13.3
Impact Factor
Theranostics 2022; 12(8):3977-3994. doi:10.7150/thno.70852 This issue Cite
Research Paper
Drug-induced self-assembled nanovesicles for doxorubicin resistance reversal via autophagy inhibition and delivery synchronism
Ministry of Educational (MOE) Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China
Abstract
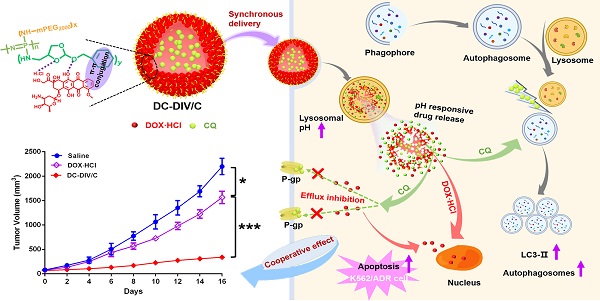
Background: As a classical autophagy inhibitor, CQ has been supposed to increase the sensitivity of tumors to chemotherapeutics. However, there exists a quite huge gap between laboratory research and clinical application, which is related to the distinct pharmacokinetic behavior of CQ to a great extent.
Methods: Based on amphiphilic copolymer PPAP, a pH-responsive drug-induced self-assembled nanovesicle, named DC-DIV/C, was constructed to load DOX⋅HCl and CQ. The physicochemical properties of DC-DIV/C were characterized. To validate the cooperative action and delivery synchronism of DOX⋅HCl and CQ, cytotoxicity, apoptosis, cellular uptake and autophagy assay were investigated in DOX⋅HCl resistant cancer cells. The pharmacokinetic character and antitumor effect of DC-DIV/C were evaluated on rats and nude mice bearing xenograft drug-resistant K562/ADR tumors, respectively.
Results: DC-DIV/C could simultaneously encapsulate DOX·HCl and CQ at the optimal ratio of 1:2. In vitro and in vivo tests confirmed that DC-DIV/C acted as an excellent vehicle for the synchronous delivery of DOX⋅HCl and CQ during the process of blood circulation, cellular uptake and intracellular release. Furthermore, CQ accomplished autophagy inhibition to reduce the IC50 of DOX⋅HCl resistant cancer cells. Consequently, DC-DIV/C exhibited the extremely improved anti-tumor effect with 84.52% TIR on K562/ADR tumor.
Conclusion: This study provides a promising and powerful strategy to achieve enhanced treatment outcomes for the precise combination therapy.
Keywords: multidrug resistance, autophagy, doxorubicin, chloroquine, nanovesicle
 Global reach, higher impact
Global reach, higher impact