13.3
Impact Factor
Theranostics 2022; 12(6):2741-2757. doi:10.7150/thno.66456 This issue Cite
Research Paper
Plaque-targeted, proteolysis-resistant, activatable and MRI-visible nano-GLP-1 receptor agonist targets smooth muscle cell differentiation in atherosclerosis
1. Case Western Reserve University, Cleveland, OH
2. Dorothy M. Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH
3. University of Maryland, Baltimore, MD
4. CVPath Institute, Inc., Gaithersburg, MD
5. NYU Langone Medical Center, New York, NY
# These authors contributed equally to this work
Abstract
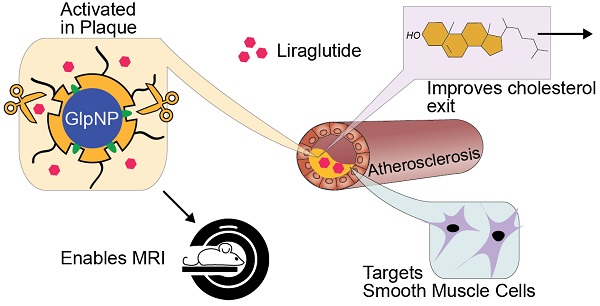
Background: Glucagon-like peptide-1 receptor (GLP-1R) agonists are powerful glycemia-lowering agents, which have systematically been shown to lower cardiovascular events and mortality. These beneficial effects were difficult to pinpoint within atherosclerotic plaque due to lack of particular specificity of such agonists to the vascular cells and an inadequate understanding of the GLP-1R expression in atherosclerosis. Here, we hypothesized that the direct engagement of the GLP-1R in atherosclerosis by targeted agonists will alleviate vascular inflammation and plaque burden, even at a very low dose.
Methods: The expression of GLP-1 receptor (GLP-1R, Glp1r mRNA) in human lesions with pathologic intimal thickening, Apoe-/- mouse atheroma and cultured immune/non-immune cells was investigated using genetic lineage tracing, Southern blotting and validated antisera against human GLP-1R. Protease-resistant and “activatable” nanoparticles (NPs) carrying GLP-1R agonist liraglutide (GlpNP) were engineered and synthesized. Inclusion of gadolinium chelates into GlpNP allowed for imaging by MRI. Atherosclerotic Apoe-/- mice were treated intravenously with a single dose (30 µg/kg of liraglutide) or chronically (1 µg/kg, 6 weeks, 2x/week) with GlpNP, liraglutide or control NPs, followed by assessment of metabolic parameters, atheroma burden, inflammation and vascular function.
Results: Humal plaque specimens expressed high levels of GLP-1R within the locus of de-differentiated smooth muscle cells that also expressed myeloid marker CD68. However, innate immune cells under a variety of conditions expressed very low levels of Glp1r, as seen in lineage tracing and Southern blotting experiments examining full-length open reading frame mRNA transcripts. Importantly, de-differentiated vascular smooth muscle cells demonstrated significant Glp1r expression levels, suggesting that these could represent the cells with predominant Glp1r-positivity in atherosclerosis. GlpNP resisted proteolysis and demonstrated biological activity including in vivo glycemia lowering at 30 µg/kg and in vitro cholesterol efflux. Activatable properties of GlpNP were confirmed in vitro by imaging cytometry and in vivo using whole organ imaging. GlpNP targeted CD11b+/CD11c+ cells in circulation and smooth muscle cells in aortic plaque in Apoe-/- mice when assessed by MRI and fluorescence imaging. At a very low dose of 1 µg/kg, previously known to have little effect on glycemia and weight loss, GlpNP delivered i.v. for six weeks reduced triglyceride-rich lipoproteins in plasma, plaque burden and plaque cholesterol without significant effects on weight, glycemia and plasma cholesterol levels.
Conclusions: GlpNP improves atherosclerosis at weight-neutral doses as low as 1 µg/kg with the effects independent from the pancreas or the central nervous system. Our study underlines the importance of direct actions of GLP-1 analogs on atherosclerosis, involving cholesterol efflux and inflammation. Our findings are the first to suggest the therapeutic modulation of vascular targets by GlpNP, especially in the context of smooth muscle cell inflammation.
Keywords: GLP-1, atherosclerosis, nanomedicine, MRI, smooth muscle cells
 Global reach, higher impact
Global reach, higher impact