13.3
Impact Factor
Theranostics 2022; 12(15):6548-6575. doi:10.7150/thno.74305 This issue Cite
Review
Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications
1. Jiangsu Key Laboratory of Medical Science and Laboratory Medicine, Institute of Stem Cells, School of Medicine, Jiangsu University, Zhenjiang, China.
2. Department of Clinical Laboratory, The Affiliated Yixing Hospital of Jiangsu University, Yixing, China.
3. Aoyang Institute of Cancer, Affiliated Aoyang Hospital of Jiangsu University, 279 Jingang Road, Suzhou, Jiangsu, China.
#These authors contributed equally to this work.
Received 2022-4-22; Accepted 2022-8-25; Published 2022-9-6
Abstract
Extracellular vesicles, especially small extracellular vesicles (sEVs) are now accepted as important messengers in cell-to-cell communication and as a promising drug delivery platform. They are involved in nearly all physiological and pathological processes and are involved in disease diagnosis and therapy. However, their heterogeneity of physicochemical properties and functions is not fully understood, which hinders further clinical applications. To obtain highly bioactive sEVs with both high yield and purity, will certainly facilitate their future study and application. This review informs up-to-date research on frequently-used and cutting-edge technologies of sEVs isolation and makes a deep comparison and analysis of different methods, including their advantages, limitations and applications. Pending questions about the inherent property of these small vesicles as well as isolation strategies are discussed. Additionally, an overview of their applications in disease diagnosis and treatment, including some of the on-going clinical trials, are also reviewed.
Keywords: Small extracellular vesicles, exosomes, isolation techniques, clinical applications
Introduction
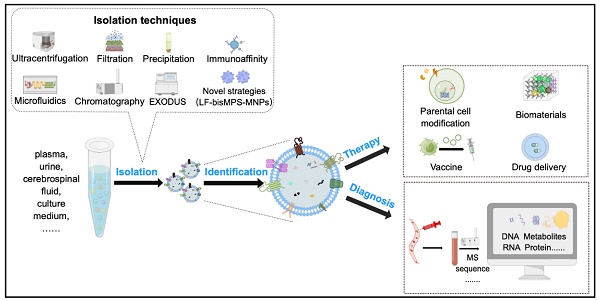
Extracellular vesicles (EVs) are a heterogeneous group of membrane-structured vesicles that are actively released by almost all types of cells and are found in various human body fluids such as blood, urine, saliva and ascites. Small extracellular vesicles (sEVs) usually refers to EVs smaller than 200 nm in diameter, and are the representative EV types most widely studies for their roles in different physiological and pathological conditions. They are broadly reported to transfer bioactive components (nucleic acids and proteins) from donor to recipient cells, thus mediating information exchange between cells [1]. A growing number of studies have shown that sEVs play an important part in occurrence, diagnosis, and treatment of diseases and also as a new nano-platform for drug delivery (Figure 1). However, there are several challenges that still exist for the clinical applications of sEVs. For example, there is currently no standardization in the techniques for storage [2], dosage, and administration of sEVs [3]. More importantly, the heterogeneity in the physicochemical properties and functions of sEVs is not fully understood. Additionally, the biological fluids where the sEVs circulate also contains various particles with properties overlapping those of the sEVs. Therefore, particular isolation techniques are of great importance since they are largely related to the physicochemical properties and contents of sEVs [4]. Although EV separation methods are constantly updated, most of the currently available isolation techniques usually do not guarantee the purity and yield of the EVs at the same time; meanwhile, destruction of vesicle integrity is a risk that may hinder the accuracy of subsequent experiments. Some isolation techniques are multi-step and time-consuming, with low repeatability, which makes them unable to meet the actual clinical requirement [5, 6]. In this review, we will focus on the existing isolation technologies in detail, discussing frequently-used as well as novel methods, and analyze their advantages and disadvantages. In addition, the challenges and future directions for research and clinical applications are also discussed. This review is aimed to provide the audience, whether an experienced researcher or a new hand in the field of EVs, with a full-scale understanding of sEVs isolation strategies to further facilitate their study and the future translational applications from bench to bedside.
EV Characteristics, Biogenesis, and Cargos
EVs are lipid bilayer-encapsulated nanoparticles with a size of 50-1000 nm [7] that are present in different biological fluids [8], such as blood [9], urine [6], cerebrospinal fluid [10] and others [11]. The obtained EVs may contain proteins, nucleic acids, lipids and metabolites [12], which might be similar or different from that of their cells of origin. The components can vary depending on distinct regulatory sorting mechanisms of their producing cells [13]. EVs can transfer these contents from donor to recipient cells, mediating information exchange between cells [14, 15]. EVs are shown to be highly heterogeneous in both structures and biological functions [16]. The classification criteria for the subtypes of EVs have not yet been unified. According to MISEV2018 [17], EVs can be divided into medium/large EVs (>200 nm) and small EVs (<200 nm) based on their physical properties of EVs. EVs can also be classified into apoptotic bodies (50-1000 nm in diameter), microvesicles (MVs) (100-1000 nm), and exosomes (40-160 nm, average~ 100 nm) based on their origin. Another way to classify EVs is according to their biological composition such as the presence of the surface protein CD63 [18]. Additionally, prevailing conditions are used to distinguish EVs as large oncosomes, hypoxic EVs, and podocyte EVs. Beyond that, as of now, there are still many EV particles whose functions and contents are undiscovered, and therefore, need to be characterized.
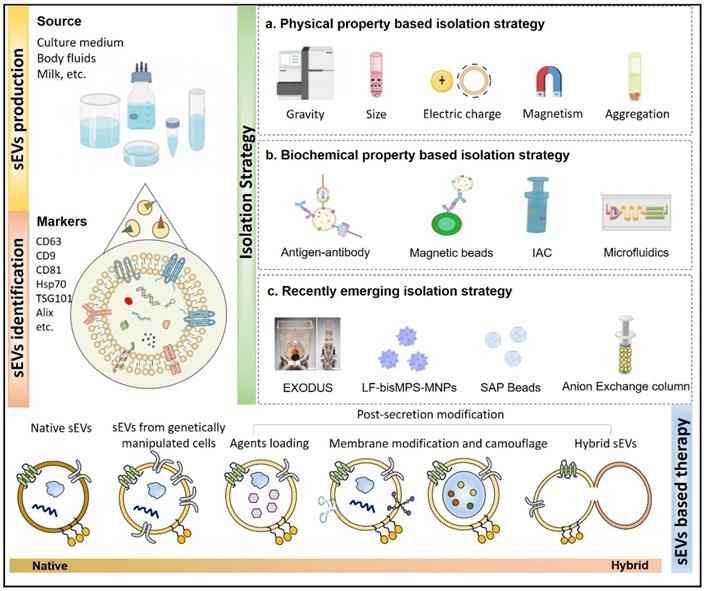
Isolation and modification of sEVs. sEVs can be isolated from cell or tissue culture medium, body fluids such as blood, urine, hydrothorax, ascites, milk, even beer and juice from plants. The isolated sEVs, particularly exosomes are usually found to express markers like CD63, CD81, CD9, Hsp70, TSG101, Alix and negatively express proteins such as calnexin. The existing methods developed to separate these vesicles are generally based on their physical or biochemical properties. Natural sEVs as well as tailored vesicles can bring great potential in disease treatment.
Biogenesis and Cellular uptake of EVs. Apoptotic bodies are formed by membrane folding, invagination and shedding with organelles and nuclear debris. MVs are formed by directly outward budding of plasma membranes. As for exosomes, firstly, the invagination of the plasma membrane forms a cup-shaped structure that includes cell surface proteins and some components such as proteins, lipids, and metabolites in the extracellular environment, that is, early sorting endosomes (ESE). ESE then develops into late sorting endosomes (LSEs), which invaginate to form intraluminal vesicles (ILVs), while components in the cytoplasm also enter the ILVs, and then LSEs form multivesicular bodies (MVBs). Finally, the MVBs fuse with the plasma membrane and release exosomes. The released exosomes taken up by recipient cells mainly through three ways: (1) Exosomes bind to cell membrane surface receptors. (2) Exosomes fuse directly with the cell membrane to release the contents. (3) Exosomes directly enter the cytoplasm in a complete form through cell pinocytosis or phagocytosis [19]. The complex biogenesis, selection and transfer mechanism are responsible for the high heterogeneity of sEVs, which brings uncertainty and challenges to the standardization of isolation methods.
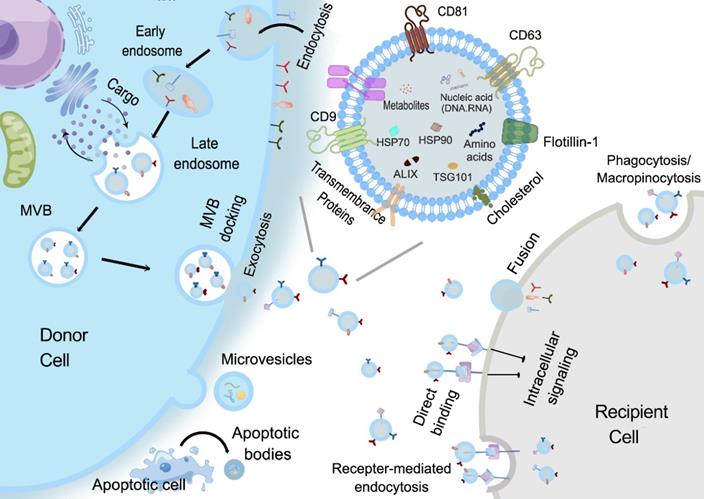
With respect to the biogenesis of EVs, apoptotic bodies are released by dying cells, which are seldomly used for study possibly due to their large and uneven particle size. MVs are formed by the direct outward budding of plasma membranes [18]. Presently, most studies are focused on the potential of sEVs, especially exosomes in regenerative medicine. The specific process of exosomes biogenesis is recognized as a “swallow and spit” process. At the very beginning, the invagination of the plasma membrane forms a cup-shaped structure termed early sorting endosome (ESE) containing cell surface proteins and other biological substances (e.g., proteins, lipids, and metabolites). ESE then develops into late sorting endosomes (LSEs), which invaginates to form intraluminal vesicles (ILVs), packaging cytoplasmic contents. LSEs then form multivesicular bodies (MVBs) that finally fuse with the plasma membrane and release the exosomes [19]. Compared with other types of EVs, exosomes are smaller in size and have specific markers such as CD9, CD63, CD81, HSP70, HSP90, Flotillin 1 and TSG101 [20, 21] (Figure 2). Given that latest guidelines suggest the use of “EVs” to generally denote a heterogeneous extracellular vesicle population, and “exosomes” are defined as small extracellular vesicles that are released upon the exocytosis of MVBs filled with ILVs, in this review, the general term “small extracellular vesicles (sEVs)” in this review will be used as defined in the latest MISEV guideline.
Structurally, the sEVs phospholipid membrane bilayer provides a natural protection for their cargos and makes them highly biocompatible and favorable for cell-cell communication [22]. The exposed phosphatidylserine regulates various pathophysiological processes, including inflammation, immune responses, coagulation, and neuronal regeneration [23], while the glycoconjugates (including proteoglycans and glycoproteins) participate in cell growth, migration, differentiation, tumor invasion, host-pathogen interactions, and transmembrane signaling [24]. Moreover, some membrane proteins can also be inherited from their parent cells thus maintaining certain targeting properties. The specific cargos transported by the sEVs can include proteins, nucleic acids and metabolites, which could reflect the status of their parental cells [25]. Although the exact mechanisms associated with distinct cargo sorting in sEVs are still unclear, several possible ways for contents loading have been discovered. Proteins can be sorted into MVBs by the regulation of tetraspanin-enriched microdomains or in an ubiquitin-dependent manner with the assistance of endosomal sorting complex required for transport (ESCRT). RNAs are shown to gain entrance with the help of factors such as Ago2, hnRNPA2B1, HuRand adenylation at the 3' end of miRNAs. In short, the protective and partial targeting abilities of sEVs, as well as their dynamic and specific cargo, bestow on them the potential to be ideal candidates for disease diagnosis and therapy [25-28].
EV separation techniques
Given their multiple functions and clinical translation potential, to obtain sEVs with high yield and quality is of great significance. Currently, many techniques have been developed for sEVs separation which largely dependent on their biophysical and/or biochemical traits, such as the size, density, shape as well as specific surface markers. Both the inspection or research requirements and the complexity of the biological fluids where sEVs circulating should be taken into careful consideration when one particular method is chosen for the vesicle isolation. As for the complexity of samples, many non sEVs interferences such as lipoprotein in plasma, uromodulin (Tamm-Horsfall protein) in urine and surfactants bronchoalveolar lavage fluid [17], show the potential to co-isolate with sEVs to influence the subsequent observation. Specific clinical or research demands should also be considered. When using size exclusion chromatography (SEC) method, the product may be contaminated by abundant serum proteins but the yield is high, which makes SEC a suitable way for research that requires more on quantity, such as RNA analysis [29]. Plasma samples used for liquid biopsy requires small sample volume with high yield [30]. A batch of commercial kits based on precipitation thus become good options. Moreover, the selection of isolation technique also affects the structural integrity and functional activity of sEVs [31]. For example, sEVs isolated by different methods (e.g. ultracentrifugation and SEC) show discrepant function in endothelial cell migration [31]. Therefore, to choose the most appropriate method to isolate sEVs and even the subpopulations can allow better understanding of the vesicle biology and function before their clinical translation. Ideal isolation strategy with high-purity, high-yield, structural and functional integrality is still urgently needed [22, 32]. The most frequently-used and cutting-edge sEVs isolation techniques will be discussed in detail in the following sections (Table 1).
Comparison of sEVs isolation methods
| Strategy | Principle | Time | Purity | Advantages | Disadvantages | Sample | References |
|---|---|---|---|---|---|---|---|
| Differential ultracentrifugation | According to particle density, size and shape | >4 h | Medium (with the coprecipitation and non-exosome contaminants) | Simple operation, low cost, suitable for large samples and high yield | Low repeatability, long time-consuming and destroying the integrity of sEVs | Plasma, urine, culture medium | [136] |
| Density gradient centrifugation | Mainly based on particle density | >16 h | High | Improved purity compared to UC | complex operation | Plasma, urine, culture medium | [37, 49] |
| Rate zone ultracentrifugation | Mainly based on particle size | >16 h | High | Improved purity compared to UC | The operation must strictly control the time | Plasma, urine, culture medium | [46] |
| Size exclusion chromatography | Porous stationary phase for separation by particle size | 0.3 h | High | Maintain sEVs integrity, high yield and simple operation | Suitable for low upper limit of sample volume, need to be combined with other methods, high equipment cost and long time-consuming | Plasma, urine, Culture medium, cerebrospinal fluid (Universal for almost all biological fluids) | [56, 60, 65, 136, 137] |
| Precipitation | Changing the solubility and dispersibility of particles by using hydrophilic polymers | 0.3-12 h | Low | High yield, simple operation, suitable for large samples | Low purity (affected by polymer) | Culture medium | [63, 138, 139] |
| Ultrafiltration | Using filtration membranes, the separation is based on particle size. Particles flow vertically to the membrane (vertical flow) | Generally <4 h | Low | Short time, simple operation, no need for equipment and additional separation reagents | Lower purity and higher rate of consumables (particles may clog the filtration membrane) | Fetal bovine serum, culture medium, Urine (10 kDa MWCO), Plasma (50 kDa MWCO) | [37, 61, 140] |
| Circulating tangential flow filtration (TFF) system | Compared to the TFF, there is an additional peristaltic pump that sends the flow to the membrane into a continuous loop. | - | High | Compared with the improved purity of UC, the isolated sEVs have higher biological activity | Adaptability to various types of biological fluids (such as plasma) is unclear | Culture medium | [78] |
| Hydrostatic filtration dialysis | Filtration-Concentration-Dialysis | - | - | Suitable for large samples. Compared with UC, the purity is improved, the sample loss is reduced, the yield is improved, and the operation is simple. | Efficiency may decrease when sample volume is greater than 200 mL | Urine, Culture medium | [79, 141] |
| Combined Phospholipid Affinity Method | Harnessing specific interactions between metal and phosphate groups on lipid bilayers | 3-5 h | Medium | Less time-consuming than UC, with comparable purity | May also clog the filter membrane | urine | [81] |
| AF4 | Cross flow perpendicular to the parabolic flow pattern, separated by particle size | 4-5 h | High | Maintain sEVs integrity, high purity, and reproducibility | High requirements for equipment and operators, not suitable for large samples | Culture medium UF and SEC purified EVs from urine Plasma and serum (350 μm spacer, 10 kDa regenerated cellulose membrane) | [38, 142] |
| IAC-AsFlFFF system | Cross flow perpendicular to the parabolic flow pattern, separated by particle size | 4-6 h | High | Highly reproducible, automated, and can process multiple samples simultaneously | Suitability for other samples is unclear | Plasma (350μm spacer, 10 kDa regenerated cellulose membrane) | [72] |
| AF4/UV-MALS | Cross flow perpendicular to the parabolic flow pattern, separated by particle size | 4-6 h | High | high repeatability | Suitability for other samples is unclear | Urine | [70] |
| Immunoaffinity capture technology | Specific binding of capture molecules to sEVs surface markers | 4-20 h | High | High purity to isolate specific sEVs subtypes | Low yield, high cost, disrupts sEVs biological function | Plasma, culture medium | [38, 139, 143] |
| Label-free microfluidics | Mainly chip technology designed according to sEVs physical properties (acoustic, electrical.) | - | High | Guaranteed sEVs integrity, simple operation, low cost, high repeatability, and broad application prospects | Still exploring | Plasma, culture medium | [109] |
| Synthetic peptide (Vn96) based isolation method | Specific affinity of Vn96 and HSP | - | High | High efficiency, high output, low cost, high versatility | Still exploring | Plasma, urine, culture medium and animal plasma | [119] |
| Chromatography-Based Systems | Separation based on the negative Zeta potential of the sEVs surface | - | - | Simple operation, adapts to a wide range of sample volumes, and maintains sEVs integrity | Susceptible to charged species in different biological fluids. | Culture medium | [144] |
| Magnetic bead-based ion exchange technology | ditto | - | - | ditto | ditto | Culture medium | [95] |
| Separation technology based on chitosan | ditto | - | - | ditto | ditto | Culture medium, Urine, Saliva | [104] |
| EXODUS | Introducing double-coupled harmonic oscillations into a double-film filter configuration to generate shear waves, separated primarily by particle size | - | High | Short time-consuming, relatively high yield and purity, suitable for a wide range of sample volumes, maintaining sEVs integrity, low cost, and scalability. | exploring | Plasma, urine, saliva, culture medium, tears | [122] |
| Separation technology based on chimeric nanocomposites | Physical absorption, electrostatic interactions, and biometric interactions | - | High | Higher yield and purity, better biological integrity, no need for expensive equipment | It's hard to completely distinguish it from other types of EVs. | Culture medium, urine | [123] |
| Based on SAP technology | Separation according to the water absorption properties of SAP | - | Low | Improve the sensitivity of liquid biopsies and preserve sEVs integrity | This technology is mainly concentrated, and the separation purity is low | Culture medium, urine | [129] |
| Anion exchange method | Separation based on negative surface charge of sEVs, elution at low NaCl concentration | - | High | High purity, high biological activity, high yield | unknown | Culture medium | [133] |
Ultracentrifugation (UC)
Differential Ultracentrifugation
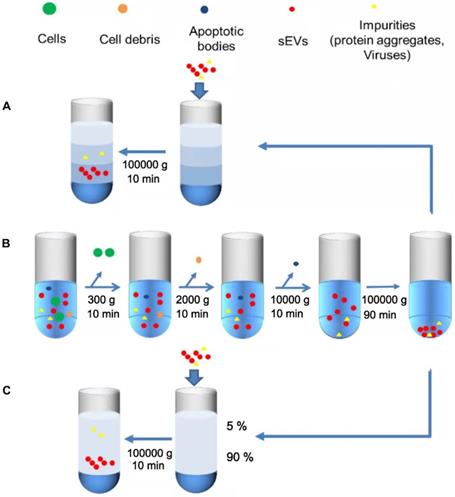
Differential ultracentrifugation is the most commonly used “gold standard” technique for sEVs isolation [30], which involves the fractionation and separation of substances with different densities and sizes by using different centrifugal speeds and forces (Figure 3B). The first few simple steps are performed to remove dead cells, cell debris, and large extracellular vesicles [22]. The pellet thus obtained is resuspended in PBS, and a final ultracentrifugation step is performed to eliminate contaminating proteins. Centrifugation speed is selected according to the experimental requirements, and the temperature is maintained at 4 °C throughout the process to ensure that protease, DNase, and RNase is inactive [33]. Finally, the characterization analysis of sEVs can be performed [34]. This technique is simple to operate [35], low-cost, does not require extensive expertise or additional materials, which makes it reproducible and suitable for large-volume samples. Ye et al. has performed a characterization experiment using flow cytometry and showed that when sEVs were separated from plasma, UC had the highest isolation purity compared to other size- based or precipitation methods. Therefore, UC can be preferentially selected when separating sEVs from plasma [36]. However, the results obtained by ultracentrifugation are relatively difficult to control, and easy to be affected by the type of biological material, the specific type of rotor, and the centrifugation time [37]. In particular, when these features are not properly documented, the comparison between studies will not be convincing [38]. Other limitations exist such as long time-consumption [39] and large output variation, which may also be affected by different operations [40]. Under the external force of high-speed rotation, the structural and biological integrity of sEVs could be impaired [41]. The quantitative and qualitative variation of samples could affect the accuracy of subsequent observations [42]. The low particle recovery rate also makes this method unsuitable for small sample separations [43, 44]. More importantly, while differential ultracentrifugation is now recognized as a high-purity isolation strategy, there is a possibility of co-segregation of contaminants, such as residual soluble protein [31, 40] and enriched particles with indistinguishable density or size, including microvesicles, non-vesicles, protein aggregates, and lipoproteins [45, 46]. This method has been further improved by using isopycnic gradient ultracentrifugation. Another study described a new optimization method based on diluting serum with PBS to reduce viscosity, and prolonging the first UC cycle, followed by four more UC cycles. This approach was experimentally shown to remove 95% of serum proteins with no significant loss of sEVs when compared with size exclusion chromatography (SEC), along with providing sEVs of high purity [29].
Simplified illustration of ultracentrifugation of sEVs. A. Isopycnic density gradient centrifugation. First, the samples are centrifuged at 300 ×g, 2000 ×g and 10,000 ×g to remove larger cells, cell debris and dead cells. Secondly, the sEVs are isolated by ultracentrifugation twice at a speed of more than 100,000 ×g. B. Differential Ultracentrifugation. Impurities are firstly removed by low-speed centrifugation (such as UC), and then the separated samples are added to the constructed density medium (3%, 35%, 45%, 90%) for separation. C. Rate zone ultracentrifugation. Construct high-density media (90%) at the bottom of the test tube as a buffer, operating steps are similar to DGC.
Isopycnic density gradient centrifugation
Isopycnic density gradient centrifugation, an improvement on differential ultracentrifugation, is a density-based isolation technique that uses a density gradient tube and is based on the principle that objects with a specific density will remain suspended in a liquid layer with similar density after centrifugation [47] (Figure 3A). Isodensity centrifugation is a good solution for the problem of co-precipitation that is caused by overlapping physical properties when using UC for sEVs isolation. By constructing a density gradient medium, such as a sucrose medium, which gradually increases from top to the bottom of the centrifuge tube, sEVs settle along with the corresponding isodensity area under centrifugal force, and most contaminants are thus removed [22]. Although both the purity and isolation efficiency are improved when compared with UC [48], isodensity centrifugation still possesses several disadvantages, such as the relatively complicated operation and expensive equipment [37]. Suchi Gupta and colleagues have proposed a one-step sucrose cushion-buffered centrifugation (SUC) method, where body fluid or cell supernatant are added directly onto the sucrose cushion, thereby removing the previous preconcentration step, and then the sucrose cushion is collected and centrifuged to obtain sEVs after dilution with PBS. This method can improve the yield and integrity of sEVs produced [49]. Kang Li et al. improved the existing method by proposing cushioned-density gradient ultracentrifugation (C-DGUC), where iodixanol buffer is used as a density gradient medium for the concentration of sEVs, which are further isolated by density ultracentrifugation. The iodixanol buffer can better maintain the physical and biological integrity of sEVs better. Moreover, as iodixanol is biologically inert and compatible, it need not be removed, eliminating an additional step. C-DGUC greatly improves sEVs yield and purity, and it has been demonstrated that sEVs can be extracted from plasma and urine, which is promising for clinical research and diagnosis [42].
Rate zone ultracentrifugation
Compared with isodensity ultracentrifugation, rate zone ultracentrifugation (RZC) is mainly based on particle diameter and can be used to separate particles with the same density but different diameters (Figure 3C) [50]. For example, RZC can separate platelets from EV fractions [51]. RZC has previously been used to isolate viruses, DNA [52], and other nanoparticles [51]. The density of the medium in the RZC centrifuge tube should be a linear gradient from top to bottom of the tube, which is lower than that of the experimental sample. In addition, the linear gradient must be more viscous than the samples to prevent any mixing with the gradient when loading the sample, thus ensuring that the distance and speed of the particles moving in the centrifuge tube are mostly dependent on the particle diameter [53]. However, all the particles will settle to the bottom of the tube as long as the time is sufficient; therefore, it is necessary to control the time strictly and place a high-density medium at the bottom of the tube as a buffer zone [46].
Size Exclusion Chromatography
Size Exclusion Chromatography (SEC) is a widely recognized method that uses polymers to form a porous stationary phase in a chromatographic column. sEVs are separated according to differences in path length of different sized molecules or particles. The path length includes both the excluded volume outside the bead and the included volume that incorporates part of the bead volume (Figure 4A). When compared with UC, the physical structure and biological functions of the SEC separated sEVs are more complete [54]. SEC can also efficiently separate sEVs from soluble contaminants [40] with simple operation and no additional pretreatments are required, and considering its sample compatibility, SEC is suitable for various biological fluids [55]. In addition, sEVs do not interact with the stationary phase during SEC isolation, which reduces sample damage, resulting in relatively high yields [46].
Recent studies report the development of an assay to compare multiple isolation techniques (UC, precipitation, and SEC), and proved that SEC is the best method for sEVs purification from cerebrospinal fluid and plasma [56]. However, the narrow application range of size exclusion chromatography makes it unfit for large-volume samples [40]. Kaloyan et al found that a higher yield could be obtained by using SEC when compared with UC; however, the purity was relatively low [31]. Although SEC can partially remove co-separated contaminants, such as part of HDL and small molecule proteins, it is difficult to remove lipoproteins (Chylomicrons and VLDL) particularly with overlapping sizes [57-59]. Some improvements have been proposed, including two SEC columns to separate large exosomes (l-exo), exosomes (m-exo) and small exosomes (s-exo) from human urine samples [59, 60]. Guo et al. proposed a simple dichotomic SEC, where they selected the CL-6B column, and performed optimization of the bed volume, raising it from the original 10 mL to 20 mL, and also replaced multiple elution steps with two large elution steps to simplify the complexity of the operation [43]. This improvement is more suitable for isolation of sEVs and proteins from FBS, human serum, and FBS-free cell culture supernatants. It has been experimentally demonstrated that this method can improve the reproducibility of applications in clinical settings while obtaining high-quality sEVs with high particle recovery [43]. A combination of ultrafiltration and size exclusion chromatography performs well giving both high yield and purity [61].
Precipitation
Precipitation is a separation method based on the dispersibility of the buffer where the sEVs are located. Hydrophilic polymers, such as polyethylene glycol (PEG), are usually used as a highly hydrophilic polymers that interacts with surroundings to create a hydrophobic microenvironment, thus enabling the precipitation of the sEVs [62, 63] (Figure 4B). The specific operations include steps to remove large contaminants, such as cell debris and apoptotic bodies, and precipitation operations. Precipitation has a higher yield than UC, and does not require specialized equipment, and is simple to implement [63]. Precipitation also preserves the structural integrity and biological function of sEVs [64], and can fit in a wide range of starting volumes from 100 μL to several milliliters. Notably, precipitation is particularly useful for small-volume samples, and is extensively used for RNA analysis of EV fractions [65]. A large number of precipitation-based commercial kits are currently available, such as the Total Exosome Isolation kit (Invitrogen), ExoquickTM (System Biosciences), Exoprep (HansaBioMed), miRCURRY (QIAGEN), ExoGAG (NasaBiotech), Pure Exo (101 Bio), Exosome precipitation solution (Immunostep) and the Total sEVs isolation reagent (Thermo Fisher Scientific) [38]. However, it is difficult to separate polymers such as PEG from sEVs, which may affect the results of subsequent research. PEG-separated sEVs are also contaminated with co-precipitated substances, especially some plasma lipoproteins and non-sEVs vesicles, making PEG ineffective for separating plasma [63]. Moreover, cell viability was reduced in samples separated using PEG when compared with UC, indicating that the co-precipitated substances may have toxic or antagonistic effects. [22]. The contamination of co-precipitated substances can be reduced by adding a high-efficiency pre-filtration step with a 0.22-micron filter or a post-precipitation purification step [66].
Asymmetric flow field flow fractionation
Asymmetric flow field flow fractionation (AsFIFFF4, AF4) is a technique for separating EV subtypes, as well as sEVs [67, 68] (Figure 4C). The AF4 flat channel is composed of two plates, the upper wall of the AF4 channel is a water-impermeable polycarbonate glass plate, and the lower channel plate is breathable, made of porous stainless-steel frit material, with a polyester trapezoidal separator and an ultrafiltration membrane in the middle. Bottom channel plate and ultrafiltration membrane can form agglomeration walls [69]. The size-based isolation is achieved by a transverse flow perpendicular to the parabolic flow pattern. The particles to be analyzed flow towards the channel floor or accumulation wall under the action of the transverse flow. At the same time, the particles diffuse to the center of the channel under Brownian motion. Depending on the diffusion coefficient, the molecules are separated into different laminar flows [69, 70]. Smaller particles have higher diffusion coefficients and therefore are separated later than larger particles. This technique is a gentle isolation strategy without strong shearing force so that maintains sEVs structural and biological integrity and allows the isolation of EV subtypes. AF4 has now been shown to isolate sEVs from cell culture supernatants and human serum [71]. Optimization of the parameters of AF4 such as cross flow gradient, focusing time, sample ultrafiltration conditions, plasma volume, and injection volume, can improve reproducibility and resolution, and separate lipoproteins from EVs [71]. AF4 can also be used for resolve the complexity of heterogeneous nanoparticle subpopulations. For example, Zhang et al identified two subpopulations of exosomes and non-membrane nanoparticle “exomeres” by using this method [68]. However, this technique requires specific equipment and personnel with expertise to regulate and optimize various parameters (e.g. cross-flow velocity, channel height, and membrane type) [38]. Moreover, AF4 is not suitable for large-volume samples, which need to be pre-concentrated by UF, UC or sEVs isolation kits. Combined use of immunoaffinity chromatography (IAC) and AF4 can be employed for the automated isolation of CD9+ and CD61+ sEVs [72]. The IAC-AsFlFFF system provides highly reliable and reproducible separations as the relative standard deviations of EVs yield between fractionation cycles are only 2.9-4.2% and can automatically process up to 18 plasma samples per day [72]. Moreover, asymmetric flow field fractionation coupled with UV and multi angle light scattering (AF4 /UV-MALS) can be used to separate sEVs from urine with a high degree of reproducibility, while determining their size, quantity and purity of the isolated urine SEVs, and also enabling isolation analysis of sEVs subtypes [70].
Ultrafiltration
Ultrafiltration (UF) is an isolation technique based on the size of sEVs [33]. UF uses membranes with molecular weight cut-off (MWCO) ranging from 10-100 kDa [59], filtration membranes are usually made of cellulose, polyethersulfone or hydrogenated salts, among which cellulose film is mostly used [38]. sEVs from a large amount of raw material are concentrated into a small volume sample so that they can be suitable for subsequent isolation and purification steps [37] (Figure 4D). UF is simple to handle with no additional need of special equipment, and can separate sEVs with well-defined particle sizes by adjusting the pore size of the filtration membrane [73]. It is reported that ultrafiltration has the highest recovery rates for particles smaller than 100 nm, including sEVs, and it improves sEVs yield and isolation efficiency with a shorter processing time compared to UC [74], perhaps it can be one of the alternative methods to UC [37].
Simplified diagram of various sEVs separation techniques. A. Size-exclusion chromatography, B. Precipitation, C. Asymmetric flow field flow fractionation (AF4), D. Ultrafiltration, E. Tangential flow filtration (TFF), F. Circulating tangential flow filtration, G. Immunoaffinity capture technology, H. Magnetic bead-based ion exchange technology, I. Chitosan based separation techniques.
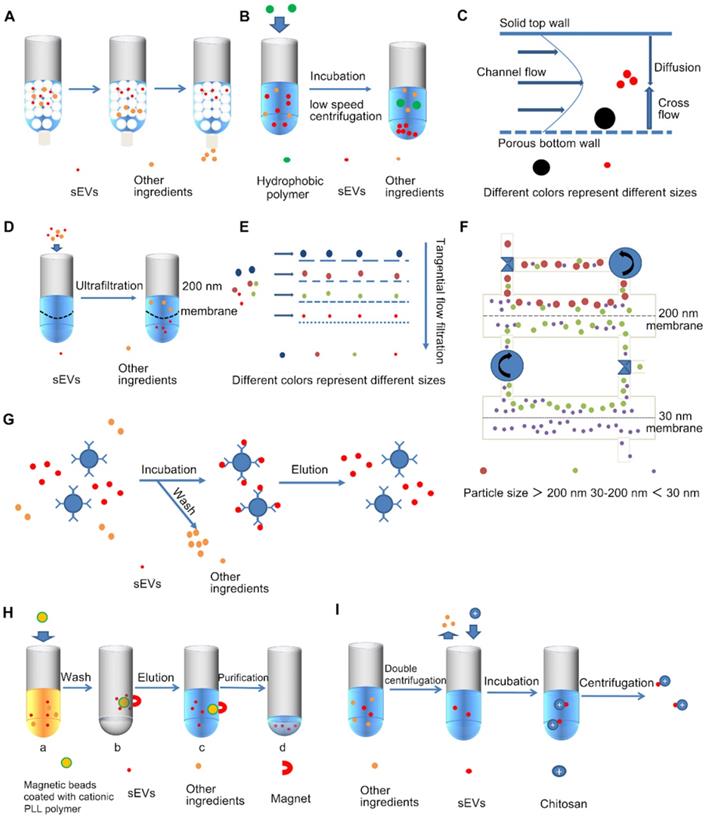
There are also two devices with simple operation and high isolation efficiency named microfilters configured in series [75] and continuous filtration. Tandem configuration filtration uses two filters with different filtration pore sizes in series, a 200 nm filtration membrane on top and a 20 nm one below, leaving large particles (larger than 200 nm apoptotic bodies) in the upper layer, small particles (smaller than 20 nm proteins) in the layer below, while the desired ingredient (sEVs) in the middle layer. Based on the time-consuming continuous filtration operation, “ExoMir™ Exosome Isolation” isolation kit was developed [76]. However, non-sEVs still can be co-separated together with sEVs. Particularly, interaction with the membrane as vesicles passing through the membrane can lead to clogging of the pores of the filtration membrane, resulting in a high rate of damage to the filtration membrane and increased cost [22]. Shear forces also disrupt the integrity of sEVs [35]. Researchers have subsequently developed a tangential flow filtration (TFF) [77], the particles flow along the membrane are parallel to the membrane surface, rather than flow to the membrane or perpendicular to the membrane, which can cleverly avoid clogging problems [38] (Figure 4E). The experiments proved that compared with UC-sEVs, the production of TFF-sEVs was increased by 18 times, and its anti-apoptotic effect was also improved [74].
On this basis, Kim et al. proposed a circulating tangential flow filtration (TFF) system (Figure 4F) [78], which consists of two membranes with pore sizes of 200 nm and 30 nm connected to a peristaltic pump. This system has higher isolation purity than single cycle TFF. Compared with ExoQuick, it can better ensure the integrity of sEVs structure and biological functions [78]. If the pore size of the filtration membrane is adjusted, it is possible to separate particles with a well-defined particle size. Also, Luca Musante et al. proposed a new sEVs isolation technique, hydrostatic filtration dialysis, which can concentrate samples and adapt to large-volume samples. It can also eliminate the influence of soluble protein on purity, and reduce the amount of loss, and the yield is better than UF [79]. UF is suitable for application together with other methods, such as UF-LC ultrafiltration combined with size exclusion liquid chromatography, can obtain purer and structurally complete sEVs than UC [41]. There is also a sEVs total isolation chip (ExoTIC) based on ultrafiltration technology [80], which is easy-to-operate and high-yield [22]. Xiang et al. proposed an ultrafiltration-TiO2 series method combining ultrafiltration and phospholipid affinity-based EV isolation. The phospholipid affinity-based method used the specific interaction between the metal and phosphate groups on the lipid bilayer for separation [81]. This hybrid method is fast, capable of processing a large number of urine samples and produces high-purity EVs, and can be considered as an alternative method for processing urine samples [81].
Immunoaffinity capture
Immunoaffinity capture technology is primarily based on sEVs membrane surface protein markers such as CD9, CD63, CD81, CD82, annexins, programmed cell death 6 interacting protein, Rab5, and epithelial cell adhesion molecules [22, 82] (Figure 4G). Several immunoaffinity capture-based methods have been developed using microtiter plates, affinity columns or magnetic beads [82, 83]. Immunoaffinity capture is especially suitable for isolating EV subtypes based on markers rather than isolating all EVs at one time with a relatively low yield but high purity [38]. When studying specific EV subpopulations, after performing UC or SEC to isolate sEVs, immunoaffinity capture can efficiently isolate specific EVs. For example, when conducting immunocapture of melanoma-derived exosomes from plasma, morphologically intact and biologically active sEVs can first be obtained by mini-size exclusion chromatography (miniSEC), and then specific tumor-targeted antibodies, antigen peptide epitope chondroitin sulfate peptidoglycan4 (CSPG4) monoclonal antibody is then used to precisely isolate MTEX [84]. Related commercial kits have also been developed, such as the exoRNeasy Serum/Plasma Kit (Qiagen, Hilden Germany), which is widely used to purify sEVs-derived total RNA from serum/plasma. However, due to the low yield and high cost of this technology, it is difficult to apply it on a large scale [37]. Notably, while the yield here is low for total EVs, for a particular subpopulation that is isolated, the yield is relatively high [17]. Importantly, the specific binding of immunoaffinity capture is hard to reverse, thus affecting subsequent experiments. Use of molecules with reversible binding can avoid such problems. For instance, the use of Tim4 peptide; the specific binding of Tim4 is Ca2+-dependent, and it can bind to phosphatidylserine that is specifically expressed on the surface of sEVs, and the Tim4 peptide is immobilized on magnetic beads for isolation. Finally, sEVs can be dissociated from the beads by adding Ca2+ chelators [85]. A cleavable-linked antibody immobilization method can also be used. Kang et al. propose an sEVs-specific dual-mode immunofiltration (ExoDIF) device that introduces 3,3'-Dithiobis (sulfosuccinimidylpropionate, DTSSP) on the surface of the antibody and immobilizer, this linkage can be cleaved by tris(2-carboxyethyl) phosphine (TCEP) or dithiothreitol (DTT), resulting in the release of the sEVs [39]. In addition, Zhu et al. developed a column-based CD9-antibody-immobilized HPLC immunoaffinity chromatography (CD9-HPLC-IAC) technique, which can separate sEVs in real-time from trace serum (40 μL) within 30 minutes [86]. The advantages of the method are that it is small scale, high efficiency, and can be monitored real-time. Compared with UC and SEC methods, the contamination of proteins and apolipoproteins from blood is greatly reduced, and the purity is improved [86]. At the same time, this method also ensures the integrity of sEVs, and the immunoaffinity method is optimized in all aspects.
Charge-based separation techniques
sEVs are negatively charged particles varying from their origin cells since different cells have different charges and are highly heterogeneous, which may affect the isolation efficiency [87]. Therefore, the distinct methods should be selected by considering different cell sources. A number of methods have been developed based on the negatively charged properties of sEVs.
Chromatography-based systems
The chromatography-based systems mainly rely on the interaction between the zeta negative potential of the sEVs membrane and the positively charged anion exchanger, which allows the dissociation of EVs from the positively charged medium by increasing the buffer ionic strength (by introducing a high salt concentration). Applications like anion exchange chromatography (AIEC) involves the use of a monolithic column with quaternary amine functionality (strong anion exchanger) [88] and diethylaminoethyl cellulose resin (weak anion exchanger) [89]. This method shows high operability and scalability; however, it is currently mostly used for cell culture medium as many biological fluids contain complex components and charged substances [59].
In addition, a novel chromatographic method, developed by Ken Marcus and colleagues, proposes a separation and purification strategy using hydrophobic interaction chromatography (HIC) which uses a polyester capillary channel polymer fibrous phase [90]. Capillary channel polymer (C-CP) fibers show a certain special structure as when packed in the form of columns, the fibers interdigitate to form numerous 1-4 μm channels, providing high permeability for fluid flow [90]. It has hydrodynamic advantages combined with a high degree of chemical separation versatility and can also modify the fiber surface to affect high ligand densities for ion exchange (cations and anions) and affinity chromatography [91]. Hydrophobic exosome surfaces adhere to the weakly ionized surface of poly (ethylene terephthalate) (PET) fibers, making HIC a selective method for exosome isolation [90]. The method has been extended to a more clinically beneficial EV isolation workflow that uses a 1cm C-CP fiber connected to a micro shift tip to allow solid phase extraction (SPE) of EV in a bench centrifuge [92]. The sEVs isolated by this method have good integrity, relatively low cost, high yield, and high cost performance compared to differential centrifugation (DC). Most importantly, they have recently discovered that this method can also separate sEVs from LDL, greatly improving the purity [93]. In addition, studies have demonstrated that this method can also isolate sEVs from human urine, saliva, cervical mucus, serum, and goat milk matrices, with a high degree of generality. It can be seen that chromatography-based methods do hold great potential for clinical application in the future [94].
Magnetic bead-based ion exchange technology
Kim et al. proposed a magnetic bead-based ion exchange technology, ExoCAS-2 (sEVs clustering and scattering), which is a flowable resin that can freely move and adsorb counter-ionic objects in the liquid phase [95]. sEVs can be separated by adhering to magnetic beads coated with polycationic polymers. The specific process is shown in Figure 4H [95]. This method relies primarily on magnetic, particle-based mobile ion exchange resins that can easily isolate high-purity and high-yield sEVs with well-controlled bead size, uniform polymer coating, and magnetic operation, making it highly reproducible and repeatable. It is also scalable for a wide range of sample volumes [95]. Moreover, compared with the traditional fixed resin, this flowable resin can reduce the loss of sEVs due to the reduction of the flow rate and the sEVs yield can be improved by optimizing the final washing and elution steps. Experiments show that the highest washing efficiency can be obtained by using buffer solution (pH=6), and the highest elution efficiency can be obtained by using 1M NaCl buffer [95]. The method is simple and time-consuming and it is worth of attention that this technique cannot be applied to urine for high concentrations of chloride ions, which affect sEVs capture [95].
Chitosan based isolation techniques
A new charge-based method has recently been discovered to separate sEVs from a variety of biological fluids using the polysaccharide chitosan (Figure 4I). Chitosan is an alkaline deacetylated derivative of chitin [96], it is a linear cationic polyelectrolyte polysaccharide with biological properties such as biocompatibility, non-immunogenicity, biodegradability and low toxicity [97, 98]. Chitosan shows various biological uses in viral infection treatment and bone repair. [99-101]. Chitosan can be soluble and protonated in an acidic environment. The high positive charge of chitosan easily attracts sEVs, which carry a net negative zeta potential, ranging from about -10 mV to -20 mV [102, 103]. The biological fluid is first subjected to a two-step pretreatment, to remove cells, debris, and large particles. Then adding chitosan into the pretreated biological fluid to incubate the chitosan-sEVs complex. Finally, the chitosan-sEVs complex is centrifuged and subjected to subsequent research [104]. Awanit Kumar et al used chitosan to isolate sEVs from cell culture conditioned medium (CCM), human plasma and various body fluids, respectively, and analyzed the proteomics of sEVs in chitosan-isolated CCM, urine and saliva, which in turn demonstrated that chitosan can separate sEVs from biological fluids. Each biological fluid has different physicochemical complexities, indicating the compatibility of chitosan isolation technique in various types of biological fluids [104]. Besides, chitosan immobilized on magnetic beads separate sEVs, thus proving the versatility and adaptability of chitosan to sEVs isolation platforms [104].
As a matter of fact, the zeta potential of sEVs changes as the ionic strength of the environment, and the surface charge of chitosan can also change by adjusting its formulation. For example, the acidic formulation of chitosan provides more positive charges than the neutral one. This is also the reason why the chitosan acid formula is more effective in separating sEVs. Given that different biological fluids possess distinct physicochemical properties, such as the protein and urea content, which can affect the efficiency of chitosan for sEVs isolation, it is necessary to select different formulations of chitosan based on the sample origin [104]. Chitosan-mediated sEVs isolation uses low-speed centrifugation, which may have little physical damage to sEVs function when compared to ultracentrifugation. As for the comparison with immunoaffinity or antibody-based capturing, chitosan is safer and nearly non-toxic which has been proved by locally use (wound healing) and even diet for decades. In general, the isolation of sEVs from chitosan not only ensures its integrity, but also has a low technical cost, and the most important thing is that chitosan is relatively safe to apply in vivo [105, 106], thus making it a fairly promising strategy for sEVs isolation [104].
Microfluidics
Microfluidic technology integrates sEVs isolation, detection, and analysis in miniaturized chips [107] with the benefits of reduced time, high sensitivity, specificity and high production [108]. Besides, not much sample volume and reagents are required to support the whole process and the on-site analytical capabilities make it a user-friendly, clinically reliable and cost-effective choice for sEVs isolation [33]. The microfluidic platform is also widely used for DNA, protein and virus isolation [37]. Currently, there are two main types of microfluidics based EV isolation strategies, namely, label-based and label-free isolation technologies [109].
Label-based microfluidics
The basic principle of label-based microfluidic technology is kind of similar to that of immunoaffinity capturing. Capturing molecules, such as antibodies and aptamers, can specifically bind to corresponding lipid components or proteins on the surface of EVs on the basis of chemical or physical properties [109]. Modified magnetic beads or nanomaterials and antibodies/aptamers immobilized on the surface of microchannels are commonly used for EV isolation [107]. Liu et al. proposed a novel EV capture strategy based on dip-pen nanolithography technology, which efficiently isolates sEVs by carrying antibodies that specifically recognize surface markers of sEVs (Figure 5A) [110]. Furthermore, there are still many label-based microfluidic technologies for isolating sEVs based on specific surface markers [33]. The separation sensitivity can be improved by modulating the structure-specific and chemical properties of the microfluidic channel to increase the surface area for the interaction between the microfluidic channel and sEVs. ExoTIC is a novel development that is an exosome total isolation chip device. ExoTIC uses a simple filtration method to isolate intact sEVs in the 30-200 nm size range by a nonporous membrane. The yield of sEVs by ExoTIC yields 4-1000 times higher sEVs than UC [80]. Moreover, other researchers also demonstrated that this technology is also a modular platform which can be adapted to a variety of samples and can classify heterogeneous populations of cancer cell line EVs by sizes [80]. However, the cost of labeling is relatively high, the labeling operation is complex, and the capturing molecules are also likely to change the physical and biological properties of the sEVs [33, 109].
Label-free microfluidics
In order to reduce the influence of labeling on subsequent experiments, label-free isolation strategy has been proposed, which is mainly dependent on the physical properties of sEVs, such as size, electrical properties, and deformability [33]. A growing number of label-free microfluidic platforms have been developed, including methods based on sieving, deterministic lateral displacement, field flow, and entrainment fractionation, viscoelastic, acoustic, inertial, electrical, and centrifugal force [38, 109]. The greatest benefit of using label-free strategy is to avoid the destruction of sEVs, guaranteeing their structural and biological integrity. Besides, it also simplifies the operational steps to save time and cost, improves application reproducibility and reduces the risk of contamination. On the other hand, label-free isolation cannot completely remove contaminants such as proteins and cell debris. This technology is still in the process of advancing from the micro-scale to the nano-scale, but a combination of technologies provides optimized outcome, such as DLD sorter coupled with a spiral inertial microfluidic sorter in series, inertial microfluidics combined with an integrated membrane filter, or an external dielectrophoretic force, inertial microfluidic sorter combined with an acoustic sorter [109]. It is believed that the continuous efforts on microfluidics may lead it to a better future for clinical application.
Acoustic-related microfluidic technology is mainly based on the variant acoustic force received by particles with different sizes [107, 111, 112]. For example, Zhao et al. developed a unidirectional disposable acoustofluidic platform based on a unidirectional interdigital transducer (IDT) [113] with high isolation efficiency and versatility. Electrically related microfluidics is developed on the basis of electric field strength and particle charge, such as the employment of electrophoresis (Figure 5B) [114] and dielectrophoresis (DEP) [115]. The centrifugal microfluidic system is a lab-on-a-disc integrated with two nanofilters (Exodisc), which mainly perform separation based on particle size, and can fully automate the separation of EVs in the size range of 20-600 nm in a short time [116]. Inertial isolation [117] takes the advantage of inertial migration, where the particle size determines inertial resistance and viscous resistance, so that isolation can be performed according to particle size. Viscoelastic isolation can be achieved by the size-dependent elastic lift in viscoelastic fluid media [109, 118].
Synthetic peptide (Vn96) based isolation method
Anirban Ghosh et al. designed a series of peptides (venceremins, or Vn peptides) and discovered a new class of peptides (Vn96), which showed nucleotide-independent specific affinity for typical heat shock proteins [119]. Various experiments demonstrated that Vn peptides can specifically and efficiently capture HSP-containing sEVs from cell culture growth media, plasma, and urine. sEVs were first isolated from the samples by ultracentrifugation, the isolated samples were incubated with Vn96 overnight at 4 °C with rotation, then centrifuged at 17,000 × g for 15 min at 4 °C, and finally washed three times with PBS [119], The characteristics of the final product were similar to those obtained from UC isolation, which proved the reliability of the technique. Irene V Bijnsdorp et al. found that this method is more convenient, time-saving and has higher yields than UC [120]. Notably, Vn96 peptide can be combined with HSPs from various species; therefore, this method is not only suitable for human body fluid samples (such as plasma and urine) [121], but also suitable for animal samples (mouse and dog plasma), making it applicable in animal experiments for basic medical research. Overall, the Vn96 peptide isolation method can isolate sEVs of clinical value and outperform current isolation methods in terms of efficiency, cost, and platform versatility, and can also be applied to basic research in animal models [119].
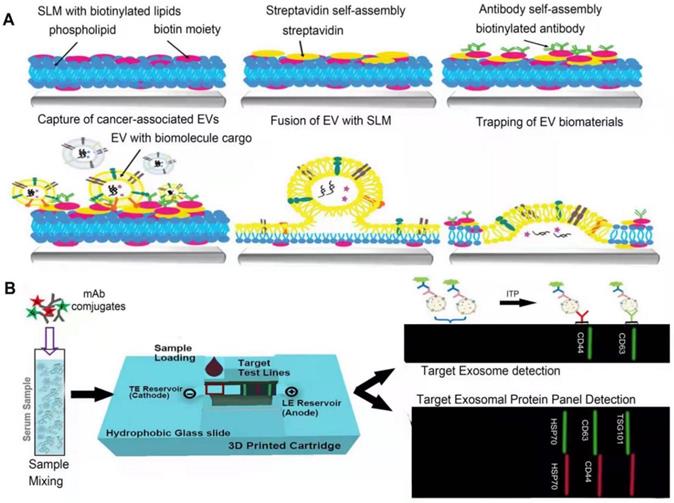
Simplified illustration of microfluidics methods. A. Scheme of lipid membranes microarrays. Biotinylated SLM arrays are firstly fabricated using lipid dip-pen nanolithography (L-DPN), overlaid with streptavidin. Then, biotinylated antibodies (ABs) are bound to the SLM array. Finally, the separation can be achieved by exposing the array to a solution of sEVs with the marker of interest. Adapted with permission from [110], copyright 2021 John Wiley and Sons. B. A paper-based isotachophoresis (ITP) technology. Place the paper strip on a glass slide with both ends dipped into the container, and then mount the ITP box on a fluorescence microscope stage equipped with a DFC310 digital color camera, below the 4× objective. After loading the sample, a voltage of 150 V was applied to the anode and the cathode can be grounded to start the ITP experiment, monitoring targeted enrichment with labeled fluorescence. Adapted with permission from [114], copyright 2020 Elsevier.
Other new isolation strategies
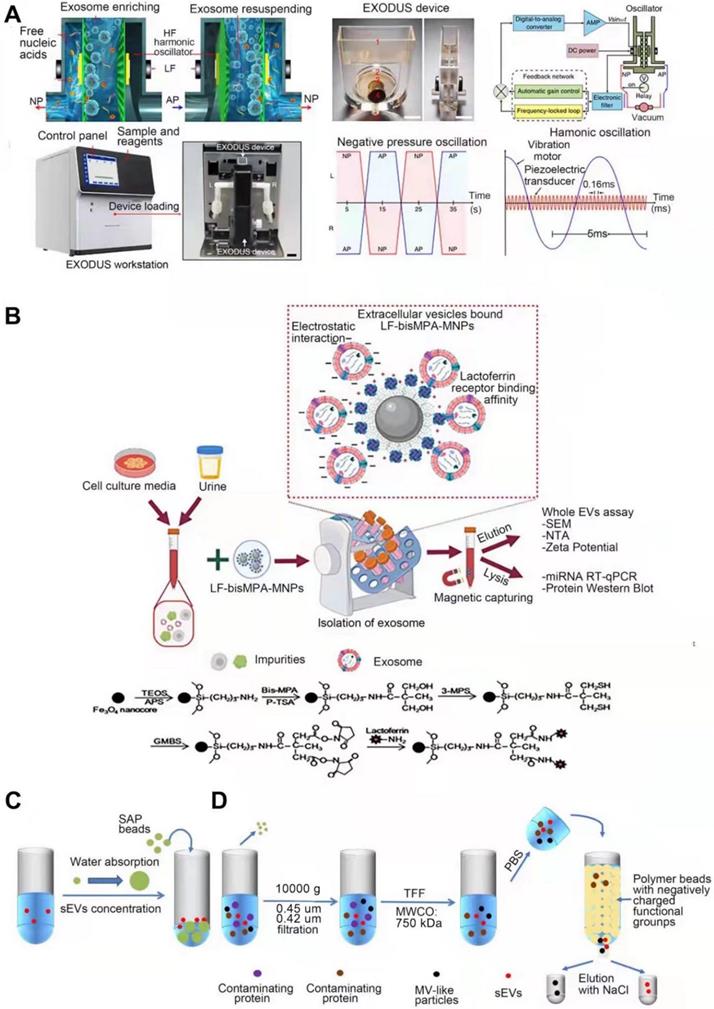
EXODUS
Liu et al. developed a technique “EXODUS” (Figure 6A) that could automatically isolate sEVs from various biological fluids without labeling [122]. With negative pressure oscillation and a dual coupled oscillator that vibrates the membrane, highly efficient isolation of exosomes can be achieved. The two coupled oscillators generate a double frequency transverse wave across the membrane, making EXODUS superior to other isolation techniques in speed, purity, and yield. Periodic negative pressure oscillations (NPOs) in the device can effectively avoid the aggregation of particles such as contaminant proteins and vesicles, on the membrane, along with the possible clogging of the pores in the nanoporous membrane by these particles [122]. In addition, during the isolation process, due to the negative pressure, the integrity of the physicochemical properties of exosomes are able to be reserved. Different harmonic oscillators, such as NPO alone or combined lactoferrin, can be set up on the EXODUS unit to combine different harmonic oscillators. Liu and colleagues also applied EXODUS to plasma, urine, culture medium, and even tears, demonstrating the adaptability of this technology to different types and volumes of biological fluids [122]. Overall, this technique compares favorably with others in terms of speed, purity, and yield, and has no limitation on specimen volume, enabling isolation and purification of SEVs in a noninvasive and cost-effective manner [122]. However, the current EXODUS platform is limited to single-channel isolation. Large-scale biological research will be facilitated by implementing a series of EXODUS devices with automated reagent distribution and sample collection for multi-sample processing [122]. The smaller nanopore size of EXODUS can be further studied to specifically isolate more sEVs subtypes. Integrating EXODUS with downstream assays can also be explored. For example, Liu Fei et al combined EXODUS with MALDI-TOF MS to obtain highly sensitive proteomic fingerprints of intact sEVs from 20 μL of human plasma. They also applied EXODUS to isolate sEVs from urine samples of kidney and bladder cancer patients for transcriptional profiling, which demonstrated the feasibility of the clinical application of EXODUS [122].
Chimeric nanocomposites-based technology
Chimeric nanocomposites of lactoferrin conjugated 2,2-bis(methylol) propionic acid dendrimer-modified magnetic nanoparticles (LF-bis-MPA-MNPs) are reported to efficiently separate sEVs from human urine [123] (Figure 6B). This method is mainly based on physical absorption, electrostatic interaction and biorecognition of sEVs [123]. The N-terminus of LF is cationic and hydrophobic, maintaining a net positive charge at high pH level (8.0-8.5) [124] while the phosphatidylserine (PS, a negatively charged lipid) of sEVs provides a net negative charge [125], allowing sEVs to bind to lactoferrin (LF). LF also interacts with glycosaminoglycans and chondroitin sulfate proteoglycans [126], the van der Waals force providing the driving force for physical absorption. GAPDH expressed on the surface of sEVs acts as a receptor for LF [127]. These three strategies enable efficient isolation of sEVs, after which the LF-sEVs complex can be dissociated by eluent buffer (pH~10.6) to further purify sEVs [128]. sEVs isolated from conditioned medium or urine are subsequently subjected to following identification. Compared to the existing methods, this method has better isolation speed, efficiency, yield and purity, and can also maintain the integrity and biological functions of sEVs, with no requirements for expensive equipment is required [123]. However, when this method is selected to isolate sEVs, it is difficult to completely distinguish them from other types of extracellular vesicles.
SAP -based technology
Yang et al. developed the first single step, equipment free method of sEVs concentration by using high water absorption polymer (SAP) beads (Figure 6C) [129], which are able to absorb small molecules including water through nano-sized channels while sEVs are excluded [130, 131], leading to the final concentration [129]. In addition, SAP can absorb several contaminants, such as proteins, thereby improving the purity of sEVs. During this process, no external forces act on sEVs thereby maintaining their integrity. This method is mainly used for concentration, and the isolation purity is not that high. It needs to be used in combination with other high-efficiency isolation techniques, such as SEC. Experiments have shown that this method is versatile for various biological fluids and media, such as urine and plasma [129]. It can also adapt to samples of different volumes and concentrations by adjusting the absorption time and the concentration of SAP, and the integrity of the sEVs remains intact during this process. This method can also be applied for the detection of biomarkers transported by sEVs. For example, Yang et al. found that in the process of using nanoscale oligonucleotide probes to detect sEVs miRNA [132], SAP can absorb the free probes, amplifying the detection of probe-miRNA hybridization signal, reducing background signal and improving detection sensitivity [129]. Further, this method can be widely used in liquid biopsy to improve its sensitivity of biopsy and facilitate disease diagnosis [129]. The advantages, such as being simple-to-operate, cost-effective, and equipment-free, make SAP-based sEVs isolation hold great promise for popularization and application [129].
Simplified illustration of sEVs emerging isolation techniques for sEVs. A. EXODUS. Adapted with permission from [122], copyright 2021 Springer Nature. B. Chimeric nanocomposites. Adapted with permission from [123], copyright 2022, John Wiley and Sons. C. SAP-based technology. D. Anion exchange-based method.
The methods used most often for sEV isolation are illustrated comparing their performance in purity, yield, and processing time. DGC, density gradient centrifugation; SEC, size exclusion chromatography; TFF, tangential flow (cross-flow) filtration; dUC, differential ultracentrifugation.
Anion exchange-based method
A new separation method based on anion exchange column chromate graphy was reported (Figure 6D) [133]. This method is used mainly to isolate and purify sEVs and other EVs by using anion exchange column chromatography followed by elution at high (0.3 M ~ 0.5 M) and low (0.15 M ~ 0.3 M) NaCl concentrations, respectively. The protein removal rate of this method was over 99.97% [133]. The principle of anion exchange resin separation is described in the “Charge-based separation” section above. This method can efficiently isolate sEVs, and the selection of elution conditions as the key to step. sEVs present weaker affinity for phosphatidylserine (PS)-binding proteins AnnexinV and lactadherin than other EVs [134], and have low surface sialic acid content [133]. These factors allow sEVs to have a relatively weak negative charge, thereby distinguishing them from other EVs particles. The EVs obtained by elution at two different NaCl concentrations were analyzed by proteomics, DNA, morphological size, miRNA distribution, zeta potential values, target cells, and surface glycosylation [133]. The results indicated that the particles obtained at low NaCl concentrations (0.15 M~0.3 M) were sEVs for they have sEV-specific proteins, including late endosome-related proteins, integrin and rab family of proteins, as well as functional miRNAs [133]. They also show biological activity to prevent tumor metastasis by depleting the mesenchymal cell population in primary tumor lesions [135]. The particles obtained at high NaCl concentrations (0.3 M~0.5 M) were microvesicle (MV)-like particles [133]. This isolation method can produce sEVs with high purity on a large scale without affecting their biological activity, providing an efficient method for the research and clinical application of sEVs.
Cocktail strategy
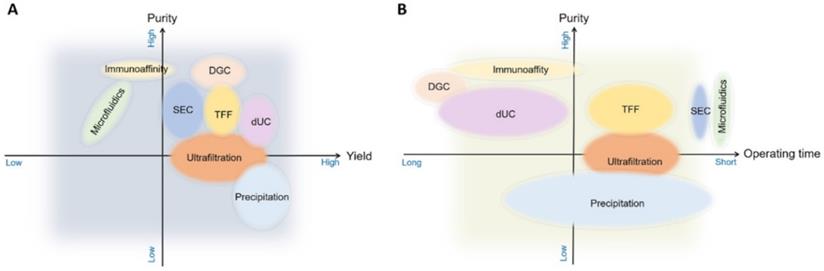
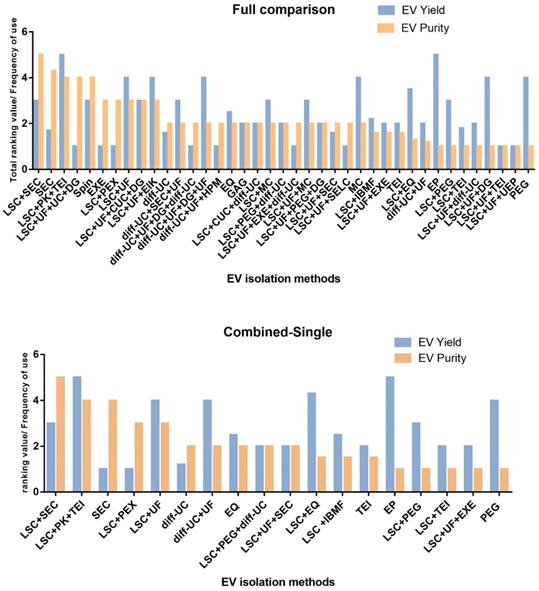
Single methods for sEVs isolation show great variability in many aspects, particularly in yield and purity (Figure 7). A cocktail strategy employs optimal combination of methods by sharing their complementary advantages in order to achieve the purpose of high yield and high purity. For example, when sEVs are obtained with centrifugation, which is based on physical properties such as density, co-isolation of substances overlapping with sEVs in terms of density and other physical properties cannot be avoided. Therefore, a complementary method can be used to sequentially remove the co-separated contaminants. It has been shown that the combined isolation strategy is clearly superior to individual isolation methods in terms of achieving higher purity and yield. Size exclusion chromatography is recommended as an initial step followed by low speed centrifugation, the combination of these two methods can achieve the highest sEVs purity while maintaining a reasonable sEVs yields (Figure 8) [38]. Combinations can also be selected according to different research objectives. Despite the fact that the combinatorial method is suitable for sEVs isolation from highly heterogeneous and complex biological materials and improves the yield and purity, the resulting operation time is relatively long and large samples volumes may be required. These limitations imply that the combinatorial strategy is more likely to be used for cell culture supernatants, but might not be suitable for clinical research and small sample volumes, such as the samples for non-invasive liquid biopsy [38].
Comparison of sEVs isolation methods in terms of purity and yield, sorted from highest to lowest purity. Adapted with permission from [38], copyright 2021 Elsevier. A. Comparison of all sEVs isolation methods, B. Comparison of combined sEVs isolation methods and single sEVs isolation methods. (diff)UC: differential ultracentrifugation, DGUC: density gradient (ultra) centrifugation, CCS: cell culture supernatant, EIK: Exosome isolation kit Pan (immunoaffinity based on CD9, CD63 & CD81), CUC: cushioned-density (ultra)centrifugation, EP: Exoprep (precipitation method), EXE: exoEASY, EQ: Exoquick (precipitation method), GAG: ExoGag (glycosaminoglycan precipitation method), HPM: Heparin/polymer coated microspheres, IBMF: Immunity-based microfluidics (antigen: annexin), LSC: low- speed centrifugation (< 80,000 x g regardless of time), MC: miRCURY (precipitation method), SEC: size-exclusion chromatography, PEG: polyethylene glycol (precipitation method), PEX: Pure Exo isolation kit, PK: proteinase K treatment, SELC: size-exclusion liquid chromatography, TEI: Total exosome isolation kit, UEP: Urine exosome precipitation (and RNA isolation) kit, Spin: EXOspin (SEC plus precipitation method), UF: ultrafiltration.
Challenges and outlooks of sEVs and their isolation techniques
Unsolved problems with respect to sEVs
Despite the remarkable application prospects, there are still questions that are worth discussing. Intrinsic problems of sEVs, such as their heterogeneity in physico-chemical properties, are not well understood and can derivatively influence the choice of different isolation techniques. It is also hard to say which method is the best option since there are too many measuring indicators to be measured, such as yield, purity, and isolation efficiency. From what we've seen so far, choice of the most appropriate methods must be based on each specific research requirement, application scenarios, and sample types. A cocktail strategy by using multiple isolation methods together to improve the purity and/or production is also recommended (Table 2).
What are the possible causes of the functional heterogeneity of sEVs?
The heterogeneity of sEVs is reflected in their physical properties and biological functions. The biological function of sEVs can be cognized as the effect exerted by the cargo-mediated information exchange between cells or organs [145], which implies that many factors, such as the originating cell, the target cell, as well as the microenvironment, jointly shape the sEV functions [19]. For example, it is possible that the same group of sEVs have different roles in different target cells. MSC-derived sEVs promote cell growth in diabetic foot [146] while inhibit cell proliferation in lung cancer [147]. The function of sEVs is also affected by the body's microenvironment [148]. Patient-derived sEVs are usually shown to carry disease markers, pro-inflammatory factors and generate other destructive effectors, while sEVs derived from healthy people can reduce inflammation, be antioxidant and present other protective effects [148]. sEVs functions may also depend on their origin cells [19], possibly due to their distinctive biogenesis and/or content loading mechanism in mother cells [149], resulting in differences in cargo composition of sEVs. Cancer and non-cancer cell-derived sEVs showed a different expression level of HSP70 on surface membrane [150]. However, there are currently no specific markers to distinguish sEVs from different cell types from the same individual; sEVs from mixed origin existing in plasma are hard to be precisely separated [151]. The functional heterogeneity of sEVs may also be affected by factors that are even currently unknown. More efforts should be made to discover the mechanism that is responsible for the functional heterogeneity, based on which, more accurate isolation techniques could be developed.
How to identify EV subtypes?
As mentioned above, currently EV subtypes are mainly classified according to their physical properties (size), biological origin, or their surface markers. The currently reported subtypes are as follows, exosomes (40-160 nm, marker proteins are ESCRT complex proteins and CD63), microvesicles and oncosomes (50-10,000 nm, marker proteins are Annexin A1 and ARF6), migrasomes (500-3000 nm, marker protein is TSPAN4), secretory autophagosomes/amphisomes (size not determined, marker protein is LC3), exomeres (<50 nm, marker protein is unknown), retroviral-like particles (size not determined, marker proteins are Arc1 and Arc2), exophers (1,000-10,000 nm, with labeled protein as Phosphatidylserine, LC3, and Tom20), apoptotic bodies (50-5000 nm, with labeled Phosphatidylserine) [152]. Among them, exosomes, microvesicles (MVs) and apoptotic bodies are the three subtypes that have been thoroughly studied. MISEV2018 states that the subtypes of EVs can be classified according to their size (e.g., by filtration, which must be combined with another method, such as SEC, to eliminate non-EV components), density, their surface protein, sugar, or lipid composition (immunal or other affinity separations, including flow cytometry of large particles) or other biophysical properties such as surface charge [17]. However, the analysis of EV subtypes remains a great challenge due to the high heterogeneity in their physical properties, composition, and biogenesis. For example, research showed that the presence and abundance of tetraspanins (CD9, CD63, and CD81) on the surface of exosomes from different cells are heterogeneous [153]. This also shows that transmembrane proteins such as CD9 may not be effective markers for sEVs recognition when performing isotype analysis of EVs secreted by different cells [153]. Studies have shown that in addition to the above subtypes, there are still a large number of particles whose characteristics are unclear. For example, proteomic analysis has reported a high degree of molecular diversity. Purified EVs from a single cell source contains more than 1000 protein signals, which indicates that there are still many undetected EV subtypes [154]. Since the resolution thresholds are lower than the standards followed by most optical imaging methods, these undetected subtypes are not easily characterized and identified [155]. Choi et al. found that nano-flow cytometry combined with high-resolution microscopy can improve the resolution of EVs subtypes [155]. Other researchers proposed an optimized isolation characterization method, an ultracentrifugation-hollow-fiber flow field-flow fractionation orthogonal approach, which has promising results for purification and differentiation of EVs subtypes. The specific operations are as follows: firstly, large EVs and small EVs are distinguished by differential ultracentrifugation, and then these subgroups are analyzed by the HF5 method using UV, fluorescence and multi angle laser scattering as detectors. sEVs are further separated by density gradient centrifugation (DGC), and then analyzed by HF5 multiple detection. The density-dominant isolation principle of DGC is orthogonal to the hydrodynamic radius-dominant isolation principle of HF5, and two-dimensional isolation can be obtained when these two techniques are used together; the size, density, and composition (protein and nucleic acid) of EVs subtypes can be analyzed more comprehensively, and new methods for better purification and localization of EV subtypes can be developed [156]. Identifying EVs subtypes is of great significance for developing isolation techniques and improving the accuracy of downstream experiments.
Challenges and outlooks of sEVs isolation techniques
How to ensure the structural and biological integrity of isolated sEVs?
Certain existing isolation techniques inevitably destroy the structural and biological integrity of sEVs, which may largely be due to external force or properties changing caused by microenvironment, such as co-separated substances. Regarding external force, in the process of ultracentrifugation, sEVs are affected by the external force of high-speed rotation [41], and in the process of filtration isolation, extrusion through the filtration membrane can cause the mechanical damage to the extract [157]. The way to reasonably avoid external force injury is to adjust and select the appropriate external force. For example, when tangential flow filtration is used for ultrafiltration separation, if the transmembrane pressure is well controlled, the external force can be reduced. Alternatively, one could use method that are not conducive to external forces, such as SEC which depends on natural gravity-based isolation [107]. The biological functional integrity of sEVs may be affected by the physicochemical properties of the microenvironment in different biological fluids or by substances that specifically bind to sEVs. For example, in immunoaffinity centrifugation, antibodies that specifically bind to sEVs, the non-neutral pH and non-physiological elution buffers used to elute sEVs during manipulations may compromise the integrity of the biological properties [46]. A possible solution to this problem is to use substances that are easy to separate, such as Ca2+-dependent Tim4 protein [85]. In addition, the polymers used in the precipitation process also have an impact on the biological integrity of isolated sEVs. The selection and improvement of isolation methods are critical for subsequent research and observations.
How to ensure the purity of the isolated sEVs?
The low isolation purity of sEVs is mainly caused by the large number of co-isolated contaminants. In particular, the co-isolated non exosomal functional vesicles will affect the accuracy of subsequent experiments [46]. If the isolation purity of sEVs in reports is insufficient, then the research results should be carefully verified. Meanwhile, the safety of clinical applications remains to be confirmed. There are also a large number of co-isolated lipoproteins [158] that share overlapping characteristics with sEVs in terms of density, size and lipid content, and their numbers are far more than sEVs; therefore, it is difficult to separate sEVs from plasma without lipoprotein contamination. Challenges still remain in the previously proposed concepts of liquid biopsy [18]. In addition, highly heterogeneous biological samples contain substances, such as albumin, casein, and Tamm Horsfall protein [38], that overlap with sEVs in terms of physical biology, which may affect the purity of the isolated sEVs [159, 160], To improve the isolation purity, the primary problem to be solved is to make EVs classification clear, clarifying the specific differences between sEVs and other extracellular vesicles and non-vesicle components in terms of physicochemistry, such as size, density , surface protein, sugar and lipid components, and surface charge [17], which can provide a specific theoretical basis for the development of high-purity isolation technology. For example, the current immunoaffinity technology selects appropriate antibodies or ligands to specifically bind to sEVs based on their surface-specific markers to ensure that their purity can be guaranteed [38]. However, according to MISEV2018, it is currently impossible to achieve high purity and high yield at the same time. Perhaps these two parameters can be achieved by using combined methods, such as SEC combined with low-speed centrifugation, to obtain sEVs with suitable purity and yield.
Possible problems (challenges) of sEVs application in clinic
| Problems | Types | Current status | Improvement methods | Reference |
|---|---|---|---|---|
| Intrinsic problems of sEVs | Heterogeneity of sEVs | Its heterogeneity has not been standardized, and multiple properties are not discovered. Heterogeneity of sEVs in size, density, function, and origin has been reported | Keep researching | [19, 148] |
| EV subtypes | There are currently three recognized subtypes, exosomes, MVs, and apoptotic bodies. In addition, there are discovered but not fully recognized subtypes, such as exomeres. There are also some subtypes that have not been discovered. Smaller subtypes beyond existing device thresholds cannot be identified. | Nano-flow cytometry combined with high-resolution microscopy, or the recently developed HF5, can improve detection resolution. | [154, 155] | |
| Separation technology problems | Integrity of the isolated sEVs | Most of the current methods will destroy the integrity, such as UC. | Adjustment of external forces and selection of appropriate additional separation reagents can reduce integrity damage. | [46, 107, 157] |
| Purity of sEVs | Most current separation techniques cannot avoid the co-separation of some components that overlap sEVs in physicochemical properties. It is currently impossible to balance yield and purity. | Immunocapture technology, TFF and combinatorial methods are relatively superior in terms of purity. It seems to me that the technical purity that can be isolated on the basis of sEVs-specific properties is relatively high. | [38] | |
| Reproducibility of sEVs isolation method | The current low reproducibility of separation technology is still a major challenge to limit the application. | Reduce manual work and operational complexity, such as EXODUS. | [38] | |
| sEVs storage method | Selection of storage temperature and time | Most current storage methods may alter sEVs. | Try to use freshly isolated sEVs, and if you must store them, try to store them at -80°C for a short period of time. | [2] |
| Problems in the application process | The safety of clinical application | Security cannot be guaranteed. | Any therapeutic application of sEVs requires transparent reporting of data on vesicle manufacturing and characterization, appropriate quality control regulations, and preclinical safety and efficacy to ensure safety in clinical applications. | [3, 148] |
| The rapid clearance of sEVs | Remaining to be improved. | Using biomaterials, hydrogels. | [172] |
How to improve the reproducibility of sEVs isolation methods?
Favorable reproducibility is when every single operation is able to maintain consistency in the quantity and quality of isolated sEVs. The most commonly used ultracentrifugation method currently lacks reproducibility, and the isolated product is affected by the inclination angle of the centrifuge, centrifugation time, sample preparation, and various culture conditions [161]. AF4 is an isolation technique with relatively high reproducibility, but when the operator optimizes the parameters of AF4, such as cross-flow gradient, focusing time, sample ultrafiltration conditions, plasma volume, and injection volume [71], human errors and variabilities might still challenge repeatability. We hold the opinion that factors such as the complexity of the operation and the use of additional isolation reagents may lead to variable results. Therefore, automated and easy-to-operate isolation techniques are most likely to guarantee repeatability; the EXODUS technology introduced above may have a high degree of repeatability [122].
How to reduce storage-induced damage to sEVs, thereby improving the possibility of clinical application?
The current sEVs storage techniques may cause damage to the concentration, content, and integrity of sEVs, affecting the accuracy of subsequent research and the possibility of clinical application. Most people currently consider 4 °C for short-term [162] and -80 °C for long-term as an acceptable way to store sEVs [138, 163]. The effect of different temperatures on the quality and stability of sEVs is unavoidable [164]. At 4 °C, sEVs may attached to the tube wall, resulting in a decrease in the number of particles, where smaller sEVs are lost, resulting in an increase in concentration [138]. In addition, the contents of sEVs change as total RNA may be lost, and most proteins are also degraded, with the surface-associated proteins (HSP70, CD63, and CD9) altered [165]. Storage at -80 °C is the traditional method recommended in the MISEV2014 guidelines, but MISEV2018 does not give a standard storage strategy as it does affect sEVs in this case while the impact is unclear [2]. It has been experimentally demonstrated that storage at -80 °C leads to a time-dependent decrease in sEVs dose and purity, an increase in sEVs size and size deformability, and a decrease in zeta potential [165], It also resulted in altered sEVs content, decreased RNA, and decreased levels of surface marker proteins (Alix, HSP70, and TSG101) [166]. Besides, cryopreservation produces ice crystals, which disrupt lipid membranes, leading to the release of sEVs contents, resulting in the loss of biological function of sEVs [164]. Additionally, freeze-thaw cycles reportedly lead to a decrease in sEVs after the first cycle and an increase in particle size with cycling, and fusion of sEVs was found using flow cytometry [2]. However, sEVs stored in semen were not affected [167], which indicated that sEVs from different sources had distinct adaptability to different storage methods, thus increasing the difficulty of standardizing sEVs storage methods. In order to alleviate the effect of freezing on sEVs, researchers have proposed mitigation methods, such as the most commonly used methods of slow freezing and vitrification. Among them, vitrification is a rapid freezing method in which a bulk solution of cryoprotective agents (CPAs) is cooled below the glass transition temperature to avoid producing ice crystals, thereby forming an amorphous matrix, which can also be defined as a very cold viscous liquid. These approaches use low-toxicity CPAs, such as trehalose and dimethyl sulfoxide (DMSO) [168], to protect sEVs [164] and reduce ice crystal formation by increasing the total concentration of all solutes in solution [169]. Lyophilization is another technique that removes water from frozen samples by vacuum sublimation and desorption [170]. When combined with CPAs, lyophilization can improve the stability of sEVs. However, recently, Stefano Geliber and others have compared different storage strategies and showed that even the aforementioned improved method still lead to the change of the yield, size and charge of sEVs after 4 weeks and 8 months, the integrity will be destroyed, and there is no improvement compared with simple storage at -80 °C [2]. However, lyophilization and addition of CPAs over a short period of time (hours) presents a significant improvement. Certainly, the two methods are incomparable since the storage time is different. To sum up, there is no perfect storage method to protect sEVs currently, so fresh samples should be used as much as possible. According to the prevailing consensus, if storage is necessary, it is recommended to store sEVs at -80 °C for a short time in a biological matrix instead of storing separate sEVs [2].
Possible problems in future applications
What are the effects of dosage and mode of administration on biosecurity? How to ensure the safety in clinical applications?
Given that it is difficult to re-separate EV subtypes with the size of 50-150 nm using current technologies, and that the functions of specific subtypes are not well understood, it is difficult to ensure the safety of applying sEVs in the clinic. It is reported that when sEVs are applied systemically, some of them may induce apoptosis of certain cells in the body, while some may induce survival, and some others may induce immune regulation [19]. Therefore, it is possible to generate side effects beyond the target organs. Local application may help in avoiding effects on other cells. However, new problems are raised as although local application is obviously safer than systemic application, sEVs are easily to be removed with either treatments within a short time [171, 172]. Furthermore, irrespective of the systemic or local treatments, the injection dosage of sEVs should be carefully considered as different doses may cause different biological effects. For example, studies have shown that when sEVs are used to treat neurodegenerative diseases, lower doses of sEVs exhibit neuroprotective effects, while high doses may be harmful to the neurons [173]. Factors such as the cell source of sEVs, the mode of administration, and the purpose of application should also be considered to determine the dose. Dhanu et al. carefully summarized the current sEVs dosage according to different isolation methods (purified or not), measurement methods (protein amount/number of particles), experimental animals (rat, mouse and pig), route of administration (systemic/local), application purpose (cardiovascular, neurological, inflammation, and cancer) and clinical trials [3]. In short, the body's scavenging effect on sEVs and their heterogeneity enhances the difficulty in determining the precise application dose [148], which requires more efforts in future studies.
How to solve the problem of rapid clearance of sEVs by the body and improve their bioavailability?
Most systemically injected sEVs are rapidly taken up by macrophages in the reticuloendothelial system and rapidly cleared from the body. This phenomenon is ubiquitous and unaffected by the mode of delivery or the cell of origin [172]. However, topical application provides hope [172]. In order to improve the half-life of sEVs, the concept of engineered sEVs has been proposed: a sustained delivery system of sEVs designed by using biomaterials, which could prospectively improve the bioavailability and the ability of sustained delivery of sEVs. The best-studied and most commonly used biomaterial is hydrogel, which is a cross-linked three-dimensional hydrophilic polymer network structure. The polymers used to make hydrogels are divided into natural (hyaluronic acid, chitosan, and gelatin) and synthetic (PEG, PLGA, pHEMA) polymers. Natural materials are usually biodegradable and highly biocompatible, with similarities to ECM, while the synthetic ones are highly tunable in terms of structure, mechanical strength, and degradation rate [174]. sEVs can be encapsulated into hydrogels in different ways, one of which is to incorporate sEVs into hydrogels by mixing sEVs with polymers in solution form and simultaneously with a cross-linking agent [175]. This technique enables in situ gelation, allowing direct injection of hydrogel components (solvent-based polymers + exosomes + cross-linkers) at the desired site [175]. Chenggui Wang et al. developed an injectable, self-healing and antibacterial peptide-based FHE hydrogel (F127/OHA-EPL) and applied this technology showing good effects on repair [176]. The injectable delivery of the hydrogel is beneficial for its minimally invasive drug delivery to the treatment site, and it has been experimentally demonstrated that the release of sEVs can be regulated by changing the physical structure of the hydrogel to suit different needs [177]. In summary, the hydrogel's degradability, tunability of physical structure, and in situ gelation make it an sEVs delivery system that can be tailored to specific application needs [175]. Hydrogel continuous delivery system can also prevent the rapid clearance of sEVs, improve their bioavailability, and can achieve maximum biological effects with a small number of sEVs. However, it also has the disadvantage of not being appropriate for all applications. For example, the cost of natural polymers is relatively high, and some synthetic polymers that are insoluble in water (such as pHEMA) usually requires the use of strong organic solvents during processing, which may damage the structural integrity of exosomes and reduce sEVs content during mixing [175]. In addition, some hydrogels may solidify at a certain temperature and pH, blocking the injecting needle, and the kinetic release condition cannot be determined in vivo [175].
Potential for clinical application of sEVs
sEVs application in diagnostics
At present, the diagnosis methods for many diseases are still invasive. For example, when using pathological biopsy to judge benign and malignant tumors, may often causes damage to the body and is not able to monitor disease status in current time. sEVs has been developed as a new strategy for the non-invasive liquid biopsy [178]. The qualitative (and) or quantitative change of sEVs content is the basis for its use as a marker in disease diagnosis. The numbers of sEVs usually abnormally accumulated in circulation and contents differs from that of healthy status. These changes can be detected by different modern techniques. For example, when the cargos such as proteins, lipids and metabolites are used as disease markers, mass spectrometry can be used to qualitatively analyze the isolated products at the early stage of isolation as well as to determine their types and sources [94, 179]. Some more advanced techniques have been developed, for example, Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) are developed to assess vesicle enrichment [17]. High-Performance chemical isotope labeling nanoflow liquid chromatography-mass spectrometry (CIL nLC-MS) was developed to analyze the metabolomics of sEVs for more sensitive and rapid detection [180]. More importantly, cargos conveyed by sEVs can be maintained and enriched in relatively good quantity, stability as well as integrity [19] and differs depending on donor cells [181]. Many studies have shown that cargos such as proteins and miRNAs in sEVs are mostly up to the parental cell types [182], for example, adiponectin can be a specific marker for adipose tissue-derived sEVs [183]. Surface proteins of sEVs can also be tissue-specific, for instance, cancer cell-derived sEVs show tumor-associated specific proteins on the surface membrane [184]. In this case, sEVs can be quantitatively altered, cargo-protected, tissue-specific and reflect the pathological and physiological state of the body, making them ideal candidates in diagnosing and monitoring diseases.
Many studies prove sEVs as diagnostic markers in early phase of diseases and can be used to reflect disease staging or the severity degree, for example, Jia et al. demonstrated that sEVs derived GAP43, neurogranulin, SNAP25, and synaptic marker protein 1 can be used for predicting the occurrence of Alzheimer's disease 5 to 7 years before cognitive impairment [185]. Other researchers found that compared with healthy children, the serum sEVs miR-21-5p increased threefold in children with new-onset type 1 diabetes, which may be helpful for the diagnosis of type 1 diabetes [186]. There are also related clinical phase 1 trials to evaluate the role of sEVs in the diagnosis of type 1 diabetes (NCT 04164966). Ling et al. found that the serum levels of miR-126 and miR-21 in patients with acute coronary syndrome (ACS) were significantly higher than those in healthy controls, and by Gensini score, they were also positively correlated with the degree of coronary stenosis in patients [187]. And related clinical phase 1 trials are underway (NCT04127591). In addition, many new methods have been developed to specifically capture and detect sEVs for disease diagnosis. For example, Chen et al. developed a DNA cascade reactor, which can quickly realize the signal amplification detection and precise in situ imaging of miRNA in exosomes, and can also realize the tracking of exosomes in living cells [181]. Liu et al. developed an “open” fluorescent aptamer sensor platform to evaluate sEVs surface proteins, and its sensitivity can be 97.37% in distinguishing benign and malignant breast cancer [184]. Even some sEVs based commercial kits has been developed to facilitate cancer diagnosis, which indicates great potential as non-invasive liquid biopsy markers for clinical and business application [188, 189] (Table 3).
Clinical Trials for Diagnostics
| NCT | Name | Status | Diseases | Sampling | Markers | Methods |
|---|---|---|---|---|---|---|
| NCT05101655 | Construction of microfluidic exosome chip for diagnosis of lung metastasis of osteosarcoma. | Enrolling by invitation | Osteosarcoma Pulmonary Metastases | Plasma | Exosome and its subgroups | Microfluidic chip technology |
| NCT05218759 | Exosomes detection for the prediction of the efficacy and adverse reactions of Anlotinib in patients with advanced NSCLC. | Not yet recruiting | Non-Small Cell Lung Cancer | Blood | Exosomal miRNA | |
| NCT04499794 | The study of exosome EML4-ALK fusion in NSCLC clinical diagnosis and dynamic monitoring. | Recruiting | Untreated Advanced NSCLC Patients, FISH Identified ALK Fusion Positive or Negative | Plasma | Exosome EML4-ALK Fusion | Exosome ALK fusion diagnosis and FISH examination |
| NCT05035134 | Application of circulating exosomes in early diagnosis and prognosis evaluation after intracerebral hemorrhage. | Recruiting | Intracerebral Hemorrhage | Plasma | Circulating Exosomes | RNA sequencing and proteome sequencing |
| NCT04127591 | Differential expression and analysis of peripheral plasma exosome miRNA in patients with myocardial infarction. | Unknown | Myocardial Infarction | Plasma | Exosomal miRNAs | Second-generation sequencing technology, qPCR |
| NCT04155359 | Clinical evaluation of the miR sentinel BCa™ Test to diagnose bladder cancer in hematuria patients. | Recruiting | Bladder Cancer | Urine | Exosomal sncRNA | The miR Sentinel™ BCa test |
| NCT03895216 | Identification and characterization of predictive factors of onset of bone metastases in cancer patients. | Recruiting | Bone Metastases | Plasma | Exosomal miRNAs and protein | Next Generation Sequencing (NGS), Triple TOF mass spectrophotometer and variation. |
| NCT04164966 | Development of novel biomarkers for the early diagnosis of Type 1 Diabetes. | Recruiting | Type 1 Diabetes | Blood | Circulating β cell-specific exosomes | Baseline Sample Characterization |
| NCT03821909 | Acquisition of portal venous CTCs and exosomes from patients with pancreatic cancer by EUS (CTCs). | Unknown | Pancreatic Cancer | The portal venous blood | Exosomal mRNA | RNA-seq |
| NCT04529915 | Multicenter clinical research for early diagnosis of lung cancer using blood plasma derived exosome. | Active, not recruiting | Lung Cancer | Plasma | Exosomes | ELISA assay, Western blotting |
Clinical Trials for Therapy
| NCT | Name | Status | Diseases | EV Type | Administration |
|---|---|---|---|---|---|
| NCT04602104 | A clinical study of mesenchymal Stem cell exosomes nebulizer for the treatment of ARDS. | Recruiting | Acute Respiratory Distress Syndrome | hMSC-Exos | Aerosol inhalation |
| NCT04270006 | Evaluation of adipose derived stem cells Exo in treatment of periodontitis (exosomes). | Unknown | Periodontitis | Adipose derived stem cells exosomes | Local injection in the periodontal pocket |
| NCT04389385 | COVID-19 specific T cell derived exosomes (CSTC-Exo). | Active, not recruiting | Corona Virus Infection Pneumonia | COVID-19 Specific T Cell derived exosomes | Aerosol inhalation |
| NCT04798716 | The use of exosomes for the treatment of acute respiratory distress syndrome or novel coronavirus pneumonia caused by COVID-19. | Not yet recruiting | Covid19, Novel Coronavirus Pneumonia, Acute Respiratory Distress Syndrome | MSC-exosomes | Intravenous injection |
| NCT04747574 | Evaluation of the safety of CD24-exosomes in patients with COVID-19 infection. | Recruiting | SARS-CoV-2 | CD24-exosomes | Aerosol inhalation |
| NCT04849429 | Intra-discal injection of platelet-rich plasma (PRP) enriched with exosomes in chronic low back pain. | Recruiting | Chronic Low Back Pain, Degenerative Disc Disease. | Platelet rich plasma (PRP) with exosomes | Intra-discal Injection |
| NCT04276987 | A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia. | Completed | Coronavirus | MSCs-derived exosomes | Aerosol inhalation |
| NCT05060107 | Intra-articular injection of MSC-derived exosomes in knee osteoarthritis (ExoOA-1) (ExoOA-1). | Not yet recruiting | Osteoarthritis, Knee | MSC-derived Exosomes | Intra-articular Injection |
| NCT03608631 | iExosomes in treating participants with metastatic pancreas cancer with KrasG12D mutation. | Recruiting | Metastatic Pancreatic Adenocarcinoma, Pancreatic Ductal Adenocarcinoma | Mesenchymal Stromal Cells-derived Exosomes with KRAS G12D siRNA | Intravenous injection |
sEVs application in therapy
At present, there are still quite a few diseases lacking effective treatment methods or favorable drug delivery platform, such as neurodegenerative diseases and cancers. Most of the therapeutic drugs cannot efficiently reach the lesion site across the blood-brain barrier, and the bioavailability of the drugs is relatively low. Therefore, plentiful studies have explored new therapeutic methods and found that sEVs can be used as potential non-cell therapeutics. sEVs share the advantage of low immunogenicity [190], low toxicity [191, 192], low tumorigenicity and perfect biocompatibility to cross certain biological barriers such as blood-brain barrier [129, 193]. For instance, mesenchymal stem cell (MSC) sEVs are found to effectively reduce myocardial injury during myocardial infarction by transferring miR-19a, targeting SOX6, activating AKT and inhibiting JNK3/caspase-3 activation [194]. Researchers found that in Alzheimer's disease, MSC sEVs derived miR-146a can be taken up by astrocytes in vivo and promoted the recovery of astrocyte function. Therefore, sEVs mediated miR-146a transfer may contribute to the correction of cognitive impairment in AD patients [195]. Xie et al. experimentally demonstrated that miR-320a-carrying sEVs may inhibit lung cancer cell growth through the SOX4/Wnt/β-catenin axis [147]. Besides, surface transmembrane proteins of sEVs enable them to target to specific recipient cells [196] by specifically binding to receptors on these recipient cells, partially endow the characteristics like tissue-specific tropism and cell-selective fusion [26]. Natural tropism to liver makes sEVs ideal therapeutics for hepatic disease. Furthermore, given their natural advantages of mediating cell communication and their high physiochemical stability and biocompatibility, sEVs are considered excellent delivery platform for various therapeutic agents such as proteins, miRNAs, siRNAs, drugs, and even nanomaterials [197]. Some tailored strategies are also been developed to enhance the targeting ability of sEVs to specific tissues and organs and to improve their repairing efficiency.
The potential of sEVs in disease diagnosis and treatment has also been demonstrated in clinical phase I trials. For example, Prof. Qu Jieming's team conducted a Phase 1 clinical trial on the effect of nebulized MSC-sEVs in alleviating lung injury. All volunteers tolerated nebulized MSC-sEVs with no adverse reactions within 7 days (NCT04313647) [198]. Afterwards, the team also conducted a phase I clinical trial of aerosolized MSC-EVs in the treatment of acute respiratory distress syndrome and carbapenem-resistant Gram-negative bacilli pulmonary infection. In addition, clinical-grade MSC-derived exosomes (iExosomes) with KrasG12D siRNA payloads have been shown to improve pancreatic cancer mouse survival in multiple animal models [199]. And launched a phase I clinical trial of iExosome's therapy for the treatment of patients with KrasG12D mutation-related pancreatic cancer (NCT03608631). When we apply sEVs to any clinical treatment, transparent reporting of vesicle manufacturing and characterization, appropriate quality control regulations, preclinical safety and efficacy data is required to ensure safety for clinical application [200] (Table 4).
Conclusion
For the past few years, the popularity of sEVs is in a sustained growth while the transformation rate of strategies from bench-to-beside is not that satisfying. To improve the dilemma ahead, one of the most important things is to continue improving and developing simple, efficient, low-cost and highly reproducible isolation techniques and standardize the isolation strategy so as to ensure the accuracy of downstream experimental results and improve the comparability of research results. Overall, recent studies describing new isolation strategies for sEVs show great progress in harvesting high quality sEVs with increasingly convenient operating steps and high-level devices. The isolated sEVs that are used in preclinical studies and clinical trials show inspiring results in disease diagnosis, monitoring, and treatment. We expect that the resolution of these key issues would enable the use of sEVs as a novel strategy for clinical application with progressive isolation techniques being developed in the near future.
Acknowledgements
The authors sincerely thank Professor Qian Hui and Professor Xu Wenrong for their advice. And this work is supported by National Natural Science Youth Foundation of China (Grant 82001975), the Natural Science Youth Foundation of the Jiangsu Province (Grant BK20190841), The Natural Science Youth Foundation of the Jiangsu Province (Grant BK20210074). The Innovation Fund on Medicine and Education Connection of Jiangsu University (JDY2022017). Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application (SS2018003), Priority Academic Program Development of Jiangsu Higher Education Institutions Project (Phase III). We would like to thank Editage (www.editage.com) for English language editing and Figdraw for picture drawing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278-94
2. Gelibter S, Marostica G, Mandelli A, Siciliani S, Podini P, Finardi A. et al. The impact of storage on extracellular vesicles: a systematic study. J Extracell Vesicles. 2022;11:e12162
3. Gupta D, Zickler AM, El Andaloussi S. Dosing extracellular vesicles. Adv Drug Deliv Rev. 2021;178:113961
4. Azkargorta M, Iloro I, Escobes I, Cabrera D, Falcon-Perez JM, Elortza F. et al. Human serum extracellular vesicle proteomic profile depends on the enrichment method employed. Int J Mol Sci. 2021;22:11144
5. Ludwig N, Whiteside TL, Reichert TE. Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci. 2019;20:4684
6. Street JM, Koritzinsky EH, Glispie DM, Yuen PST. Urine exosome isolation and characterization. Methods Mol Biol. 2017;1641:413-23
7. Urabe F, Kosaka N, Ito K, Kimura T, Egawa S, Ochiya T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am J Physiol Cell Physiol. 2020;318:C29-c39
8. Lu Y, Liu D, Feng Q, Liu Z. Diabetic nephropathy: perspective on extracellular vesicles. Front Immunol. 2020;11:943
9. Vergauwen G, Tulkens J, Pinheiro C, Avila Cobos F, Dedeyne S, De Scheerder MA. et al. Robust sequential biophysical fractionation of blood plasma to study variations in the biomolecular landscape of systemically circulating extracellular vesicles across clinical conditions. J Extracell Vesicles. 2021;10:e12122
10. Muraoka S, Jedrychowski MP, Yanamandra K, Ikezu S, Gygi SP, Ikezu T. Proteomic profiling of extracellular vesicles derived from cerebrospinal fluid of alzheimer's disease patients: a pilot study. Cells. 2020;9:1959
11. Enciso-Martinez A, Van Der Pol E, Hau CM, Nieuwland R, Van Leeuwen TG, Terstappen L. et al. Label-free identification and chemical characterisation of single extracellular vesicles and lipoproteins by synchronous Rayleigh and Raman scattering. J Extracell Vesicles. 2020;9:1730134
12. Amari L, Germain M. Mitochondrial extracellular vesicles - origins and roles. Front Mol Neurosci. 2021;14:767219
13. Jafari D, Shajari S, Jafari R, Mardi N, Gomari H, Ganji F. et al. Designer exosomes: a new platform for biotechnology therapeutics. BioDrugs. 2020;34:567-86
14. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47
15. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17
16. Vlachakis D, Mitsis Τ, Nicolaides N, Efthimiadou A, Giannakakis A, Bacopoulou F. et al. Functions, pathophysiology and current insights of exosomal endocrinology (Review). Mol Med Rep. 2021;23:26
17. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
18. Veerman RE, Teeuwen L, Czarnewski P, Gucluler Akpinar G, Sandberg A, Cao X. et al. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J Extracell Vesicles. 2021;10:e12128
19. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977
20. Shtam T, Evtushenko V, Samsonov R, Zabrodskaya Y, Kamyshinsky R, Zabegina L. et al. Evaluation of immune and chemical precipitation methods for plasma exosome isolation. PLoS One. 2020;15:e0242732
21. van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676-705
22. Alzhrani GN, Alanazi ST, Alsharif SY, Albalawi AM, Alsharif AA, Abdel-Maksoud MS. et al. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol Int. 2021;45:1807-31
23. Aheget H, Tristán-Manzano M, Mazini L, Cortijo-Gutierrez M, Galindo-Moreno P, Herrera C. et al. Exosome: a new player in translational nanomedicine. J Clin Med. 2020;9:2380
24. Freitas D, Balmaña M, Poças J, Campos D, Osório H, Konstantinidi A. et al. Different isolation approaches lead to diverse glycosylated extracellular vesicle populations. J Extracell Vesicles. 2019;8:1621131
25. Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL. et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90
26. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-9
27. Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y. et al. Regulation of exosome production and cargo sorting. Int J Biol Sci. 2021;17:163-77
28. Ju Y, Bai H, Ren L, Zhang L. The role of exosome and the ESCRT pathway on enveloped virus infection. Int J Mol Sci. 2021;22:9060
29. An M, Wu J, Zhu J, Lubman DM. Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J Proteome Res. 2018;17:3599-605
30. Coughlan C, Bruce KD, Burgy O, Boyd TD, Michel CR, Garcia-Perez JE. et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr Protoc Cell Biol. 2020;88:e110
31. Takov K, Yellon DM, Davidson SM. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential. J Extracell Vesicles. 2019;8:1560809
32. Gandham S, Su X, Wood J, Nocera AL, Alli SC, Milane L. et al. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020;38:1066-98
33. Wang J, Ma P, Kim DH, Liu BF, Demirci U. Towards microfluidic-based exosome isolation and detection for tumor therapy. Nano Today. 2021;37:101066
34. Purushothaman A. Exosomes from cell culture-conditioned medium: isolation by ultracentrifugation and characterization. Methods Mol Biol. 2019;1952:233-44
35. Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015:319-23
36. Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M. et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9:1697028
37. Zhu L, Sun HT, Wang S, Huang SL, Zheng Y, Wang CQ. et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13:152
38. Stam J, Bartel S, Bischoff R, Wolters JC. Isolation of extracellular vesicles with combined enrichment methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1169:122604
39. Kang YT, Kim YJ, Bu J, Cho YH, Han SW, Moon BI. High-purity capture and release of circulating exosomes using an exosome-specific dual-patterned immunofiltration (ExoDIF) device. Nanoscale. 2017;9:13495-505
40. Koh YQ, Almughlliq FB, Vaswani K, Peiris HN, Mitchell MD. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front Biosci (Landmark Ed). 2018;23:865-74
41. Nordin JZ, Lee Y, Vader P, Mäger I, Johansson HJ, Heusermann W. et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11:879-83
42. Li K, Wong DK, Hong KY, Raffai RL. Cushioned-density gradient ultracentrifugation (C-DGUC): a refined and high performance method for the isolation, characterization, and use of exosomes. Methods Mol Biol. 2018;1740:69-83
43. Guo J, Wu C, Lin X, Zhou J, Zhang J, Zheng W. et al. Establishment of a simplified dichotomic size-exclusion chromatography for isolating extracellular vesicles toward clinical applications. J Extracell Vesicles. 2021;10:e12145
44. Zhang W, Yu ZL, Wu M, Ren JG, Xia HF, Sa GL. et al. Magnetic and folate functionalization enables rapid isolation and enhanced tumor-targeting of cell-derived microvesicles. ACS Nano. 2017;11:277-90
45. Langevin SM, Kuhnell D, Orr-Asman MA, Biesiada J, Zhang X, Medvedovic M. et al. Balancing yield, purity and practicality: a modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019;16:5-12
46. Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L. et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684-707
47. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789-804
48. Zhang Z, Wang C, Li T, Liu Z, Li L. Comparison of ultracentrifugation and density gradient separation methods for isolating Tca8113 human tongue cancer cell line-derived exosomes. Oncol Lett. 2014;8:1701-6
49. Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK. et al. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res Ther. 2018;9:180
50. Anderson NG. An introduction to particle separations in zonal centrifuges. Natl Cancer Inst Monogr. 1966;21:9-39
51. Rikkert LG, Engelaer M, Hau CM, Terstappen L, Nieuwland R, Coumans FAW. Rate zonal centrifugation can partially separate platelets from platelet-derived vesicles. Res Pract Thromb Haemost. 2020;4:1053-9
52. Lin C, Perrault SD, Kwak M, Graf F, Shih WM. Purification of DNA-origami nanostructures by rate-zonal centrifugation. Nucleic Acids Res. 2013;41:e40
53. Lim YJ, Lee SJ. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol Commun. 2017;5:64
54. Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine. 2017;13:2061-5
55. Karimi N, Cvjetkovic A, Jang SC, Crescitelli R, Hosseinpour Feizi MA, Nieuwland R. et al. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell Mol Life Sci. 2018;75:2873-86
56. Ter-Ovanesyan D, Norman M, Lazarovits R, Trieu W, Lee JH, Church GM. et al. Framework for rapid comparison of extracellular vesicle isolation methods. Elife. 2021;10:e70725
57. Askeland A, Borup A, Østergaard O, Olsen JV, Lund SM, Christiansen G. et al. Mass-spectrometry based proteome comparison of extracellular vesicle isolation methods: comparison of ME-kit, size-exclusion chromatography, and high-speed centrifugation. Biomedicines. 2020;8:246
58. Mørk M, Handberg A, Pedersen S, Jørgensen MM, Bæk R, Nielsen MK. et al. Prospects and limitations of antibody-mediated clearing of lipoproteins from blood plasma prior to nanoparticle tracking analysis of extracellular vesicles. J Extracell Vesicles. 2017;6:1308779
59. Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636:461773
60. Zheng H, Guan S, Wang X, Zhao J, Gao M, Zhang X. Deconstruction of heterogeneity of size-dependent exosome subpopulations from human urine by profiling N-Glycoproteomics and phosphoproteomics simultaneously. Anal Chem. 2020;92:9239-46
61. Shu S, Yang Y, Allen CL, Hurley E, Tung KH, Minderman H. et al. Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles. 2020;9:1692401
62. Weng Y, Sui Z, Shan Y, Hu Y, Chen Y, Zhang L. et al. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst. 2016;141:4640-6
63. Soares Martins T, Catita J, Martins Rosa I, O ABdCES, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. 2018;13:e0198820
64. Niu Z, Pang RTK, Liu W, Li Q, Cheng R, Yeung WSB. Polymer-based precipitation preserves biological activities of extracellular vesicles from an endometrial cell line. PLoS One. 2017;12:e0186534
65. Karttunen J, Heiskanen M, Navarro-Ferrandis V, Das Gupta S, Lipponen A, Puhakka N. et al. Precipitation-based extracellular vesicle isolation from rat plasma co-precipitate vesicle-free microRNAs. J Extracell Vesicles. 2019;8:1555410
66. Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A. et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031
67. Kang D, Oh S, Ahn SM, Lee BH, Moon MH. Proteomic analysis of exosomes from human neural stem cells by flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. J Proteome Res. 2008;7:3475-80
68. Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332-43
69. Chen X, Zhang W, Dou Y, Song T, Shen S, Dou H. Applications of asymmetrical flow field-flow fractionation for separation and characterization of polysaccharides: A review. J Chromatogr A. 2021;1635:461726
70. Oeyen E, Van Mol K, Baggerman G, Willems H, Boonen K, Rolfo C. et al. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterisation of extracellular vesicles from urine. J Extracell Vesicles. 2018;7:1490143
71. Wu B, Chen X, Wang J, Qing X, Wang Z, Ding X. et al. Separation and characterization of extracellular vesicles from human plasma by asymmetrical flow field-flow fractionation. Anal Chim Acta. 2020;1127:234-45
72. Multia E, Liangsupree T, Jussila M, Ruiz-Jimenez J, Kemell M, Riekkola ML. Automated on-line isolation and fractionation system for nanosized biomacromolecules from human plasma. Anal Chem. 2020;92:13058-65
73. Heinemann ML, Vykoukal J. Sequential Filtration: A gentle method for the isolation of functional extracellular vesicles. Methods Mol Biol. 2017;1660:33-41
74. Zeng X, Yi X, Chen L, Zhang H, Zhou R, Wu J. et al. Characterization and bioassays of extracellular vesicles extracted by tangential flow filtration. Regen Med. 2022;17:141-54
75. Dehghani M, Lucas K, Flax J, McGrath J, Gaborski T. Tangential flow microfluidics for the capture and release of nanoparticles and extracellular vesicles on conventional and ultrathin membranes. Adv Mater Technol. 2019;4:1900539
76. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727
77. Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S. et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells. 2018;7:273
78. Kim K, Park J, Jung JH, Lee R, Park JH, Yuk JM. et al. Cyclic tangential flow filtration system for isolation of extracellular vesicles. APL Bioeng. 2021;5:016103
79. Musante L, Tataruch D, Gu D, Benito-Martin A, Calzaferri G, Aherne S. et al. A simplified method to recover urinary vesicles for clinical applications, and sample banking. Sci Rep. 2014;4:7532
80. Liu F, Vermesh O, Mani V, Ge TJ, Madsen SJ, Sabour A. et al. The exosome total isolation chip. ACS Nano. 2017;11:10712-23
81. Lou D, Wang Y, Yang Q, Hu L, Zhu Q. Ultrafiltration combing with phospholipid affinity-based isolation for metabolomic profiling of urinary extracellular vesicles. J Chromatogr A. 2021;1640:461942
82. Hong CS, Muller L, Boyiadzis M, Whiteside TL. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One. 2014;9:e103310
83. Oksvold MP, Neurauter A, Pedersen KW. Magnetic bead-based isolation of exosomes. Methods Mol Biol. 2015;1218:465-81
84. Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S. et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7:1435138
85. Nakai W, Yoshida T, Diez D, Miyatake Y, Nishibu T, Imawaka N. et al. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 2016;6:33935
86. Zhu J, Zhang J, Ji X, Tan Z, Lubman DM. Column-based technology for CD9-HPLC immunoaffinity isolation of serum extracellular vesicles. J Proteome Res. 2021;20:4901-11
87. Wang W, Luo J, Wang S. Recent progress in isolation and detection of extracellular vesicles for cancer diagnostics. Adv Healthc Mater. 2018;7:e1800484
88. Heath N, Grant L, De Oliveira TM, Rowlinson R, Osteikoetxea X, Dekker N. et al. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci Rep. 2018;8:5730
89. Kosanović M, Milutinović B, Goč S, Mitić N, Janković M. Ion-exchange chromatography purification of extracellular vesicles. Biotechniques. 2017;63:65-71
90. Bruce TF, Slonecki TJ, Wang L, Huang S, Powell RR, Marcus RK. Exosome isolation and purification via hydrophobic interaction chromatography using a polyester, capillary-channeled polymer fiber phase. Electrophoresis. 2019;40:571-81
91. Jiang L, Marcus RK. Microwave-assisted grafting polymerization modification of nylon 6 capillary-channeled polymer fibers for enhanced weak cation exchange protein separations. Anal Chim Acta. 2017;954:129-39
92. Jackson KK, Powell RR, Bruce TF, Marcus RK. Rapid isolation of extracellular vesicles from diverse biofluid matrices via capillary-channeled polymer fiber solid-phase extraction micropipette tips. Analyst. 2021;146:4314-25
93. Huang S, Ji X, Jackson KK, Lubman DM, Ard MB, Bruce TF. et al. Rapid separation of blood plasma exosomes from low-density lipoproteins via a hydrophobic interaction chromatography method on a polyester capillary-channeled polymer fiber phase. Anal Chim Acta. 2021;1167:338578
94. Burton JB, Carruthers NJ, Stemmer PM. Enriching extracellular vesicles for mass spectrometry. Mass Spectrom Rev. 2021 [Epub ahead of print]
95. Kim H, Shin S. ExoCAS-2: Rapid and pure Isolation of exosomes by anionic exchange using magnetic beads. Biomedicines. 2021;9:28
96. Fenice M, Gorrasi S. Advances in chitin and chitosan science. Molecules. 2021;26:1805
97. Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K. Chitosan as a bioactive polymer: Processing, properties and applications. Int J Biol Macromol. 2017;105:1358-68
98. Guo Y, Chu M, Tan S, Zhao S, Liu H, Otieno BO. et al. Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol Pharm. 2014;11:59-70
99. Abd El-Hack ME, El-Saadony MT, Shafi ME, Zabermawi NM, Arif M, Batiha GE. et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int J Biol Macromol. 2020;164:2726-44
100. Kim SJ, Nguyen VG, Kim CU, Park BK, Huynh TL, Shin S. et al. Application of chitosan as a natural disinfectant against porcine epidemic diarrhoea virus. Acta Vet Hung. 2021;69:94-9
101. Kowalczyk P, Podgórski R, Wojasiński M, Gut G, Bojar W, Ciach T. Chitosan-human bone composite granulates for guided bone regeneration. Int J Mol Sci. 2021;22:2324
102. Bellich B, D'Agostino I, Semeraro S, Gamini A, Cesàro A. "The good, the bad and the ugly" of chitosans. Mar Drugs. 2016;14:99
103. Deregibus MC, Figliolini F, D'Antico S, Manzini PM, Pasquino C, De Lena M. et al. Charge-based precipitation of extracellular vesicles. Int J Mol Med. 2016;38:1359-66
104. Kumar A, Dhadi SR, Mai NN, Taylor C, Roy JW, Barnett DA. et al. The polysaccharide chitosan facilitates the isolation of small extracellular vesicles from multiple biofluids. J Extracell Vesicles. 2021;10:e12138
105. Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V. Chitosan as a wound dressing starting material: antimicrobial properties and mode of action. Int J Mol Sci. 2019;20:5889
106. Patrulea V, Ostafe V, Borchard G, Jordan O. Chitosan as a starting material for wound healing applications. Eur J Pharm Biopharm. 2015;97:417-26
107. Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R. et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2021;9:811971
108. Cheng S, Li Y, Yan H, Wen Y, Zhou X, Friedman L. et al. Advances in microfluidic extracellular vesicle analysis for cancer diagnostics. Lab Chip. 2021;21:3219-43
109. Hassanpour Tamrin S, Sanati Nezhad A, Sen A. Label-free isolation of exosomes using microfluidic technologies. ACS Nano. 2021 [Epub ahead of print]
110. Liu HY, Kumar R, Zhong C, Gorji S, Paniushkina L, Masood R. et al. Rapid capture of cancer extracellular vesicles by lipid patch microarrays. Adv Mater. 2021;33:e2008493
111. Gu Y, Chen C, Mao Z, Bachman H, Becker R, Rufo J. et al. Acoustofluidic centrifuge for nanoparticle enrichment and separation. Sci Adv. 2021;7:eabc0467
112. Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C. et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114:10584-9
113. Zhao S, Wu M, Yang S, Wu Y, Gu Y, Chen C. et al. A disposable acoustofluidic chip for nano/microparticle separation using unidirectional acoustic transducers. Lab Chip. 2020;20:1298-308
114. Guo S, Xu J, Estell AP, Ivory CF, Du D, Lin Y. et al. Paper-based ITP technology: An application to specific cancer-derived exosome detection and analysis. Biosens Bioelectron. 2020;164:112292
115. Ayala-Mar S, Perez-Gonzalez VH, Mata-Gómez MA, Gallo-Villanueva RC, González-Valdez J. Electrokinetically driven exosome separation and concentration using dielectrophoretic-enhanced PDMS-based microfluidics. Anal Chem. 2019;91:14975-82
116. Woo HK, Sunkara V, Park J, Kim TH, Han JR, Kim CJ. et al. Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. ACS Nano. 2017;11:1360-70
117. Zhou S, Hu T, Zhang F, Tang D, Li D, Cao J. et al. Integrated microfluidic device for accurate extracellular vesicle quantification and protein markers analysis directly from human whole blood. Anal Chem. 2020;92:1574-81
118. Asghari M, Cao X, Mateescu B, van Leeuwen D, Aslan MK, Stavrakis S. et al. Oscillatory viscoelastic microfluidics for efficient focusing and separation of nanoscale species. ACS Nano. 2020;14:422-33
119. Ghosh A, Davey M, Chute IC, Griffiths SG, Lewis S, Chacko S. et al. Rapid isolation of extracellular vesicles from cell culture and biological fluids using a synthetic peptide with specific affinity for heat shock proteins. PLoS One. 2014;9:e110443
120. Bijnsdorp IV, Maxouri O, Kardar A, Schelfhorst T, Piersma SR, Pham TV. et al. Feasibility of urinary extracellular vesicle proteome profiling using a robust and simple, clinically applicable isolation method. J Extracell Vesicles. 2017;6:1313091
121. Griffiths SG, Cormier MT, Clayton A, Doucette AA. Differential proteome analysis of extracellular vesicles from breast cancer cell lines by chaperone affinity enrichment. Proteomes. 2017;5:25
122. Chen Y, Zhu Q, Cheng L, Wang Y, Li M, Yang Q. et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods. 2021;18:212-8
123. Dao TNT, Kim MG, Koo B, Liu H, Jang YO, Lee HJ. et al. Chimeric nanocomposites for the rapid and simple isolation of urinary extracellular vesicles. J Extracell Vesicles. 2022;11:e12195
124. Sohrabi SM, Niazi A, Chahardoli M, Hortamani A, Setoodeh P. In silico investigation of lactoferrin protein characterizations for the prediction of anti-microbial properties. Mol Biol Res Commun. 2014;3:85-100
125. Beit-Yannai E, Tabak S, Stamer WD. Physical exosome:exosome interactions. J Cell Mol Med. 2018;22:2001-6
126. El Yazidi-Belkoura I, Legrand D, Nuijens J, Slomianny MC, van Berkel P, Spik G. The binding of lactoferrin to glycosaminoglycans on enterocyte-like HT29-18-C1 cells is mediated through basic residues located in the N-terminus. Biochim Biophys Acta. 2001;1568:197-204
127. Malhotra H, Sheokand N, Kumar S, Chauhan AS, Kumar M, Jakhar P. et al. Exosomes: tunable nano vehicles for macromolecular delivery of transferrin and lactoferrin to specific intracellular compartment. J Biomed Nanotechnol. 2016;12:1101-14
128. Skottvoll FS, Berg HE, Bjørseth K, Lund K, Roos N, Bekhradnia S. et al. Ultracentrifugation versus kit exosome isolation: nanoLC-MS and other tools reveal similar performance biomarkers, but also contaminations. Future Sci OA. 2019;5:Fso359
129. Yang HC, Ham YM, Kim JA, Rhee WJ. Single-step equipment-free extracellular vesicle concentration using super absorbent polymer beads. J Extracell Vesicles. 2021;10:e12074
130. Wu X, Huang X, Zhu Y, Li J, Hoffmann MR. Synthesis and application of superabsorbent polymer microspheres for rapid concentration and quantification of microbial pathogens in ambient water. Sep Purif Technol. 2020;239:116540
131. Yang M, Dong W, Cheng R, Wang H, Zhao Z, Wang F. et al. Effect of highly efficient substrate modifier, super-absorbent polymer, on the performance of the green roof. Sci Total Environ. 2022;806:150638
132. Cho S, Yang HC, Rhee WJ. Simultaneous multiplexed detection of exosomal microRNAs and surface proteins for prostate cancer diagnosis. Biosens Bioelectron. 2019;146:111749
133. Seo N, Nakamura J, Kaneda T, Tateno H, Shimoda A, Ichiki T. et al. Distinguishing functional exosomes and other extracellular vesicles as a nucleic acid cargo by the anion-exchange method. J Extracell Vesicles. 2022;11:e12205
134. Matsumura S, Minamisawa T, Suga K, Kishita H, Akagi T, Ichiki T. et al. Subtypes of tumour cell-derived small extracellular vesicles having differently externalized phosphatidylserine. J Extracell Vesicles. 2019;8:1579541
135. Seo N, Shirakura Y, Tahara Y, Momose F, Harada N, Ikeda H. et al. Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9:435
136. Momen-Heravi F. Isolation of extracellular vesicles by ultracentrifugation. Methods Mol Biol. 2017;1660:25-32
137. Montaner-Tarbes S, Pujol M, Jabbar T, Hawes P, Chapman D, Portillo HD. et al. Serum-derived extracellular vesicles from african swine fever virus-infected pigs selectively recruit viral and porcine proteins. Viruses. 2019;11:882
138. Evtushenko EG, Bagrov DV, Lazarev VN, Livshits MA, Khomyakova E. Adsorption of extracellular vesicles onto the tube walls during storage in solution. PLoS One. 2020;15:e0243738
139. Dash M, Palaniyandi K, Ramalingam S, Sahabudeen S, Raja NS. Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochim Biophys Acta Biomembr. 2021;1863:183490
140. Park S, Lee K, Park IB, Kim NH, Cho S, Rhee WJ. et al. The profiles of microRNAs from urinary extracellular vesicles (EVs) prepared by various isolation methods and their correlation with serum EV microRNAs. Diabetes Res Clin Pract. 2020;160:108010
141. Xu X, Barreiro K, Musante L, Kretz O, Lin H, Zou H. et al. Management of Tamm-Horsfall protein for reliable urinary analytics. Proteomics Clin Appl. 2019;13:e1900018
142. Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, Théry C. SnapShot: extracellular vesicles. Cell. 2020;182:262-e1
143. Royo F, Zuñiga-Garcia P, Sanchez-Mosquera P, Egia A, Perez A, Loizaga A. et al. Different EV enrichment methods suitable for clinical settings yield different subpopulations of urinary extracellular vesicles from human samples. J Extracell Vesicles. 2016;5:29497
144. Pacienza N, Lee RH, Bae EH, Kim DK, Liu Q, Prockop DJ. et al. In vitro macrophage assay predicts the in vivo anti-inflammatory potential of exosomes from human mesenchymal stromal cells. Mol Ther Methods Clin Dev. 2019;13:67-76
145. Liu J, Ren L, Li S, Li W, Zheng X, Yang Y. et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783-97
146. Huang C, Luo W, Wang Q, Ye Y, Fan J, Lin L. et al. Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res. 2021;52:102235
147. Xie H, Wang J. MicroRNA-320a-containing exosomes from human umbilical cord mesenchymal stem cells curtail proliferation and metastasis in lung cancer by binding to SOX4. J Recept Signal Transduct Res. 2021:1-11
148. Skuratovskaia D, Vulf M, Khaziakhmatova O, Malashchenko V, Komar A, Shunkin E. et al. Exosome limitations in the treatment of inflammatory diseases. Curr Pharm Des. 2021;27:3105-21
149. Wen SW, Lima LG, Lobb RJ, Norris EL, Hastie ML, Krumeich S. et al. Breast cancer-derived exosomes reflect the cell-of-origin phenotype. Proteomics. 2019;19:e1800180
150. Chanteloup G, Cordonnier M, Isambert N, Bertaut A, Hervieu A, Hennequin A. et al. Monitoring HSP70 exosomes in cancer patients' follow up: a clinical prospective pilot study. J Extracell Vesicles. 2020;9:1766192
151. Pang H, Luo S, Xiao Y, Xia Y, Li X, Huang G. et al. Emerging roles of exosomes in T1DM. Front Immunol. 2020;11:593348
152. van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23:369-382
153. Kugeratski FG, Hodge K, Lilla S, McAndrews KM, Zhou X, Hwang RF. et al. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat Cell Biol. 2021;23:631-41
154. Choi D, Spinelli C, Montermini L, Rak J. Oncogenic regulation of extracellular vesicle proteome and heterogeneity. Proteomics. 2019;19:e1800169
155. Choi D, Montermini L, Jeong H, Sharma S, Meehan B, Rak J. Mapping subpopulations of cancer cell-derived extracellular vesicles and particles by nano-flow cytometry. ACS Nano. 2019;13:10499-511
156. Marassi V, Maggio S, Battistelli M, Stocchi V, Zattoni A, Reschiglian P. et al. An ultracentrifugation - hollow-fiber flow field-flow fractionation orthogonal approach for the purification and mapping of extracellular vesicle subtypes. J Chromatogr A. 2021;1638:461861
157. Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3-10
158. Brennan K, Martin K, FitzGerald SP, O'Sullivan J, Wu Y, Blanco A. et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020;10:1039
159. Smith ZJ, Lee C, Rojalin T, Carney RP, Hazari S, Knudson A. et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles. 2015;4:28533
160. Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI. et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066
161. Cvjetkovic A, Lötvall J, Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014;3:10.3402 /jev.v3.23111
162. Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S. et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632-48
163. Bosch S, de Beaurepaire L, Allard M, Mosser M, Heichette C, Chrétien D. et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. 2016;6:36162
164. Qin B, Zhang Q, Hu XM, Mi TY, Yu HY, Liu SS. et al. How does temperature play a role in the storage of extracellular vesicles? J Cell Physiol. 2020;235:7663-80
165. Maroto R, Zhao Y, Jamaluddin M, Popov VL, Wang H, Kalubowilage M. et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J Extracell Vesicles. 2017;6:1359478
166. Cheng Y, Zeng Q, Han Q, Xia W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell. 2019;10:295-9
167. Welch JL, Madison MN, Margolick JB, Galvin S, Gupta P, Martínez-Maza O. et al. Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci Rep. 2017;7:45034
168. Tegegn TZ, De Paoli SH, Orecna M, Elhelu OK, Woodle SA, Tarandovskiy ID. et al. Characterization of procoagulant extracellular vesicles and platelet membrane disintegration in DMSO-cryopreserved platelets. J Extracell Vesicles. 2016;5:30422
169. Charoenviriyakul C, Takahashi Y, Nishikawa M, Takakura Y. Preservation of exosomes at room temperature using lyophilization. Int J Pharm. 2018;553:1-7
170. Wakayama S, Ito D, Kamada Y, Yonemura S, Ooga M, Kishigami S. et al. Tolerance of the freeze-dried mouse sperm nucleus to temperatures ranging from -196 °C to 150 °C. Sci Rep. 2019;9:5719
171. Charoenviriyakul C, Takahashi Y, Morishita M, Matsumoto A, Nishikawa M, Takakura Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics. Eur J Pharm Sci. 2017;96:316-22
172. Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135-40
173. Venugopal C, Shamir C, Senthilkumar S, Babu JV, Sonu PK, Nishtha KJ. et al. Dosage and passage dependent neuroprotective effects of exosomes derived from rat bone marrow mesenchymal stem cells: an in vitro analysis. Curr Gene Ther. 2017;17:379-90
174. Brennan M, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020;30:1909125
175. Riau AK, Ong HS, Yam GHF, Mehta JS. Sustained delivery system for stem cell-derived exosomes. Front Pharmacol. 2019;10:1368
176. Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W. et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65-76
177. Nikravesh N, Davies OG, Azoidis I, Moakes RJA, Marani L, Turner M. et al. Physical structuring of injectable polymeric systems to controllably deliver nanosized extracellular vesicles. Adv Healthc Mater. 2019;8:e1801604
178. Yu D, Li Y, Wang M, Gu J, Xu W, Cai H. et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21:56
179. Jalaludin I, Lubman DM, Kim J. A guide to mass spectrometric analysis of extracellular vesicle proteins for biomarker discovery. Mass Spectrom Rev. 2021: e21749.
180. Luo X, An M, Cuneo KC, Lubman DM, Li L. High-performance chemical isotope labeling liquid chromatography mass spectrometry for exosome metabolomics. Anal Chem. 2018;90:8314-9
181. Chen J, Xie M, Shi M, Yuan K, Wu Y, Meng HM. et al. Spatial confinement-derived double-accelerated DNA cascade reaction for ultrafast and highly sensitive in situ monitoring of exosomal miRNA and exosome tracing. Anal Chem. 2022;94:2227-35
182. Garcia-Martin R, Wang G, Brandão BB, Zanotto TM, Shah S, Kumar Patel S. et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446-51
183. Garcia-Martin R, Brandao BB, Thomou T, Altindis E, Kahn CR. Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism. Cell Rep. 2022;38:110277
184. Li B, Liu C, Pan W, Shen J, Guo J, Luo T. et al. Facile fluorescent aptasensor using aggregation-induced emission luminogens for exosomal proteins profiling towards liquid biopsy. Biosens Bioelectron. 2020;168:112520
185. Jia L, Zhu M, Kong C, Pang Y, Zhang H, Qiu Q. et al. Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. Alzheimers Dement. 2021;17:49-60
186. Lakhter AJ, Pratt RE, Moore RE, Doucette KK, Maier BF, DiMeglio LA. et al. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia. 2018;61:1124-34
187. Ling H, Guo Z, Shi Y, Zhang L, Song C. Serum exosomal microRNA-21, microRNA-126, and PTEN are novel biomarkers for diagnosis of acute coronary syndrome. Front Physiol. 2020;11:654
188. Monteiro LJ, Varas-Godoy M, Monckeberg M, Realini O, Hernández M, Rice G. et al. Oral extracellular vesicles in early pregnancy can identify patients at risk of developing gestational diabetes mellitus. PLoS One. 2019;14:e0218616
189. Purghè B, Manfredi M, Ragnoli B, Baldanzi G, Malerba M. Exosomes in chronic respiratory diseases. Biomed Pharmacother. 2021;144:112270
190. Somiya M, Yoshioka Y, Ochiya T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J Extracell Vesicles. 2018;7:1440132
191. Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A. et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6:1324730
192. Gudbergsson JM, Jønsson K, Simonsen JB, Johnsen KB. Systematic review of targeted extracellular vesicles for drug delivery - Considerations on methodological and biological heterogeneity. J Control Release. 2019;306:108-20
193. Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P. et al. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172-7
194. Huang L, Yang L, Ding Y, Jiang X, Xia Z, You Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle. 2020;19:339-53
195. Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N. et al. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer's disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10:10772
196. Song Y, Li Z, He T, Qu M, Jiang L, Li W. et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. 2019;9:2910-23
197. Zeng W, Wen Z, Chen H, Duan Y. Exosomes as Carriers for Drug Delivery in Cancer Therapy. Pharm Res. 2022 [Epub ahead of print]
198. Shi MM, Yang QY, Monsel A, Yan JY, Dai CX, Zhao JY. et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J Extracell Vesicles. 2021;10:e12134
199. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503
200. Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G. et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087
Author contact
![]() Corresponding authors: Shi Hui, E-mail: shihuiedu.cn; Sun Yaoxiang, E-mail: staff2037com; Qian Hui, E-mail: lstmmmlstcom.
Corresponding authors: Shi Hui, E-mail: shihuiedu.cn; Sun Yaoxiang, E-mail: staff2037com; Qian Hui, E-mail: lstmmmlstcom.
 Global reach, higher impact
Global reach, higher impact