13.3
Impact Factor
Theranostics 2022; 12(7):3084-3103. doi:10.7150/thno.70549 This issue Cite
Research Paper
Counterintuitive production of tumor-suppressive secretomes from Oct4- and c-Myc-overexpressing tumor cells and MSCs
1. Department of Pharmacology, School of Pharmacy, Harbin Medical University, Harbin 150081, China.
2. Department of Biomedical Engineering, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202, USA.
3. Department of Orthopedic Surgery, Mie University, Mie 514, Japan.
4. Department of Comparative Pathobiology, Purdue University, West Lafayette, IN 47907, USA.
5. Indiana Center for Musculoskeletal Health, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
6. Simon Cancer Center, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
#These authors contributed equally to this study.
Abstract
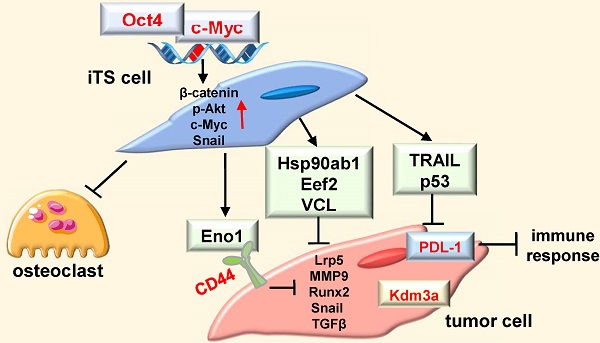
Background: Advanced breast cancer frequently metastasizes to bone, but inhibiting tumor progression in chemotherapy may occasionally enhance tumorigenesis. Here, we employed a counterintuitive approach of overexpressing Yamanaka factors (Oct4, c-Myc, Sox2, and Klf4) and examined a conditioned medium (CM)-based treatment option with induced tumor-suppressing cells (iTSCs).
Methods: In vitro proliferation and migration assays were conducted using tumor cell lines derived from breast cancer, as well as prostate and pancreatic cancers, and osteosarcoma. The tumor-suppressing capability of iTSC-derived CM was evaluated using freshly isolated breast cancer tissues and a mouse model of mammary tumors and tumor-induced osteolysis. The regulatory mechanism was evaluated using Western blotting, immunoprecipitation, pull-down, gene overexpression, and RNA interference based on mass spectrometry-based proteomics data.
Results: The overexpression of Oct4 and c-Myc in tumor cells and MSCs, but not Sox2 or Klf4, generated anti-tumor CM, which suppressed the progression of mammary tumors and tumor-induced bone loss. Notably, CM downregulated histone demethylase, and PDL-1, a blocker of T-cell-based immune responses. Whole-genome proteomics predicted enolase 1 (Eno1), Hsp90ab1, Eef2, and vinculin as extracellular tumor suppressors. Specifically, CD44 was co-immunoprecipitated with Eno1 and the silencing of CD44 suppressed Eno1's anti-tumor action. The overexpression of Oct4 and c-Myc also generated secretomes that inhibited the development of bone-resorbing osteoclasts.
Conclusions: In analogous to cell competition in which Myc-overexpressing cells in Drosophila and mouse embryos remove neighboring cells with a lower level of Myc, this study presented the possibility of eliminating tumor cells by the secretory proteomes derived from Myc/Oc4-overexpressing iTSCs.
Keywords: breast cancer, cell competition, Oct4, c-Myc, enolase 1
 Global reach, higher impact
Global reach, higher impact