN-1F12 could cross the BBB and depolymerize brain Aβ plaques into soluble Aβ species. In addition, it also avoided hepatic uptake and excreted captured Aβ species through intestinal metabolism, thereby reducing brain Aβ load and neuroinflammation and improving memory deficits of APP/PS1 mice. Furthermore, the biochemical analysis showed that HA-MMSN-1F12 did not detect any toxic side effects on the liver and kidney. Thus, the efficacy of HA-MMSN-1F12 is associated with the targeted degradation of insoluble brain Aβ plaques, avoidance of non-specific hepatic uptake, and excretion of peripheral Aβ through intestinal metabolism.
Conclusions: The study provides a new avenue for treating brain diseases by excreting disease-causing biohazards using intestinal metabolism.
Keywords: Alzheimer's disease, amyloid-β (Aβ), magnetic mesoporous silica nanoparticles, Aβ clearance, Aβ42-targeting antibody.
 Global reach, higher impact
Global reach, higher impact